Abstract
Females and males typically play different roles in survival of the species and would be expected to respond differently to food scarcity or excess. To elucidate the physiological basis of sex differences in responses to energy intake, we maintained groups of male and female rats for 6 months on diets with usual, reduced [20% and 40% caloric restriction (CR), and intermittent fasting (IF)], or elevated (high-fat/high-glucose) energy levels and measured multiple physiological variables related to reproduction, energy metabolism, and behavior. In response to 40% CR, females became emaciated, ceased cycling, underwent endocrine masculinization, exhibited a heightened stress response, increased their spontaneous activity, improved their learning and memory, and maintained elevated levels of circulating brain-derived neurotrophic factor. In contrast, males on 40% CR maintained a higher body weight than the 40% CR females and did not change their activity levels as significantly as the 40% CR females. Additionally, there was no significant change in the cognitive ability of the males on the 40% CR diet. Males and females exhibited similar responses of circulating lipids (cholesterols/triglycerides) and energy-regulating hormones (insulin, leptin, adiponectin, ghrelin) to energy restriction, with the changes being quantitatively greater in males. The high-fat/high-glucose diet had no significant effects on most variables measured but adversely affected the reproductive cycle in females. Heightened cognition and motor activity, combined with reproductive shutdown, in females may maximize the probability of their survival during periods of energy scarcity and may be an evolutionary basis for the vulnerability of women to anorexia nervosa.
Complex neuroendocrine systems control feeding and energy expenditure to ensure the presence of sufficient energy stores to weather periods of food scarcity and avoid obesity. Such regulatory systems include cognitive and motivational systems in the brain and hormones produced by endocrine and fat cells (1-3). Despite the fact that energy balance is tightly regulated, eating disorders are a major cause of morbidity and mortality in modern societies. Excessive food intake leads to obesity and dramatically increases the risk of cardiovascular disease, type 2 diabetes, and premature death (4). Laboratory animals such as rats and mice typically overeat when allowed continuous access to food, resulting in increased disease incidence and a shortened life span, compared with animals in which food intake is limited by caloric restriction (CR) or intermittent fasting (IF) (5-8). In an evolutionary context, overeating would increase the ability of the animal to survive periods of famine and reproduce and provide nourishment to offspring. At the extreme end of the spectrum is anorexia nervosa (AN), an often fatal eating disorder in which the affected individual severely restricts food intake and increases energy expenditure despite progressive emaciation (9-11). AN has been generally considered to be unique to humans and has been classified as an obsessive-compulsive psychiatric disorder in which the individual has a distorted body image (12). The biological basis of AN is presently unclear.
Many of the effects of dietary energy excess (obesity, insulin resistance, and increased mortality) and CR (decreased fat mass, morbidity, and mortality) are similar in females and males (8, 13). However, sex-dependent differences in responses to energy intake have been reported. In rodents, severe CR inhibits the reproductive cycle in females without adversely affecting male fertility (14-16). Similarly, women with AN typically exhibit amenorrhea (17, 18). The influence of energy intake on disease susceptibility may also differ in men and women. For example, premenopausal women are more resistant than men to obesity related atherosclerotic heart disease (19).
The involvement of the brain in the regulation of energy balance has been well established in studies demonstrating that neurons in the hypothalamus control appetite (20). However, recent findings suggest that higher regions of the brain, including those involved in learning and memory, influence energy balance and physiological and behavioral responses to varying energy availability (21, 22). Obese human subjects tend to perform more poorly than controls on cognitive tasks (23, 24), whereas the learning and memory ability of patients with AN is similar or superior to that of control subjects (25, 26). Some animal studies are also consistent with a detrimental effect of excessive energy intake on cognitive function (27). However, there is a lack of knowledge of the mechanisms by which the brain responds to changes in energy intake, on the one hand, and how those responses are translated into behavioral and neuroendocrine outputs on the other hand. Moreover, the possibility that changes in energy intake differentially affect the behaviors, and associated neurochemical and endocrine systems, of males and females has not been investigated. We therefore performed a detailed analysis of behavioral, neurochemical, and endocrine responses of male and female rats to high-, medium-, and very low-energy diets. Our results identify shared and sex-dependent brain and bodily responses to variations in energy intake, which suggest a biological basis for the susceptibility of females to AN.
