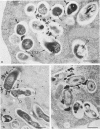
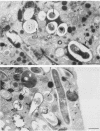
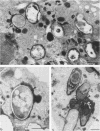
Abstract
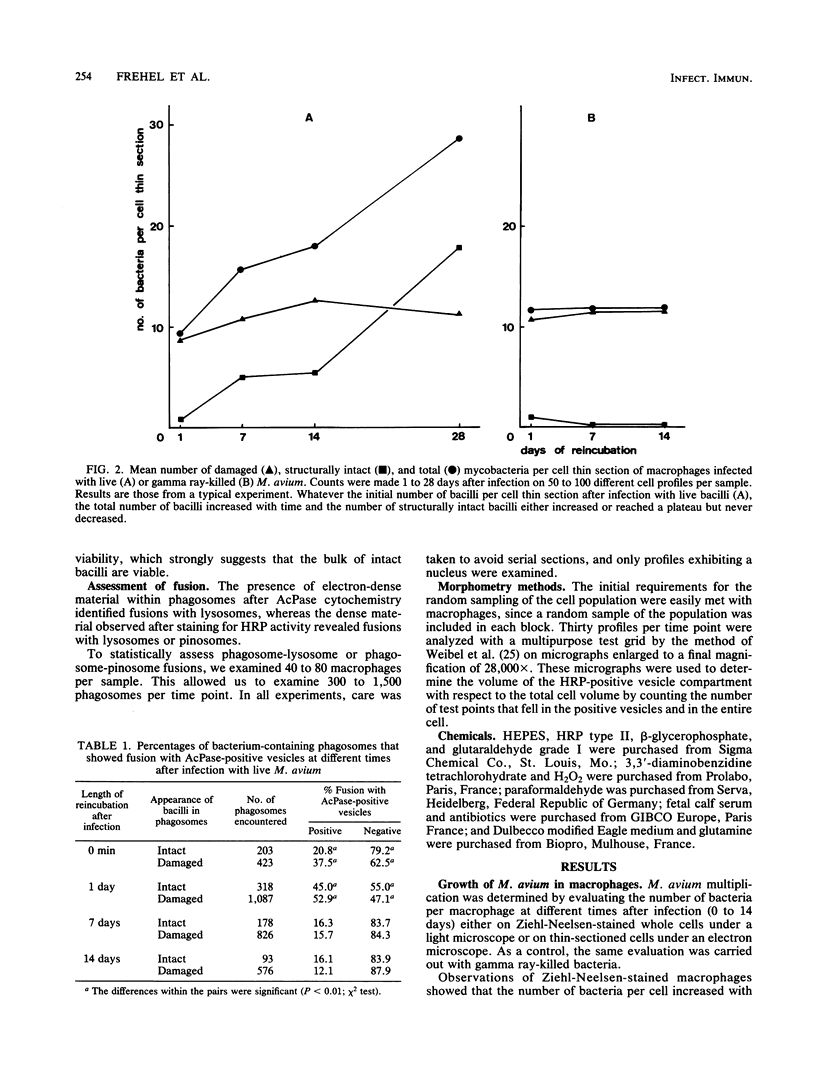
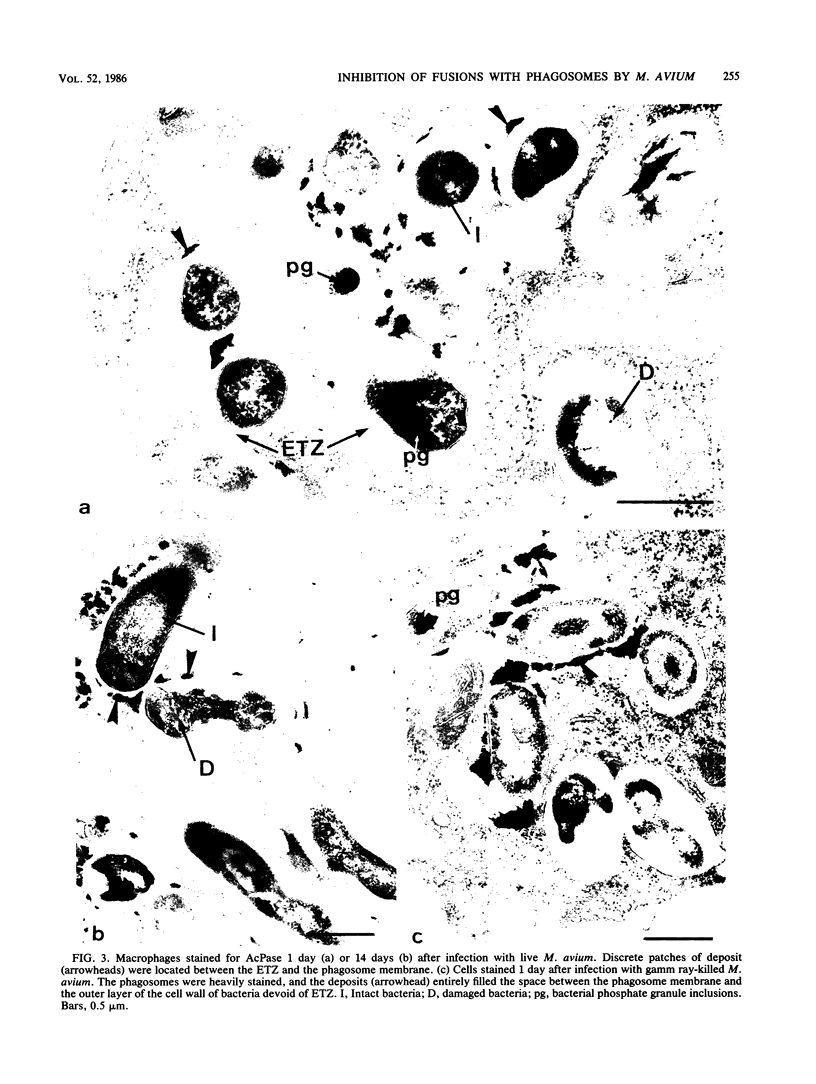
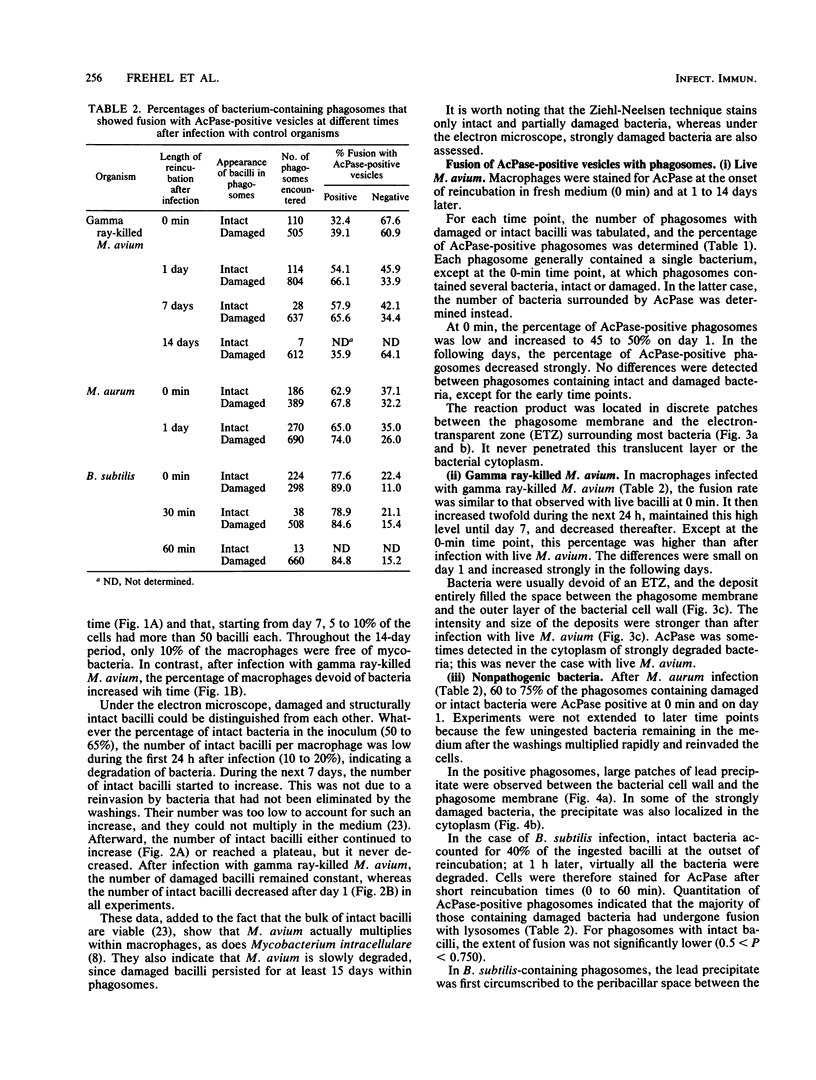
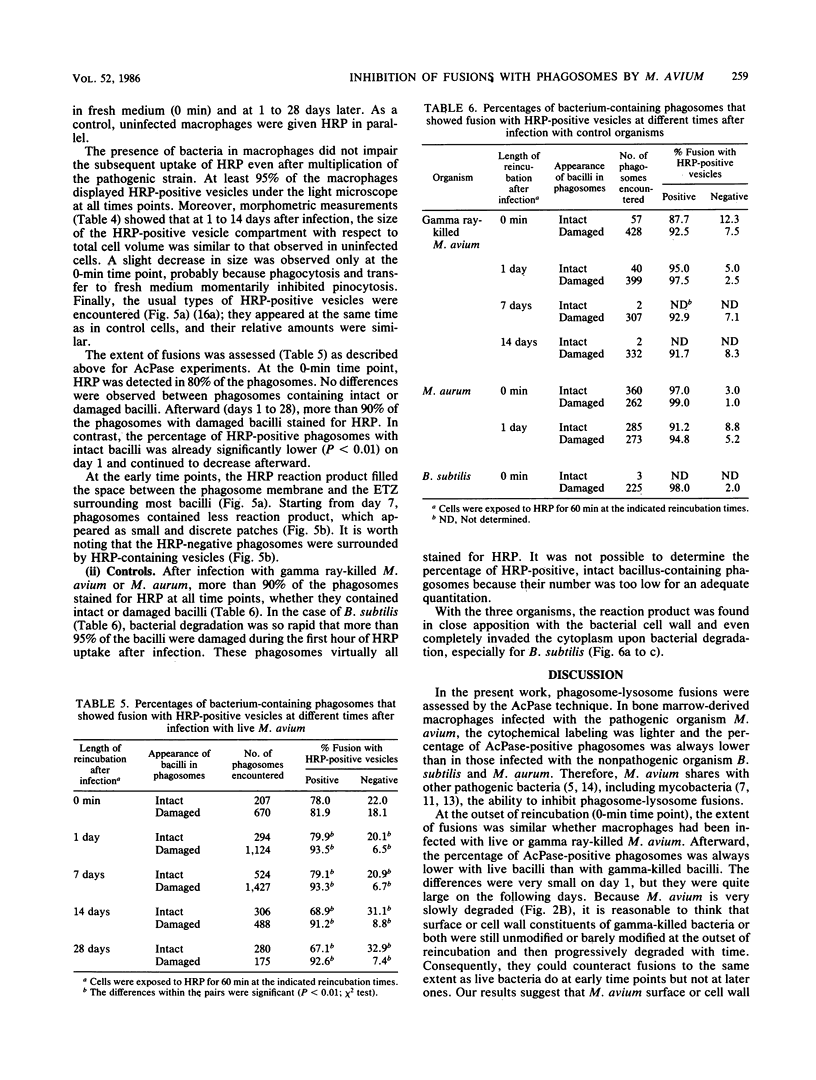
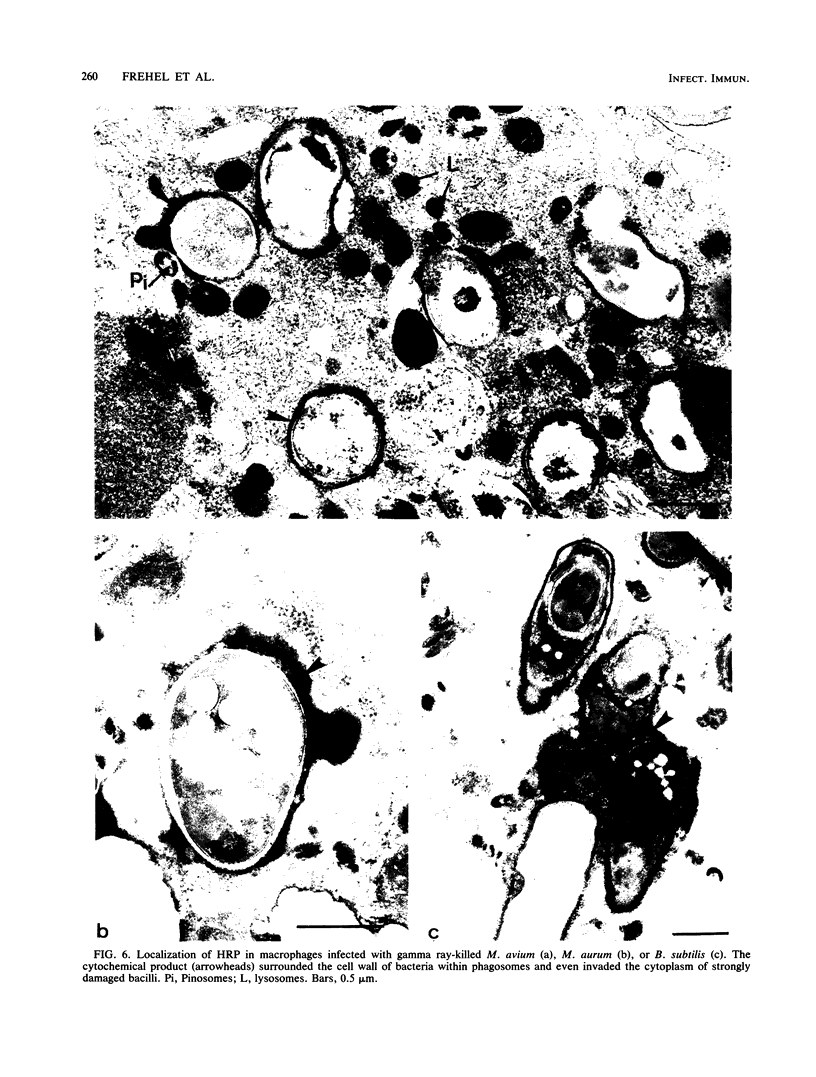
Bone marrow-derived cultured macrophages were infected with the pathogenic organism Mycobacterium avium. Immediately after infection and at 1 to 28 days later, cells either were stained for acid phosphatase activity or given horseradish peroxidase, which served as a pinocytotic marker. With the former, fusions between phagosomes and lysosomes exclusively were assessed; with the latter, those between phagosomes and both pinosomes and lysosomes were determined. As a control, similar experiments were undertaken by infecting macrophages with gamma ray-killed M. avium and the nonpathogenic live organisms Mycobacterium aurum and Bacillus subtilis. After infection with live M. avium, fusions between phagosomes and acid phosphatase-positive vesicles (lysosomes) were inhibited. The same inhibition