Abstract
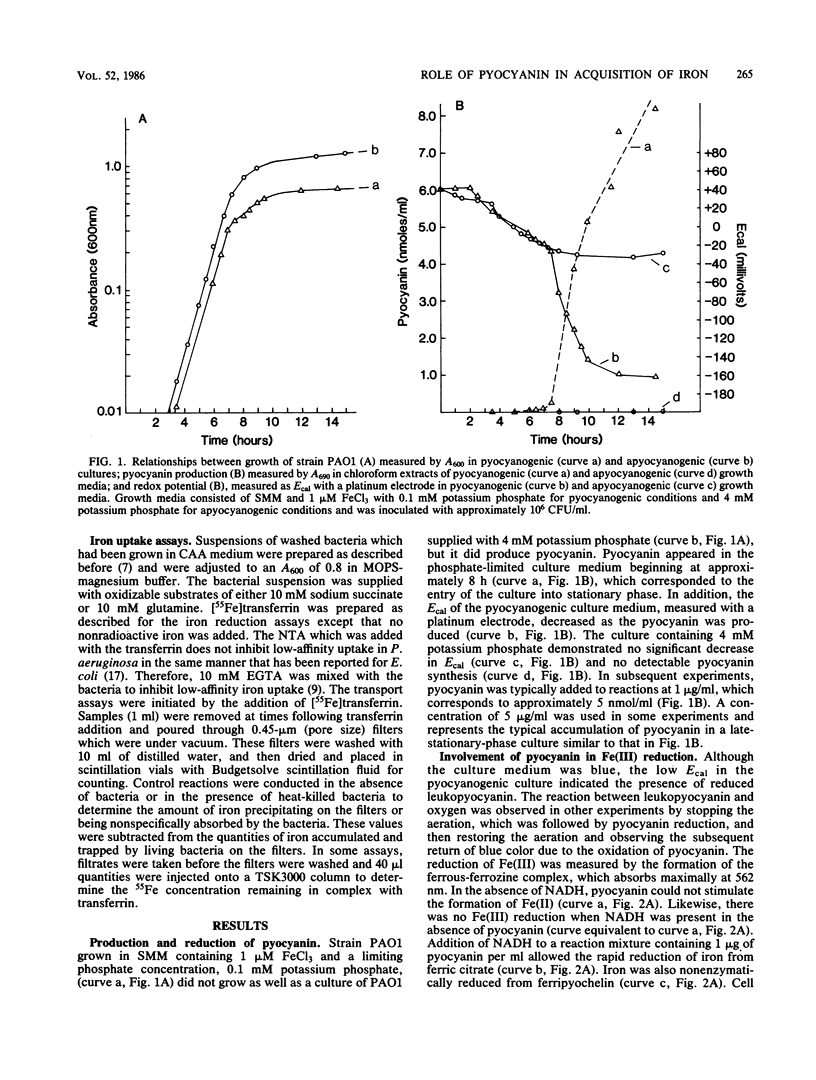
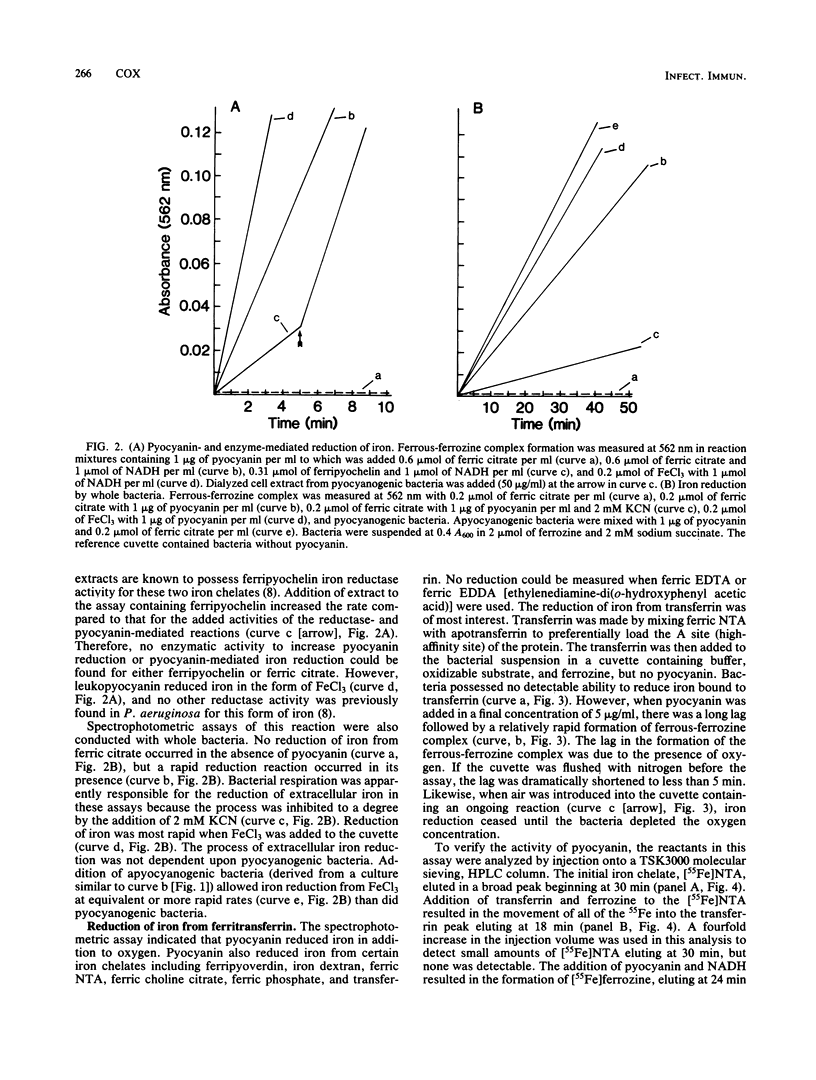
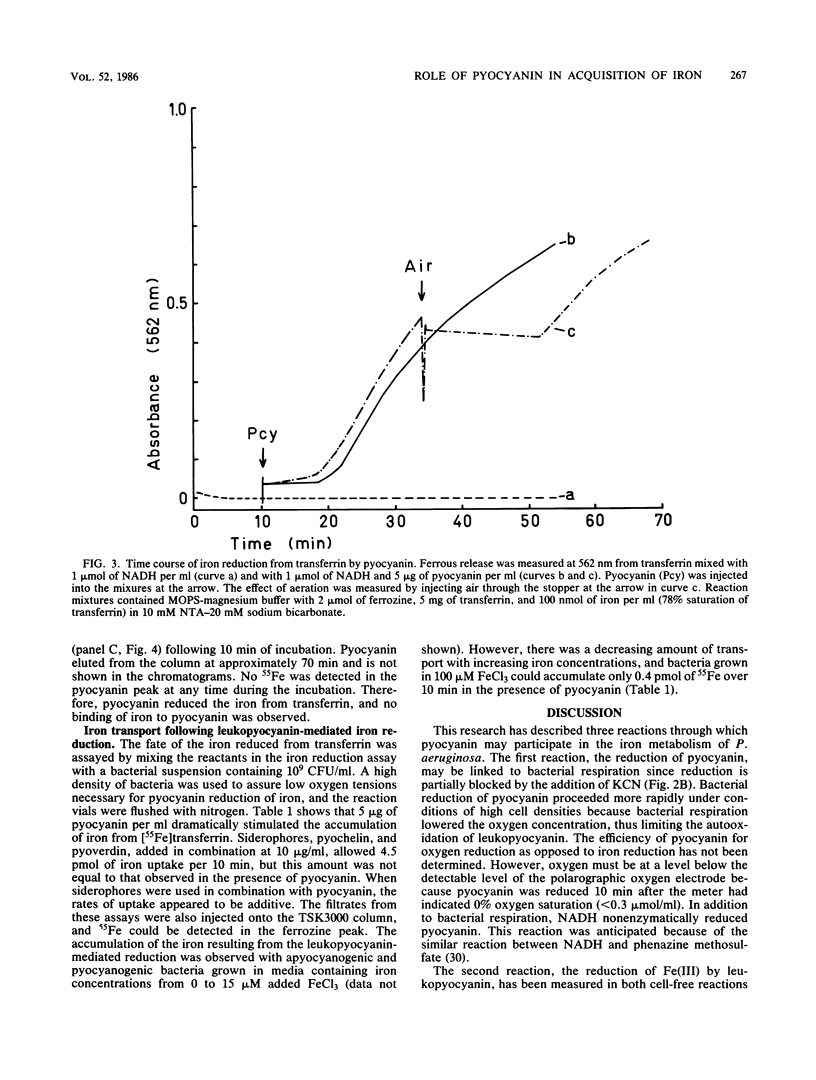
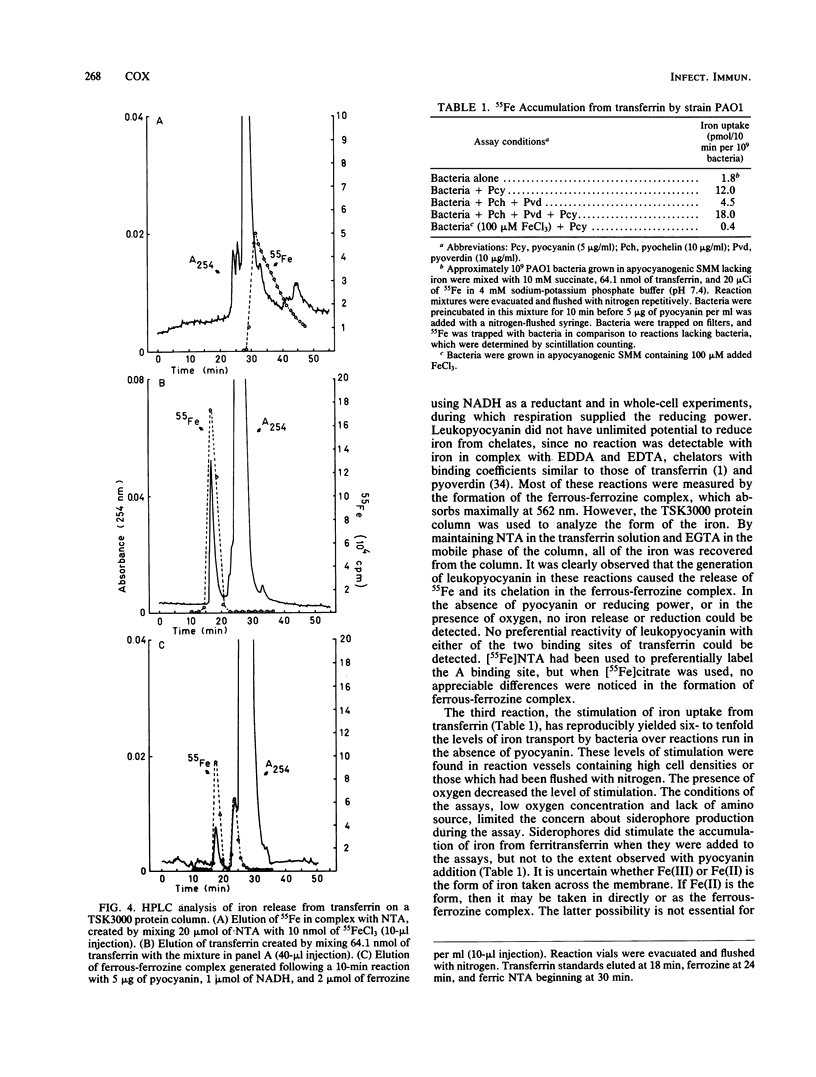
Pseudomonas aeruginosa produces a blue pigment called pyocyanin. In the presence of oxidizable substrates, bacteria reduce this pigment to a colorless product, leukopyocyanin. Pyocyanin can also be nonenzymatically reduced by NADH. Leukopyocyanin formed by cell- or NADH-mediated reduction nonenzymatically reduces oxygen or Fe(III). Pyocyanin-dependent iron reduction by whole bacterial cells was measured by the formation of the ferrous-ferrozine complex. In addition, leukopyocyanin reduced chelated Fe(III) including ferric iron in complex with transferrin, the serum iron-binding protein. High-pressure liquid chromatography was used to display the reductive removal of iron from transferrin and the accumulation of iron in the ferrous-ferrozine complex. Pyocyanin stimulated the accumulation of 55Fe from [55Fe]transferrin when it was added to bacteria incubated under low-oxygen conditions. Although bacteria grown in the presence of 100 microM FeCl3 reduced pyocyanin just as rapidly as iron-limited bacteria, these cells did not accumulate iron in the presence or absence of pyocyanin. Therefore, P. aeruginosa participates indiscriminantly in the reduction of pyocyanin, but soluble or available iron generated by the