Abstract
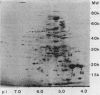
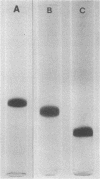
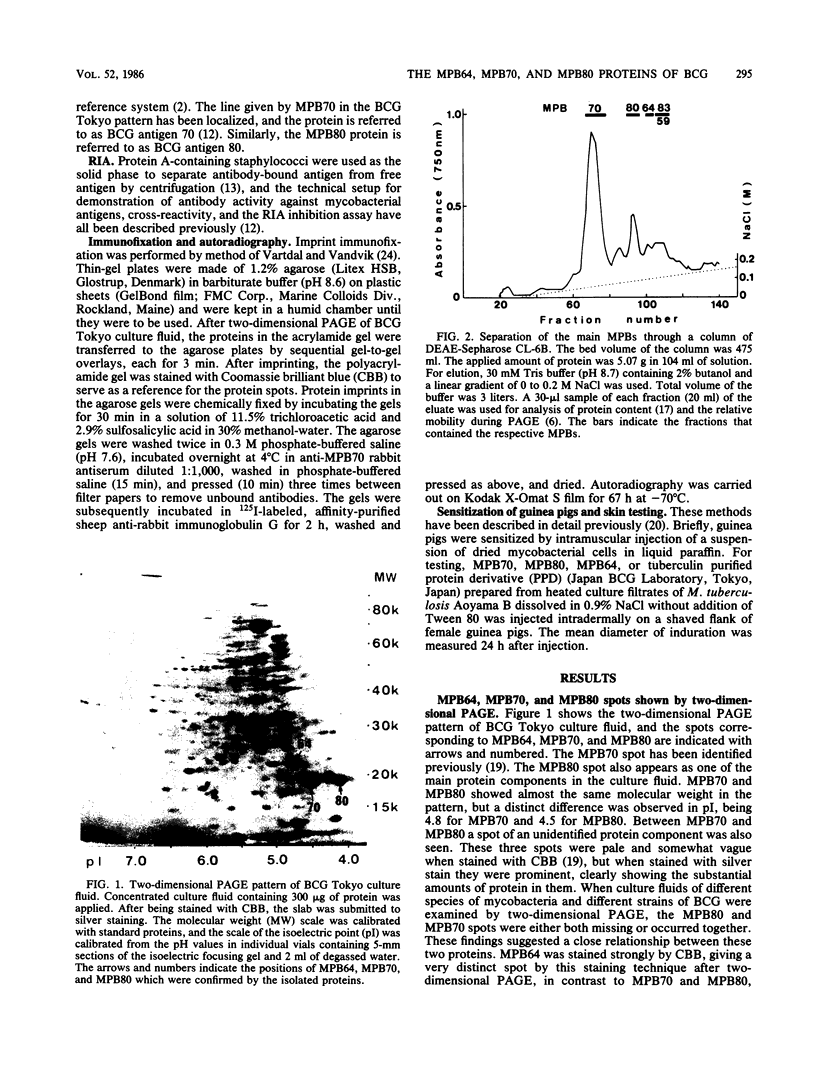
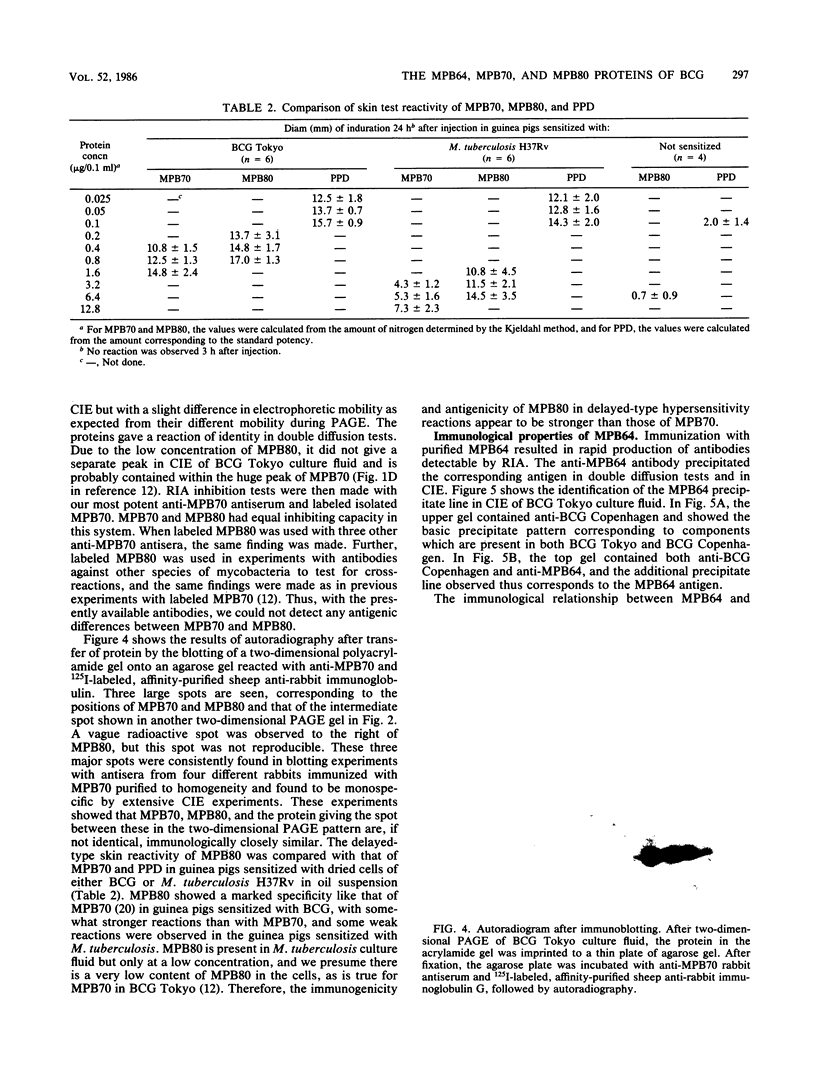
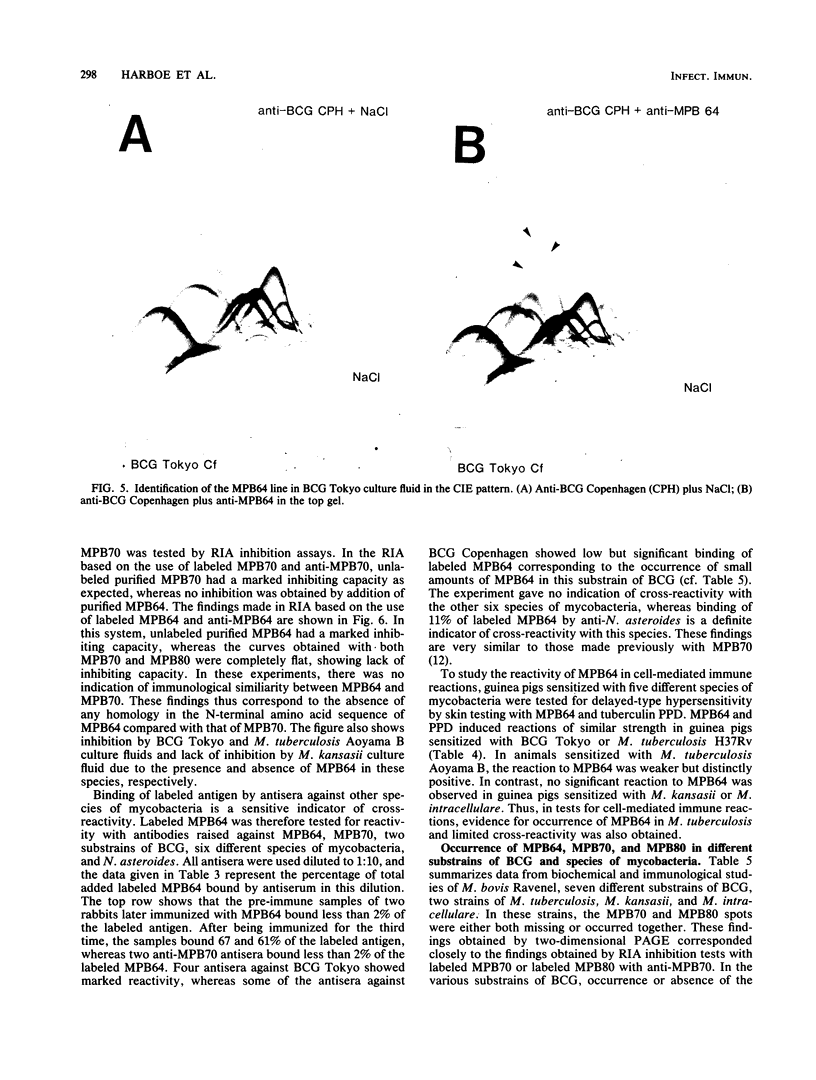
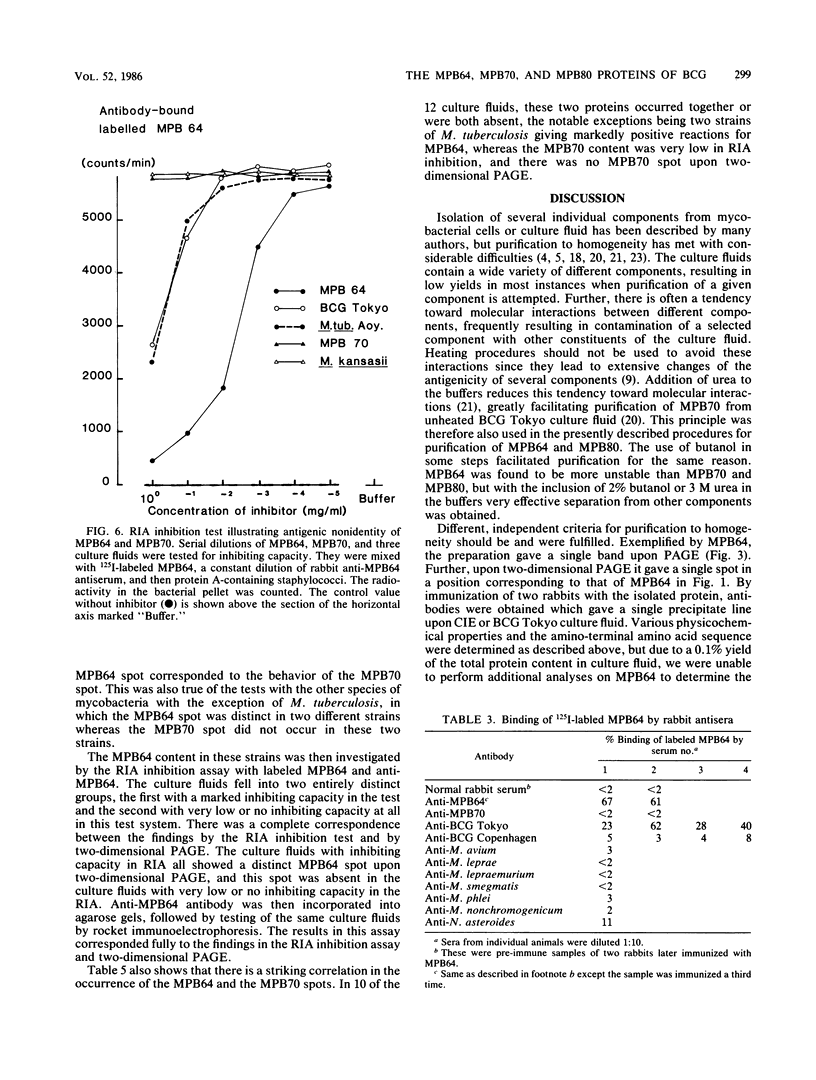
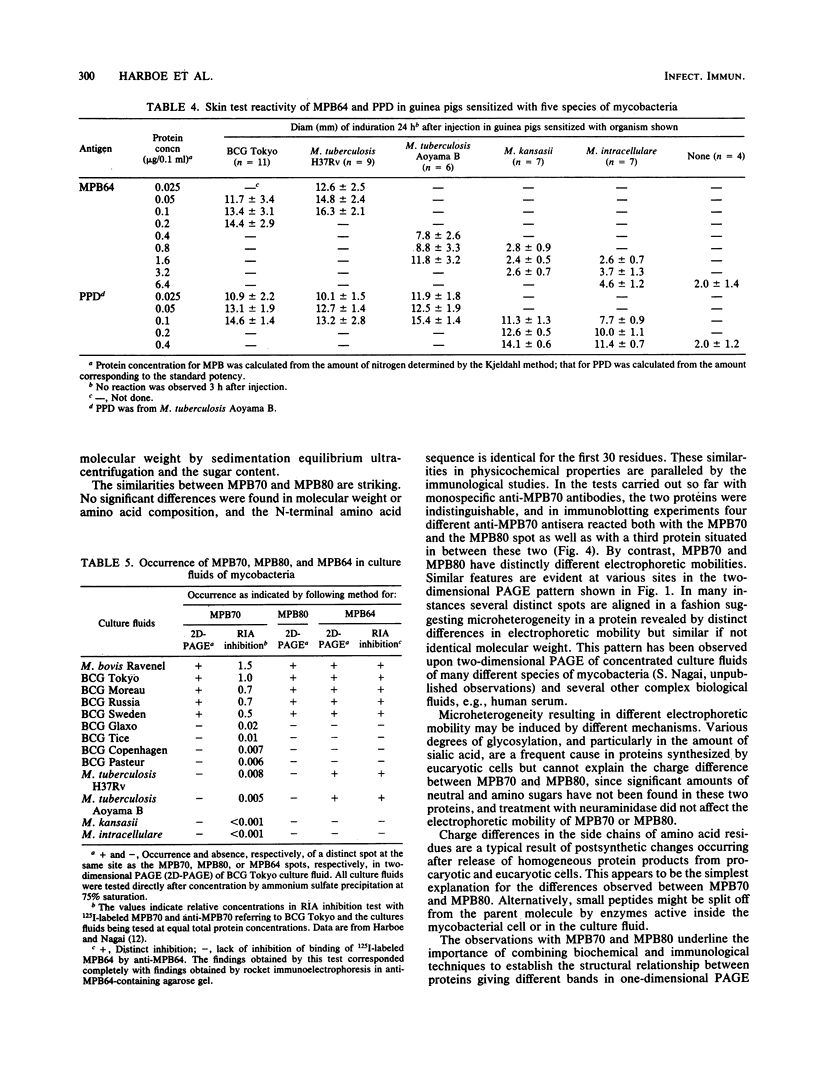
The immunogenic proteins MPB64 and MPB80 of Mycobacterium bovis BCG were purified to homogeneity and compared with MPB70. MPB70 and MPB80 showed a similar distribution in substrains of BCG, both being present in high concentrations in culture fluids of BCG substrain Tokyo, BCG Moreau, BCG Russia, and BCG Sweden and in only very small amounts in BCG Glaxo, BCG Tice, BCG Copenhagen, and BCG Pasteur. In various physicochemical properties MPB70 and MPB80 were closely similar, but MPB80 had a distinctly lower pI value. The N-terminal amino acid sequence was identical for the first 30 residues. In reactions with anti-MPB70 antibodies and delayed-type hypersensitivity skin reactions, MPB70 and MPB80 also had very similar properties. These results show that MPB70 and MPB80 are two closely similar forms of the same gene product, and postsynthetic changes probably explain the observed differences. By contrast, MPB64 had a