Materials and Methods
Animals and diets
Forty-seven male and 47 female Sprague Dawley rats were singly housed on a 12-h light, 12-h dark cycle (lights on at 0700 h). The following diets were applied to the rats beginning at 4 months of age: control (ad libitum); 20% CR, 40% CR; IF (alternate day fasting); and high-fat/high-glucose (HFG). Control, CR, and IF groups received food pellets that contained 19% protein, 64% carbohydrates, and 17% fat (yellow diet 101845; Dyets Inc., Bethlehem, PA); this food had a caloric density of 3.774 cal/g and a glycemic load per kilogram of 442. The HFG diet (red diet 101842; Dyets) contained 15% protein, 38% carbohydrates, and 47% fat. The caloric density of the HFG diet was 4.645 cal/g and its glycemic load per kilogram was 363. Food was provided (or removed in the IF group) at 1000 h daily. CR animals were fed at 1000 h daily.
Assessment of ambulatory activity and cognitive ability
Activity levels were quantitated using the Digiscan open-field activity monitor (Omnitech, Columbus, OH). Recordings were taken in 4-h intervals (from 0700 to 1000 h and again from 1900 until 2200 h). Additionally, throughout the study activity levels were also observed and scored by multiple observers at random times during the day. Using the latter semiquantitative scoring system of general activity, it was very clear early in the study that only the 40% CR and IF animals showed increased daytime activity. The rat activity levels were therefore formally measured (using the Omnitech apparatus) at one time point during this study because our observations showed that animal activity levels did not change throughout the study. Analysis of cognitive maze solving ability was preformed using a 14-unit T-maze (28). The premaze training and the 14-unit T-maze testing (Fig. 1) was started at 1200 h. Female rats were in the diestrous stage of their reproductive cycle when they were tested in the 14-unit T-maze.
Fig. 1.
Configurations of the straight runway used for premaze training and the 14-unit T-maze used for testing learning and memory. Arrows denote the correct pathway. Errors are recorded as any deviation from this pathway.
Premaze training was done using a straight runway apparatus. This one-way active avoidance chamber (2 m long) basically consisted of clear plastic sides with a diagonally oriented stainless-steel grid floor wired to receive scrambled shock from a grid foot shocker (E13–08; Coulbourn Instruments, Allentown, PA). Eight aluminum legs attached to the bottom of the runway served to elevate it above a movable wooden table. The entire apparatus was surrounded by wood walls painted gray. The rats were placed in the behavior room and allowed to acclimatize for a few hours before training in active avoidance in the straight runway and before 14-unit T-maze testing. For straight runway pretraining, each rat was removed gently by the tail from its home cage and placed into a black box with a movable rear wall and guillotine door. Identical black boxes served as start and goal boxes. The black box was placed into a start area over the grid floor in the straight runway. The guillotine door on the box was raised, and the rat was pushed gently from the black box by advancing the movable back wall forward to expel the animal into the runway. To successfully avoid foot shock (0.3 mA), the rat had to move down the runway and enter an identical black box with a guillotine door within 10 sec; otherwise, the foot shock was initiated until the rat escaped to the black goal box. Once the rat entered the goal box, a guillotine door was closed, and the rat was removed into a holding area until the next trial, 2 min later. If a rat failed to escape shock within 60 sec, it was removed from the runway to the goal box. Three failures to escape within 60 sec resulted in the rat being removed from the experiment. For this study, none of the 94 rats failed this test. On d 1 each rat received 20 massed practice trials. On d 2 each rat was trained to a criterion of 13 of 15 correct avoidances of foot shock (maximum = 20 trials).
Rats that successfully met these criteria then began training in the 14-unit T-maze on the following day. As in straight runway pretraining, each rat was removed gently from its home cage and placed into a black box that had a moveable rear wall and served interchangeably as a start and goal box. The box was placed into the start area of the 14-unit T-maze over the grid floor; the rat was pushed into the initial segment of the maze; a guillotine door from the start area was closed; and the shock avoidance contingency (10 sec; 0.3 mA) was initiated by means of the handheld switch. The rat had 10 sec to move through the first gate in the maze to avoid foot shock, or the foot shock was initiated and remained on until the rat passed through the gate to the second section of the maze. Once the rat passed through the gate and the guillotine door was closed, the shock avoidance contingency was reset, and the rat again had 10 sec to move past a second gate to a third section to avoid foot shock. This contingency was reset a total of four times as the rat traversed the five sections of the maze to arrive at the goal box in the final section. On d 3 each rat was allowed 20 trials through the maze. Failure to move through the maze within 600 sec resulted in the rat being removed and placed into the goal box. If the rat failed to meet this criterion on any of three trials, it was removed from the experiment. After each animal trial, the maze was cleaned and sterilized to prevent the generation of a scent trail on the correct path. The maze test was repeated again one month later, to test the memory retention skills of the rats. The total shock time, run time and number of route errors were recorded by computer. The data on maze errors, run time, shock duration, and shock frequency were analyzed using ANOVA. No corrections were made to acknowledge the possibility of family-wise error in this study.