pyocyanin is taken up specifically by iron-limited bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Ankenbauer R., Sriyosachati S., Cox C. D. Effects of siderophores on the growth of Pseudomonas aeruginosa in human serum and transferrin. Infect Immun. 1985 Jul;49(1):132–140. doi: 10.1128/iai.49.1.132-140.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong A. V., Stewart-Tull D. E. The site of the activity of extracellular products of Pseudomonas aeruginosa in the electron-transport chain in mammalian cell respiration. J Med Microbiol. 1971 May;4(2):263–270. doi: 10.1099/00222615-4-2-263. [DOI] [PubMed] [Google Scholar]
- Brown K. A., Ratledge C. Iron transport in Mycobacterium smegmatis: ferrimycobactin reductase (nad(p)h:ferrimycobactin oxidoreductase), the enzyme releasing iron from its carrier. FEBS Lett. 1975 May 1;53(2):262–266. doi: 10.1016/0014-5793(75)80033-0. [DOI] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- CAMPBELL J. J., MACQUILLAN A. M., EAGLES B. A., SMITH R. A. The inhibition of keto acid oxidation by pyocyanine. Can J Microbiol. 1957 Mar;3(2):313–318. doi: 10.1139/m57-035. [DOI] [PubMed] [Google Scholar]
- Cox C. D., Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun. 1985 Apr;48(1):130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Graham R. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):357–364. doi: 10.1128/jb.137.1.357-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Iron reductases from Pseudomonas aeruginosa. J Bacteriol. 