Abstract
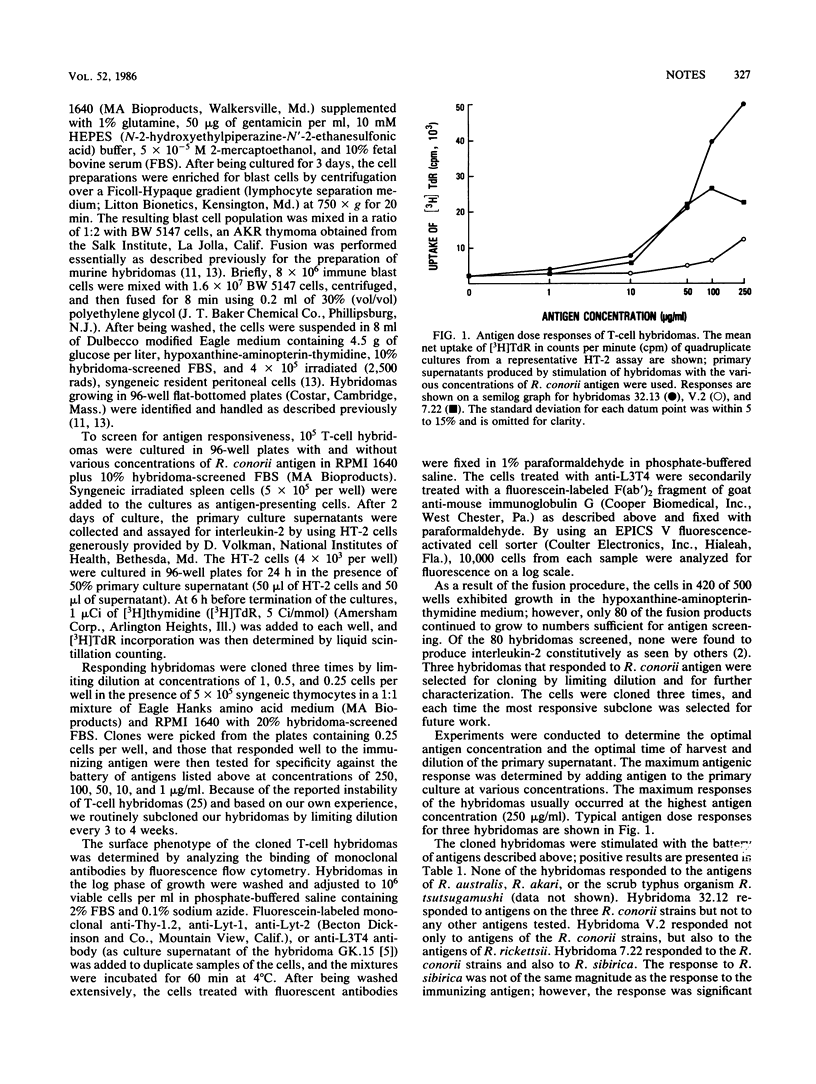
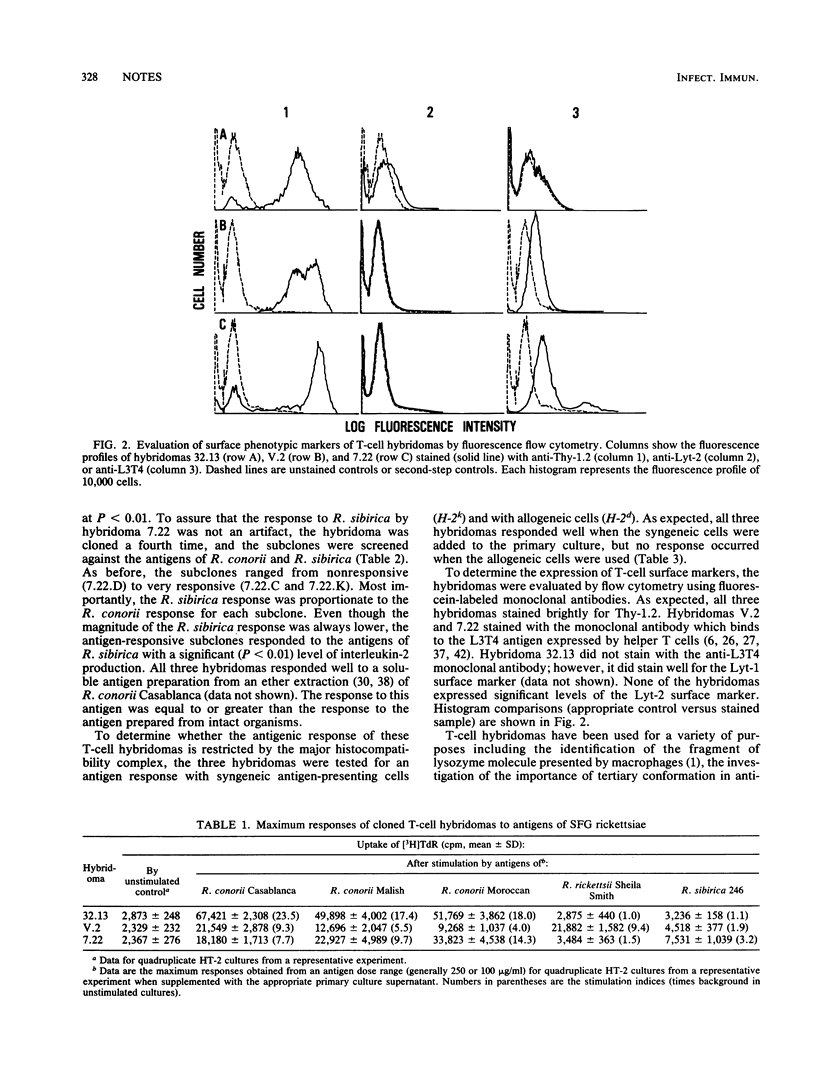
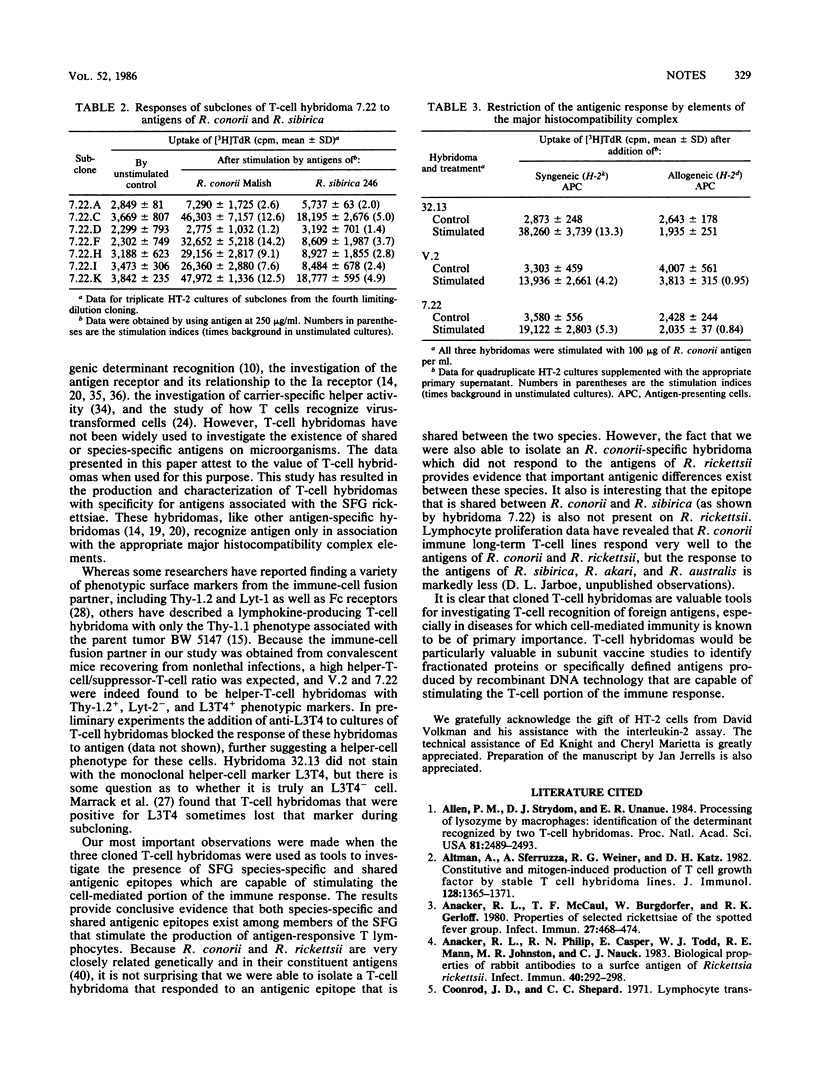
T-cell hybridomas produced by the fusion of Rickettsia conorii immune T cells to the AKR thymoma BW 5147 produced interleukin-2 when stimulated with the antigens of three different R. conorii strains. One cloned hybridoma responded only to R. conorii antigens, whereas a second and third cloned hybridoma also responded to the antigens of Rickettsia rickettsii Sheila Smith and Rickettsia sibirica 246, respectively. Antigen responses required antigen-presenting cells, and this interaction was major histocompatibility complex restricted. Fluorescence-activated cell-sorter analysis demonstrated that all three hybridomas were of the Thy-1.2+, Lyt-2- phenotype and that two of the three were L3T4+. These data demonstrated the presence of an antigenic epitope that is R. conorii species specific and other epitopes that are common to various members of the spotted fever group which can stimulate interleukin-2 production by T-cell hybridomas.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Strydom D. J., Unanue E. R. Processing of lysozyme by macrophages: identification of the determinant recognized by two T-cell hybridomas. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2489–2493. doi: 10.1073/pnas.81.8.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman A., Sferruzza A., Weiner R. G., Katz D. H. Constitutive and mitogen-induced production of T cell growth factor by stable T cell hybridoma lines. J Immunol. 1982 Mar;128(3):1365–1371. [PubMed] [Google Scholar]
- Anacker R. L., McCaul T. F., Burgdorfer W., Gerloff R. K. Properties of selected rickettsiae of the spotted fever group. Infect Immun. 1980 Feb;27(2):468–474. doi: 10.1128/iai.27.2.468-474.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., Philip R. N., Casper E., Todd W. J., Mann R. E., Johnston M. R., Nauck C. J. Biological properties of rabbit antibodies to a surface antigen of Rickettsia rickettsii. Infect Immun. 1983 Apr;40(1):292–298. doi: 10.1128/iai.40.1.292-298.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Eisemann C. S., Nypaver M. J., Osterman J. V. Susceptibility of inbred mice to rickettsiae of the spotted fever group. Infect Immun. 1984 Jan;43(1):143–148. doi: 10.1128/iai.43.1.143-148.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisemann C. S., Osterman J. V. Proteins of typhus and spotted fever group rickettsiae. Infect Immun. 