Tissue collection
Blood samples were collected from overnight fasted rats in EDTA-heparinized centrifuge tubes to prevent clotting. The blood was centrifuged at 3000 rpm for 30 min at 4 C; plasma was aspirated and was stored at −20 C. Animals were euthanized using isoflurane anesthesia followed by decapitation. Upon euthanasia, the animals were dissected to obtain the following tissues: hippocampus, striatum, cortex, cerebellum, brainstem, heart, liver, kidneys, adrenal glands, gonads, pancreas, and spleen. Tissues were flash frozen on dry ice and stored at −80 C.
Plasma lipid analysis
Plasma samples were analyzed for their triglyceride, low-density lipoprotein (LDL), high-density lipoprotein (HDL), cholesterol, and 3-hydroxybutyrate levels using an automated COBAS centrifugal analyzer (Roche Diagnostics, Indianapolis, IN) according to the kit manufacturer's specifications.
ELISA and RIA analyses
Samples for ELISA analysis were prepared in an Nonidet P-40-based lysis buffer (29). Tissue pieces were sonicated on ice in the lysis buffer, centrifuged at 14,000 rpm for 10 min, and the supernatant removed for protein content analysis. Protein concentrations were measured using a BCA protein assay reagent kit (Pierce Biotechnology, Inc., Rockford, IL) and a microplate reader (ThermoElectron, San Jose, CA). All tissue extracts were then normalized for total protein. Plasma levels of the following hormones/factors were measured according to the manufacturers' instructions using proprietary ELISA or RIA kits from the specified companies: ghrelin (RIA; Phoenix Peptides, Burlingame, CA), adiponectin (ELISA; Alpco Diagnostics, Salem, NH), leptin (ELISA; Linco, Billerica, MA), insulin (ELISA; Linco), and brain-derived neurotrophic factor (BDNF; Emax ImmunoAssay System; Promega, Madison, WI). Glucose levels were measured using a glucometer (Ascensia Elite; Bayer, Tarrytown, NY). GH, prolactin, estradiol, testosterone, and corticosterone levels were measured in plasma using RIA by Dr A. L. Parlow (National Hormone and Peptide Program, Torrance, CA).
HPLC analysis of neurotransmitters
Neurotransmitters and their metabolites [norepinephrine (NE), dihydroxyphenylacetic acid (DOPAC), dopamine (DA), 5-hydroxy indoleacetic acid (5HIAA), serotonin (5-HT), and homovanilic acid (HVA)] were measured by HPLC with electrochemical detection as described previously (30). The analytical column was a Symmetry C18 3.5 μm (4.6 × 150.0 mm; Waters, Milford, MA). The mobile phase consisted of 0.01 m sodium dihydrogenphosphate, 0.01 m citric acid, 2 mm sodium EDTA, 1 mm sodium octylsulfate, 10% methanol (pH 3.5) and was used at flow rate of 0.9 ml/min and temperature 25 C. The installation consisted of a Waters 717 Plus automated injection system, a Waters 1525 Binary pump, and Coulochem III detector (ESA, Chelmsford, MA). A Waters Breeze system was used for data collection and analysis. The concentration of neurotransmitters in the different brain regions were calculated as picograms per milligram of tissue weight.