was observed whether phagosomes contained damaged or structurally intact (presumed to be live) bacteria, except for the early time points. This inhibition was, however, partial, suggesting that some of the live bacteria are resistant to the hydrolytic enzymes of the phagolysosomal environment. Fusions between horseradish peroxidase-positive vesicles (pinosomes and lysosomes) and phagosomes depended upon the morphological state of the bacteria. Damaged bacteria did not inhibit fusions, whereas with intact bacteria, a partial inhibition which increased with time was observed. The two types of experiment suggest that viable M. avium can impair phagosome-pinosome fusions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C. M., Beaman B. L., Donovan R. M., Goldstein E. Effect of virulent and less virulent strains of Nocardia asteroides on acid-phosphatase activity in alveolar and peritoneal macrophages maintained in vitro. J Infect Dis. 1983 Jul;148(1):117–124. doi: 10.1093/infdis/148.1.117. [DOI] [PubMed] [Google Scholar]
- Brown C. A., Draper P., Hart P. D. Mycobacteria and lysosomes: a paradox. Nature. 1969 Feb 15;221(5181):658–660. doi: 10.1038/221658a0. [DOI] [PubMed] [Google Scholar]
- Davidson P. T. Treatment and long-term follow-up of patients with atypical mycobacterial infections. Bull Int Union Tuberc. 1976;51(1):257–261. [PubMed] [Google Scholar]
- Davis-Scibienski C., Beaman B. L. Interaction of Nocardia asteroides with rabbit alveolar macrophages: association of virulence, viability, ultrastructural damage, and phagosome-lysosome fusion. Infect Immun. 1980 May;28(2):610–619. doi: 10.1128/iai.28.2.610-619.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Phagocyte strategy vs. microbial tactics. Rev Infect Dis. 1980 Sep-Oct;2(5):817–838. doi: 10.1093/clinids/2.5.817. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Pratt P. F. In vitro response of murine alveolar and peritoneal macrophages to Mycobacterium intracellulare. Am Rev Respir Dis. 1983 Dec;128(6):1044–1047. doi: 10.1164/arrd.1983.128.6.1044. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A. Strain virulence and the lysosomal response in macrophages infected with Mycobacterium tuberculosis. Infect Immun. 1974 Oct;10(4):742–746. doi: 10.1128/iai.10.4.742-746.