higher molecular weight. The N-terminal amino acid sequence showed no homology with MPB70, and these two proteins showed no immunologic similarity. MPB64 and MPB70 showed only very restricted cross-reactivity with other species of mycobacteria but cross-reacted with Nocardia asteroides. The similar occurrence in eight different substrains of BCG indicated that the two proteins are influenced by similar control mechanisms, but in contrast to MPB70, MPB64 occurred in sufficient concentration in two strains of Mycobacterium tuberculosis to give a distinct spot in two-dimensional polyacrylamide gel electrophoresis of their culture fluids.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Anderson P. A. The isolation by immunoabsorbent affinity chromatography and physicochemical characterization of Mycobacterium tuberculosis antigen 5. Am Rev Respir Dis. 1978 Mar;117(3):533–539. doi: 10.1164/arrd.1978.117.3.533. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Janicki B. W. Mycobacterial antigens: a review of their isolation, chemistry, and immunological properties. Microbiol Rev. 1978 Mar;42(1):84–113. doi: 10.1128/mr.42.1.84-113.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson L. A., Morgan W. T. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27(6):1824–1828. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M. Antigens of PPD, old tuberculin, and autoclaved Mycobacterium bovis BCG studied by crossed immunoelectrophoresis. Am Rev Respir Dis. 1981 Jul;124(1):80–87. doi: 10.1164/arrd.1981.124.1.80. [DOI] [PubMed] [Google Scholar]
- Harboe M., Closs O., Bjorvatn B., Kronvall G., Axelsen N. H. Antibody response in rabbits to immunization with Mycobacterium leprae. Infect Immun. 1977 Dec;18(3):792–805. doi: 10.1128/iai.18.3.792-805.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Closs O., Svindahl K., Deverill J. Production and assay of antibodies against one antigenic component of Mycobacterium bovis BCG. Infect Immun. 1977 May;16(2):662–672. doi: 10.1128/iai.16.2.662-672.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Nagai S. MPB70, a unique antigen of Mycobacterium bovis BCG. Am Rev Respir Dis. 1984 Mar;129(3):444–452. doi: 10.1164/arrd.1984.129.3.444. [DOI] [PubMed] [Google Scholar]
- Jonsson S., Kronvall G. The use of protein A-containing Staphylococcus aureus as a solid phase anti-IgG reagent in radioimmunoassays as exemplified in the quantitation of alpha-fetoprotein in normal human adult serum. Eur J Immunol. 