Results
Body and organ weight responses to dietary energy restriction and excess
Four-month-old male and female Sprague Dawley rats were divided into five diet groups: control (ad libitum), 20% CR, 40% CR, IF, and HFG, 8–15 rats per group. The rats in the first four groups were fed a diet with a typical composition in which the majority of calories were from complex carbohydrates, whereas the HFG diet contained higher amounts of fat and glucose, resulting in a higher energy density (Fig. 2A). Rats were maintained on the diets for 6 months during which time the percentage increase in body weight was significantly greater in the male rats on the control and HFG diets, compared with the increases observed in female rats (Fig. 2C). Whereas males on the HFG regimen showed a greater increase in weight than those on the control diet, females on HGF and control diets gained similar amounts of weight. Males and females exhibited similar body weight responses to 20% CR and IF diets (a small increase in weight). Both male and female rats responded to 40% CR by an initial drop in body weight during the first 10 wk, followed by a partial recovery. With regard to energy intake, females consumed less food and fewer calories than males on all diets (Fig. 2, C and D). Energy intake was relatively stable during the experimental time period for each of the diets. Male rats consumed more calories from the HFG diet than from the control diet, whereas females consumed a similar level of total calories from the HFG and control diets (Fig. 2E).
Fig. 2.

Experimental design, diet composition, and body weight responses to energy restriction and excess. The experimental timeline for this study is displayed at the top of the figure. A and B, Relative proportions of the major nutritional groups in the control (A) and HFG diet (B) applied to male and female rats for 6 months. Panels C–E are segregated according to male (left) and female (right) denoted by the gender symbols above each series of panels. This notation is retained throughout other figures in the manuscript. C, Body weights of male and female rats in the different diet groups. All of the dietary manipulations resulted in a sustained statistically significant (P < 0.05) difference in the male animals' weight, compared with control after 2 wk of implementation. The 20% CR, 40% CR, and IF females demonstrated significant (P < 0.05) weight deviation from the control group after 3 wk. The female HFG group failed to show a sustained statistical difference to control until wk 20. D and E, The amounts of food (D) and calories (E) consumed by each group of rats. Values are the mean ± sem for 15 rats in the control diet group and eight rats in each of the other diet groups.
In addition to monitoring total body weight changes, we measured the wet weights of various organs at necropsy and normalized the organ weight to the terminal body weights of rats in the same diet group (Table 1). The weight of the adrenal gland was similar in rats on all diets; however, when normalized to body weight CR and IF diets caused a relative increase in adrenal size, the magnitude of which was greater in females, compared with males. In both males and females, the weight of the kidneys, heart, and spleen were lowest in rats on the 40% CR diet. The testicular weight was unaffected by any of the diets. In contrast, both CR diets and the IF diet caused a decrease in the size of the ovaries, and interestingly, the HFG diet also reduced ovary size.
TABLE 1.
Organ weights (wet mass and normalized mass) for male and female rats on the different dietary regimens
| Organ | Male |
Female |
|||||
|---|---|---|---|---|---|---|---|
| Wet mass (mg) |
Normalized mass (mg) |
Modulation | Wet mass (mg) |
Normalized mass (mg) |
Modulation | ||
| Adrenal (L) | |||||||
| Control | 27.92 ± 1.3 | 27.92 | 37.5 ± 1.2 | 37.5 | |||
| 20% CR | 29.42 ± 0.8 | 36.78 ± 0.6 | ↑a | 38.28 ± 2.8 | 50.15 ± 0.9 | ↑b | |
| 40% CR | 25.14 ± 0.6 | 38.97 ± 0.3 | ↑a | 32.87 ± 1.6 | 52.2 ± 2.3 | ↑b | |