1976 Jul;14(1):155–162. doi: 10.1128/iai.14.1.155-162.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W. C., Waner J. L. Serological cross-reaction and cross-protection in guinea pigs infected with Rickettsia rickettsii and Rickettsia montana. Infect Immun. 1980 May;28(2):627–629. doi: 10.1128/iai.28.2.627-629.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher L. H., Schroer J. A., Chan C., Shevach E. M. Fine specificity of cloned insulin-specific T cell hybridomas: evidence supporting a role for tertiary conformation. J Immunol. 1983 Dec;131(6):2868–2874. [PubMed] [Google Scholar]
- Goldsby R. A., Osborne B. A., Simpson E., Herzenberg L. A. Hybrid cell lines with T-cell characteristics. Nature. 1977 Jun 23;267(5613):707–708. doi: 10.1038/267707a0. [DOI] [PubMed] [Google Scholar]
- Harwell L., Skidmore B., Marrack P., Kappler J. Concanavalin A-inducible, interleukin-2-producing T cell hybridoma. J Exp Med. 1980 Oct 1;152(4):893–904. doi: 10.1084/jem.152.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber-Katz E., Schwartz R. H., Matis L. A., Hannum C., Fairwell T., Appella E., Hansburg D. Contribution of antigen-presenting cell major histocompatibility complex gene products to the specificity of antigen-induced T cell activation. J Exp Med. 1982 Apr 1;155(4):1086–1099. doi: 10.1084/jem.155.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Matis L. A., Hecht T. T., Samelson L. E., Longo D. L., Heber-Katz E., Schwartz R. H. The fine specificity of antigen and Ia determinant recognition by T cell hybridoma clones specific for pigeon cytochrome c. Cell. 1982 Aug;30(1):141–152. doi: 10.1016/0092-8674(82)90020-4. [DOI] [PubMed] [Google Scholar]
- Howard M., Burgess A., McPhee D., Metcalf D. T-cell hybridoma secreting hemopoietic regulatory molecules: granulocyte-macrophage and eosinophil colony-stimulating factors. Cell. 1979 Dec;18(4):993–999. doi: 10.1016/0092-8674(79)90211-3. [DOI] [PubMed] [Google Scholar]
- Jerrells T. R., Eisemann C. S. Role of T-lymphocytes in production of antibody to antigens of Rickettsia tsutsugamushi and other Rickettsia species. Infect Immun. 1983 Aug;41(2):666–674. doi: 10.1128/iai.41.2.666-674.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Jarboe D. L., Eisemann C. S. Cross-reactive lymphocyte responses and protective immunity against other spotted fever group rickettsiae in mice immunized with Rickettsia conorii. Infect Immun. 