1974 Jan;4(1):29–33. doi: 10.1002/eji.1830040108. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Bjune G., Stanford J., Menzel S., Samuel D. Mycobacterial antigens in antibody responses of leprosy patients. Int J Lepr Other Mycobact Dis. 1975 Oct-Dec;43(4):306–306. [PubMed] [Google Scholar]
- LIND A. Serological studies of mycobacteria by means of diffusion-in-gel techniques. 3. A difference in precipitinogenic content found in substrains of BCG. Int Arch Allergy Appl Immunol. 1960;17:1–9. doi: 10.1159/000229107. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Minden P., Kelleher P. J., Freed J. H., Nielsen L. D., Brennan P. J., McPheron L., McClatchy J. K. Immunological evaluation of a component isolated from Mycobacterium bovis BCG with a monoclonal antibody to M. bovis BCG. Infect Immun. 1984 Nov;46(2):519–525. doi: 10.1128/iai.46.2.519-525.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K., Nagai S., Kinomoto M., Haga S., Tokunaga T. Comparative studies with various substrains of Mycobacterium bovis BCG on the production of an antigenic protein, MPB70. Infect Immun. 1983 Feb;39(2):540–545. doi: 10.1128/iai.39.2.540-545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S., Matsumoto J., Nagasuga T. Specific skin-reactive protein from culture filtrate of Mycobacterium bovis BCG. Infect Immun. 1981 Mar;31(3):1152–1160. doi: 10.1128/iai.31.3.1152-1160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S., Nagasuga T., Matsumoto J. Tuberculin peptide from culture filtrate of Mycobacterium tuberculosis. Am Rev Respir Dis. 1980 Mar;121(3):551–557. doi: 10.1164/arrd.1980.121.3.551. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Vartdal F., Vandvik B. Imprint immunofixation of electrofocused immunoglobulins. J Immunol Methods. 1983 Aug 12;62(1):23–29. doi: 10.1016/0022-1759(83)90106-0. [DOI] [PubMed] [Google Scholar]
- Widebäck K., Kronvall G., Bjorvatn B., Closs O., Harboe M. Comparative studies of antigen 21 in Mycobacterium and Nocardia species: possible taxonomic relationships with Mycobacterium leprae. Infect Immun. 1980 Nov;30(2):413–420. doi: 10.1128/iai.30.2.413-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G. L., Jr, Roberts D. B. Two-dimensional immunoelectrophoresis of mycobacterial antigens. Comparison with a reference system. Am Rev Respir Dis. 1974 Feb;109(2):306–310. doi: 10.1164/arrd.1974.109.2.306. [DOI] [PubMed] [Google Scholar]