| IF | 29.12 ± 1.1 | 45.13 ± 0.3 | ↑b | 33.12 ± 1.4 | 40.0 ± 1.8 | ↑a | |
| HFG | 33.85 ± 0.5 | 31.14 ± 0.2 | ↑a | 37.0 ± 2.3 | 40.33 ± 2.1 | ↑a | |
| Kidney (L) | |||||||
| Control | 1703.53 ± 84.5 | 1703.53 | 1035.66 ± 42 | 1035.66 | |||
| 20% CR | 1517.62 ± 93.9 | 1897 ± 49 | ↑a | 944.25 ± 33 | 1236.9 ± 30 | ↑a | |
| 40% CR | 1260.62 ± 40.5 | 1953.9 ± 42 | ↑b | 747.25 ± 28 | 1188.1 ± 28 | ↑a | |
| IF | 1495.5 ± 81.3 | 1869.4 ± 37 | ↑a | 981.12 ± 18 | 1187.1 ± 18 | ↑a | |
| HFG | 1807.37 ± 79 | 1671.4 ± 28 | ↑a | 968.75 ± 22 | 1055.9 ± 16 | ↑ | |
| Gonad (L) | |||||||
| Control | 2125.73 ± 88 | 2125.73 | 75.4 ± 2.3 | 75.4 | |||
| 20% CR | 2176.62 ± 76 | 2720.7 ± 54 | ↑b | 61.75 ± 4.5 | 80.8 ± 3.1 | ↑a | |
| 40% CR | 2163.75 ± 65 | 3353.8 ± 48 | ↑c | 63.12 ± 4.3 | 100.3 ± 2.9 | ↑b | |
| IF | 2186.12 ± 98 | 2732.7 ± 71 | ↑a | 60.87 ± 5.8 | 73.6 ± 3.5 | ↓ | |
| HFG | 2116.5 ± 74 | 1957.7 ± 43 | ↓a | 65.5 ± 3.6 | 71.3 ± 2.9 | ↑a | |
| Heart | |||||||
| Control | 1911.76 ± 58 | 1911.76 | 1142.66 ± 32 | 1142.66 | |||
| 20% CR | 1710.37 ± 66 | 2137.9 ± 25 | ↑a | 1089.62 ± 28 | 1427.4 ± 18 | ↑a | |
| 40% CR | 1528.62 ± 94 | 2369.4 ± 27 | ↑b | 970.5 ± 15 | 1543.1 ± 13 | ↑a | |
| IF | 1637.25 ± 75 | 2046.3 ± 32 | ↑a | 1067.87 ± 22 | 1292.1 ± 21 | ↑a | |
| HFG | 1873.25 ± 43 | 1732.8 ± 28 | ↓b | 1063 ± 36 | 1158.7 ± 33 | ↑ | |
| Spleen | |||||||
| Control | 885.53 ± 24 | 885.53 | 700.53 ± 18 | 700.53 | |||
| 20% CR | 841.12 ± 28 | 1051.4 ± 23 | ↑b | 630 ± 22 | 825.3 ± 15 | ↑b | |
| 40% CR | 633.5 ± 33 | 981.9 ± 19 | ↑a | 482.12 ± 37 | 766.5 ± 31 | ↑a | |
| IF | 795.87 ± 43 | 994.8 ± 37 | ↑a | 609.12 ± 28 | 968.5 ± 22 | ↑b | |
| HFG | 946.5 ± 55 | 875.5 ± 29 | ↓ | 717.12 ± 26 | 781.6 ± 15 | ↑a | |
Arrows pointing upward denote an increase, whereas arrows pointing downward denote a decrease. Values are mean ± sem (n = 8–15 rats/group).
P < 0.05.
P < 0.01.
P < 0.001.
Females increase their ambulatory activity more than males in response to energy restriction
Despite their abnormally low calorie intake, women with AN typically exhibit high levels of activity, including vigorous exercise (11). To determine whether there are sex differences in the effects of dietary energy restriction on spontaneous activity levels in rats, we monitored the movements of male and female rats in the control, 40% CR, and IF groups during 4-h periods in the day and night. Females responded to 40% CR with a highly significant 4-fold increase in daytime ambulatory activity, whereas males exhibited a 2-fold increase in daytime activity (Table 2). The daytime activity of females was doubled in response to IF, whereas the IF diet did not affect the activity level of males. Nighttime activity levels of males and females were unaffected by dietary energy restriction (Table 2).
TABLE 2.
Daytime ambulatory activity is increased in response to energy restriction
| Female |
Male |
|||
|---|---|---|---|---|
| Day | Night | Day | Night | |
| Control | 58.5 ± 12.4 | 237.2 ± 36.7 | 74.7 ± 16.5 | 306.4 ± 29.5 |
| 40% CR | 233.8 ± 38.5a | 236.9 ± 20.4 | 199.9 ± 28.3b | 245.5 ± 12.7 |
| IF | 124.3 ± 14.5b | 301.7 ± 25.4 | 77.9 ± 26.5 | 252.1 ± 11.9 |
Ambulatory counts/4 h. Values are mean ± sem (n = 8–15 rats/group).
P < 0.001.
P < 0.01.
Energy restriction perturbs reproductive physiology in females
Because the energy-restricted diets resulted in decreased size of gonads in females, but not males, we evaluated the reproductive cycle in females and circulating levels of relevant hormones in both sexes. Uterine activity was monitored daily with vaginal smear tests; cyclicity was scored as regular, irregular (more than but less than 3 d altered in the typical estrous cycle), or absent (Fig. 3A). The mild energy-restriction diets (20% CR and IF) significantly increased the proportion of animals displaying irregular cycling patterns, whereas the 40% CR animals displayed an almost complete loss of estrous cyclicity. The females on the HFG diet continued to cycle but exhibited an increase in cycle irregularity.