1986 Mar;51(3):832–837. doi: 10.1128/iai.51.3.832-837.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Development of specific and cross-reactive lymphocyte proliferative responses during chronic immunizing infections with Rickettsia tsutsugamushi. Infect Immun. 1983 Apr;40(1):147–156. doi: 10.1128/iai.40.1.147-156.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J., Kubo R., Haskins K., White J., Marrack P. The mouse T cell receptor: comparison of MHC-restricted receptors on two T cell hybridomas. Cell. 1983 Oct;34(3):727–737. doi: 10.1016/0092-8674(83)90529-9. [DOI] [PubMed] [Google Scholar]
- Kenyon R. H., Ascher M. S., Kishimoto R. A., Pedersen C. E., Jr In vitro guinea pig leukocyte reactions to Rickettsia rickettsii. Infect Immun. 1977 Dec;18(3):840–846. doi: 10.1128/iai.18.3.840-846.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon R. H., Pedersen C. E., Jr Immune responses to Rickettsia akari infection in congenitally athymic nude mice. Infect Immun. 1980 May;28(2):310–313. doi: 10.1128/iai.28.2.310-313.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokorin I. N., Kabanova E. A., Shirokova E. M., Abrosimova G. E., Rybkina N. N., Pushkareva V. i. Role of T lymphocytes in Rickettsia conorii infection. Acta Virol. 1982 Jan;26(1-2):91–97. [PubMed] [Google Scholar]
- Lakow E., Tsoukas C. D., Vaughan J. H., Altman A., Carson D. A. Human T cell hybridomas specific for Epstein Barr virus-infected B lymphocytes. J Immunol. 1983 Jan;130(1):169–172. [PubMed] [Google Scholar]
- Lonai P., Rechavi G., Arman E., Givol D. Retention and loss of immunoglobulin heavy chain alleles in helper T cell hybridoma clones. EMBO J. 1983;2(5):781–786. doi: 10.1002/j.1460-2075.1983.tb01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek T. R., Schmidt J. A., Shevach E. M. The murine IL 2 receptor. III. Cellular requirements for the induction of IL 2 receptor expression on T cell subpopulations. J Immunol. 1985 Apr;134(4):2405–2413. [PubMed] [Google Scholar]
- Misfeldt M. L., Hanna E. E. Complementation of the plaque-forming cell responses of T-cell-deficient nude mice by a T-cell hybridoma. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1813–1817. doi: 10.1073/pnas.78.3.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutman T. B., Ottesen E. A., Fauci A. S., Volkman D. J. Parasite antigen-specific human T cell lines and clones. Major histocompatibility complex restriction and B cell helper function. J Clin Invest. 