1980 Jan;141(1):199–204. doi: 10.1128/jb.141.1.199-204.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol. 1980 May;142(2):581–587. doi: 10.1128/jb.142.2.581-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Rinehart K. L., Jr, Moore M. L., Cook J. C., Jr Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4256–4260. doi: 10.1073/pnas.78.7.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A., Jr, Lascelles J. Reduction of iron and synthesis of protoheme by Spirillum itersonii and other organisms. J Bacteriol. 1977 Feb;129(2):815–820. doi: 10.1128/jb.129.2.815-820.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK L. H., DEMOSS R. D. On the biosynthesis of pyocyanine. J Bacteriol. 1959 Jun;77(6):776–782. doi: 10.1128/jb.77.6.776-782.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedheim E. A. The effect of pyocyanine on the respiration of some normal tissues and tumours. Biochem J. 1934;28(1):173–179. doi: 10.1042/bj0280173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. The inducible citrate-dependent iron transport system in Escherichia coli K12. Biochim Biophys Acta. 1973 Nov 30;330(1):90–101. doi: 10.1016/0005-2736(73)90287-3. [DOI] [PubMed] [Google Scholar]
- Gentry M. J., Smith D. K., Schnute S. F., Werber S. L., Weinberg E. D. Pseudomonas culture longevity: control by phosphate. Microbios. 1971 Dec;4(15):205–215. [PubMed] [Google Scholar]