Fig. 3.

Dietary energy restriction disrupts reproductive physiology in females. Panel A, The ability of female Sprague Dawley rats to perform a 4-d estrous cycle was assessed in the presence of the four dietary alteration paradigms. Irregular estrous cycling represents cycles with between 1 and 3 altered days in the pattern. Nonestrous cycle animals displayed no regularity during their typical 4-d cycle. Percentage distribution of animal numbers per cycling group are represented. Statistical significance of the relative values were calculated relative to the control proportions using a Student's t test. Panels B–D, Plasma levels of estradiol (panel B), testosterone (panel C), and corticosterone (panel D) in male and female rats that had been maintained on the indicated diets. C, Control. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Levels of estradiol were significantly decreased in males on all three energy-restriction diets as well as the HFG diet; estradiol levels were also decreased in females on the 40% CR and HFG diets (Fig. 3B). Testosterone levels were significantly increased in males on the IF diet and in females on the 40% CR diet but were decreased in females on the HFG diet (Fig. 3C). In males, corticosterone levels were elevated only in response to the 40% CR diet, whereas in females corticosterone levels were significantly elevated in response to all three energy-restriction diets (Fig. 3D), suggesting a relative hyperactivation in females of the adrenal stress response to reduced energy availability.
Sex-dependent effects of energy intake on plasma lipids
In humans excessive energy intake is associated with a proatherogenic lipid profile characterized by elevated levels of total and LDL cholesterol and triglycerides (31). We found that total cholesterol levels were decreased in males on 20% CR, 40% CR, and IF diets and in females on 20% CR and 40% CR diets (Fig. 4A). LDL levels were decreased in males on all three energy-restriction diets and in females on 40% CR and IF diets (Fig. 4C). HDL levels were unaffected by any of the diets (Fig. 4D). Triglyceride levels in males and females were unaffected by energy restriction diets; however, the HFG diet resulted in a significant increase in triglyceride levels in males but not females (Fig. 4B). Levels of 3-hydroxybutyrate were significantly decreased in male and female rats on 20% CR and IF diets but were not significantly affected by the 40% CR and HFG diets (Fig. 4E). Collectively, these data suggest that atherogenic profiles of both males and females are improved by dietary energy restriction.
Fig. 4.
Dietary energy intake modifies plasma lipid levels similarly in males and females. Each bar (panels A–E) represents the mean ± sem lipid/lipid-metabolite/lipoprotein level for the control, 20% CR, 40% CR, IF, and HFG diet. Control animal data are represented by black bars, 20% CR animals by boxed bars, 40% CR animals by striped bars, IF animals by gray bars, and HFG animals by white bars. This notation and its position in the figure is continued throughout the remaining figures in the manuscript. Panel A, Circulating cholesterol concentration. Panel B, Circulating triglyceride. Panel C, Circulating LDL. Panel D, HDL. Panel E, 3-Hydroxybutyrate (3-HB). Statistical significance, compared with control animals, was estimated using a non-paired Student's t test (n = 8–15 rats/group). C, Control. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
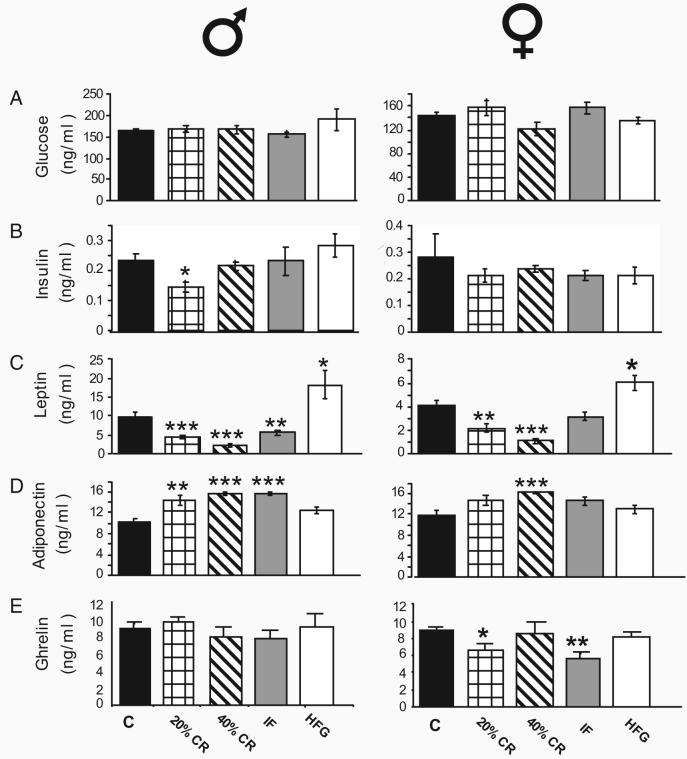
Effects of dietary energy intake and sex on hormones involved in energy metabolism
Fasting plasma glucose concentrations were similar in males and females and were not significantly different among the diet groups (Fig. 5A). Plasma insulin levels tended toward being lower in males and females on both CR diets, although the reduction reached statistical significance only in males on the 20% CR diet (Fig. 5B). In both males and females, plasma leptin levels were decreased in response to all three energy-restriction diets, with the lowest levels occurring in the 40% CR groups (Fig. 5C). The HFG diet caused a significant increase in leptin levels in both males and females. Adiponectin levels were significantly greater in males on all three energy restriction diets and in females on the 40% CR diet (Fig. 5D). Adiponectin levels in males and females on the HFG diet were not different from levels in the control diet groups. Whereas plasma ghrelin levels in males were unaffected by energy intake, ghrelin levels in females on the 20% CR and IF diets were significantly decreased (Fig. 5E).