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M. Studies of lysosomal enzymes in macrophages. III. Lysosomal enzyme activities in cultured macrophages infected with some species of mycobacteria. Hiroshima J Med Sci. 1981 Jun;30(2):99–107. [PubMed] [Google Scholar]
- Lang T., de Chastellier C. Fluid phase and mannose receptor-mediated uptake of horseradish peroxidase in mouse bone marrow-derived macrophages. Biochemical and ultrastructural study. Biol Cell. 1985;53(2):149–154. doi: 10.1111/j.1768-322x.1985.tb00362.x. [DOI] [PubMed] [Google Scholar]
- Lowrie D. B., Aber V. R., Jackett P. S. Phagosome-lysosome fusion and cyclic adenosine 3':5'-monophosphate in macrophages infected with Mycobacterium microti, Mycobacterium bovis BCG or Mycobacterium lepraemurium. J Gen Microbiol. 1979 Feb;110(2):431–441. doi: 10.1099/00221287-110-2-431. [DOI] [PubMed] [Google Scholar]
- Malmgren L., Olsson Y. A sensitive histochemical method for light-and electron-microscopic demonstration of horseradish peroxidase. J Histochem Cytochem. 1977 Nov;25(11):1280–1283. doi: 10.1177/25.11.915248. [DOI] [PubMed] [Google Scholar]