- HELLINGER E. Requirements for pyocyanine production by Pseudomonas aeruginosa (Schroeter) Migula. J Gen Microbiol. 1951 Oct;5(4):633–639. doi: 10.1099/00221287-5-4-633. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Mechanism of the antibiotic action pyocyanine. J Bacteriol. 1980 Jan;141(1):156–163. doi: 10.1128/jb.141.1.156-163.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. M., Campbell J. J. A new resuspension medium for pyocyanine production. Can J Microbiol. 1969 Jun;15(6):595–598. doi: 10.1139/m69-101. [DOI] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Korth H. Einfluss von Eisen und Sauerstoff auf die Pigmentbildung bei verschiedenen Pseudomonas-Spezies. Arch Mikrobiol. 1971;77(1):59–64. [PubMed] [Google Scholar]
- Mann S. Zur Identifizierung und Redoxfunktion der Pigmente von Pseudomonas aureofaciens und P. iodina. Arch Mikrobiol. 1970;71(4):304–318. [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D., Goodnight S. A. Iron requirement for longevity of Pseudomonas cultures. Antonie Van Leeuwenhoek. 1970;36(1):73–80. doi: 10.1007/BF02069010. [DOI] [PubMed] [Google Scholar]
- Williams H. L., Conrad M. E. A one-tube method for measuring the serum iron concentration and unsaturated iron-binding capacity. J Lab Clin Med. 1966 Jan;67(1):171–176. [PubMed] [Google Scholar]