Fig. 5.
Some energy-regulating hormones are differentially affected by dietary energy restriction in males and females. Plasma levels of glucose (panel A), insulin (panel B), leptin (panel C), adiponectin (panel D), and ghrelin (panel E) were measured in male and female rats that had been maintained for 6 months on the indicated diets. Values are the mean ± sem. C, Control. *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared with the control value.
Sex-dependent effect of dietary energy restriction on GH levels
Previous studies have documented effects of CR on prolactin (PRL) and GH levels in male rats (32) and on PRL levels in humans (33). However, whether the latter hormones are differentially affected by energy restriction and/or excess in males and females is unknown. PRL levels were significantly decreased in male rats in the IF group, compared with the control group, but were unaffected by CR or HFG diets (Fig. 6A). There were no significant effects of diet on PRL levels in female rats, although there was a trend toward increased levels in the HFG group. Plasma GH levels were highly sensitive to energy restriction in male rats, being 60–80% lower than control diet levels in both the CR groups and the IF group (Fig. 6B). In contrast, GH levels in females were significantly elevated in the 20% CR group but not different from the control diet levels in the 40% CR and IF groups. The HFG diet had no significant effect on GH levels in males or females.
Fig. 6.
Sex-dependent effects of dietary energy restriction on plasma GH levels. Plasma prolactin (panel A) and GH (panel B) levels in male and female rats that had been maintained for 6 months on the indicated diets. Values are the mean ± sem (n = 8–15 rats/group). C, Control. *, P < 0.05; **, P < 0.01, compared with the control value.
Energy restriction enhances cognitive performance only in females
Studies of rodents have shown that lifelong CR can improve the learning and memory abilities of old males (34). To elucidate possible sex-dependent effects of dietary energy intake on cognition, we subjected male and female rats in all five diet groups to two different tests of learning and memory, active avoidance and a 14-unit T-maze. We suspected that any sex differences and/or dietary differences in learning and memory behavior would be subtle and would be noticeable only in complex behavioral tasks. Therefore, we chose a complex task (the 14-unit T-maze), which allowed us to investigate sex and/or dietary differences in complex maze-solving behavior. The active avoidance test was performed before the acquisition trials in the 14-unit T-maze; a memory retention trial was completed 1 month after the acquisition trials. There were no significant effects of sex or diet on the number of trials taken to learn the nature of the applied aversive stimulus in the active avoidance test (Fig. 7A).
Fig. 7.

Active avoidance trials (for premaze testing) and shock time and shock frequency in the 14-unit T-maze. Panel A, Results of active avoidance trials. The times of male (M) and female (F) rats that had been maintained on the indicated diets to escape from a foot shock administered up to 25 successive times are shown. Panels B and C, Shock frequencies (panel B) and shock times (panel C) for individual trials of male and female rats in the acquisition phase of the 14-unit maze test. Panels D and E, Shock frequencies (panel D) and shock times (panel E) for individual trials of male and female rats in the retention phase of the 14-unit maze test. Values are mean ± sem (n = 8–15 rats/group). C, Control.