- Milatovic D. Antibiotics and phagocytosis. Eur J Clin Microbiol. 1983 Oct;2(5):414–425. doi: 10.1007/BF02013898. [DOI] [PubMed] [Google Scholar]
- Myrvik Q. N., Leake E. S., Wright M. J. Disruption of phagosomal membranes of normal alveolar macrophages by the H37Rv strain of Mycobacterium tuberculosis. A correlate of virulence. Am Rev Respir Dis. 1984 Feb;129(2):322–328. [PubMed] [Google Scholar]
- Redmond W. B., Ward D. M. Media and methods for phage-typing mycobacteria. Bull World Health Organ. 1966;35(4):563–568. [PMC free article] [PubMed] [Google Scholar]
- Ryter A., De Chastellier C. Phagocyte--pathogenic microbe interactions. Int Rev Cytol. 1983;85:287–327. [PubMed] [Google Scholar]
- Ryter A., Frehel C., Rastogi N., David H. L. Macrophage interaction with mycobacteria including M. leprae. Acta Leprol. 1984 Oct-Dec;2(2-4):211–226. [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- de Chastellier C., Lang T. Exchange of material between the extracellular medium and macrophage phagosomes containing different species of bacteria including mycobacteria. Acta Leprol. 1984 Oct-Dec;2(2-4):249–257. [PubMed] [Google Scholar]