1984 Jun;73(6):1754–1762. doi: 10.1172/JCI111384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman J. V., Eisemann C. S. Surface proteins of typhus and spotted fever group rickettsiae. Infect Immun. 1978 Sep;21(3):866–873. doi: 10.1128/iai.21.3.866-873.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman J. V., Parr R. P. Plaque Formation by Rickettsia conori in WI-38, DBS-FRhL-2, L-929, HeLa, and Chicken Embryo Cells. Infect Immun. 1974 Nov;10(5):1152–1155. doi: 10.1128/iai.10.5.1152-1155.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L. The role of Ia molecules in the activation of T lymphocytes. I. The activation of an IL 1-dependent IL 2-producing T cell hybridoma by Con A requires an interaction, which is not H-2-restricted, with an Ia-bearing accessory cell. J Immunol. 1982 Oct;129(4):1360–1366. [PubMed] [Google Scholar]
- Roehm N. W., Marrack P., Kappler J. W. Antigen-specific, H-2-restricted helper T cell hybridomas. J Exp Med. 1982 Jul 1;156(1):191–204. doi: 10.1084/jem.156.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson L. E., Schwartz R. H. T cell clone-specific alloantisera that inhibit or stimulate antigen-induced T cell activation. J Immunol. 1983 Dec;131(6):2645–2650. [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter J., Sriram S., Rassenti L., Chou C. H., Fritz R. B., Steinman L. Characterization of T cell lines and clones from SJL/J and (BALB/c x SJL/J)F1 mice specific for myelin basic protein. J Immunol. 1985 Apr;134(4):2322–2327. [PubMed] [Google Scholar]
- Tzianabos T., Palmer E. L., Obijeski J. F., Martin M. L. Origin and structure of the group-specific, complement-fixing antigen of Rickettsia rickettsii. Appl Microbiol. 1974 Sep;28(3):481–488. doi: 10.1128/am.28.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman D. J., Matis L. A., Fauci A. S. Development and characterization of interleukin-2-independent antigen-specific human T cell clones that produce multiple lymphokines. Cell Immunol. 1984 Oct 15;88(2):323–335. doi: 10.1016/0008-8749(84)90165-5. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Montenegro M. R., Hegarty B. C., Tringali G. R. Rocky Mountain spotted fever vaccine: a regional need. South Med J. 1984 Apr;77(4):447–449. doi: 10.1097/00007611-198404000-00009. [DOI] [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]