For the 14-unit maze test, the number of route errors, total shock time, and shock frequency were measured for each animal over the 20 acquisition or five retention trials. There were no significant differences observed between any of the male dietary groups for the acquisition shock frequency or shock time (Fig. 7, B and C). In contrast to the males, the 40% CR females showed significantly reduced shock frequency and shock time in the acquisition trials (Fig. 7, B and C). In the retention trials, there was no effect of diet on shock frequency or shock time in males (Fig. 7, D and E). However, females on the 40% CR diet responded to a significantly lower shock frequency than did females on any of the other diets (Fig. 7D).
There was a robust sex difference in the effect of CR on the number of route-finding errors made during the acquisition phase of the maze test. Diet had no significant effect on acquisition error number in males (Fig. 8A). In contrast, females on 20% CR and 40% CR diets made significantly fewer errors than females on the control diet (Fig. 8A). Not only did the CR diets decrease the number of errors made by females, the females on CR diets also completed the maze in a significantly shorter time period than females on the control diet or males on CR diets (Fig. 8B). There were no diet or sex differences in numbers of errors or task completion times in the retention trials of the T-maze test (Fig. 8, C and D). Therefore, females respond to CR with a heightened state of cognitive ability, compared with males.
Fig. 8.
Dietary energy restriction improves performance of female, but not male, rats in a 14-unit T-maze cognitive test. Acquisition errors (panel A), acquisition times (panel B), retention errors (panel C), and retention times (panel D) of male and female rats that had been maintained for 5 months on the indicated diets. Values are the mean ± sem (n = 8–15 rats/group). C, Control. *, P < 0.05; **, P < 0.01.
In future experiments we plan on further investigating this sex difference in the effect of CR on the number of route-finding errors in female rats by using other behavioral tasks, such as the novelty preference test and the Y-maze.
Dietary energy intake differentially affects hippocampal dopamine levels in males and females
The monoamine neurotransmitters NE, DA, and 5-HT are believed to play important roles in the regulation of energy intake (35), stress responses (36), and behavior (37). To determine whether the sex differences in physiological and behavioral responses to dietary energy intake are associated with differential changes in neurotransmitters, we measured levels of NE; DA; 5-HT; the DA metabolites DOPAC and HVA; and the 5-HT metabolite 5-HIAA in the hippocampus, cerebral cortex, cerebellum, striatum, and brainstem of male and female rats in each diet group (Tables 3-7).
TABLE 3.
Monoamine neurotransmitter and metabolite levels in the hippocampus of male and female rats with different dietary energy intakes
| Neurotransmitter | Male mean ± sem (pg/mg) |
Female mean ± sem (pg/mg) |
|
|---|---|---|---|
| NE | |||
| Control | 3531.4 (±127.8) | 3744.5 (±427.1) | |
| 20% CR | 3428.3 (±218.4) | 3285.9 (±143.3) | |
| 40% CR | 4479.9 (±474.7) | 4610.7 (±246.2) | |
| IF | 3579.3 (±149.4) | 3751.7 (±471.7) | |
| HFG | 3459.8 (±136.8) | 3991.1 (±638.6) | |
| DOPAC | |||
| Control | 999.6 (±10.3) | 2402.2 (±204.6) | |
| 20% CR | 1560.9 (±288.1) | 1820.2 (±188.9) | |
| 40% CR | 2565.7 (±358.1)↑a | 1974.4 (±466.6) | |
| IF | 2477.4 (±90.7) | 2809.4 (±133.9) | |
| HFG | 955.3 (±85.6) | 2092.8 (±236.8) | |
| DA | |||
| Control | 106.7 (±16.2) | 318.6 (±92.5) | |
| 20% CR | 128.3 (±40.0) | 120.0 (±43.3) | |
| 40% CR | 82.4 (±6.9) | 97.9 (±7.9)↓a | |
| IF | 71.5 (±4.7) | 163.6 (±65.6) | |
| HFG | 121.0 (±37.1) | 53.9 (±5.9)↓b | |
| 5HIAA | |||
| Control | 1863.0 (±56.9) | 2637.9 (±360.1) | |
| 20% CR | 1728.6 (±190.0) | 2047.7 (±99.9) | |
| 40% CR | 2233.9 (±139.5)↑a | 2829.1 (±333.2) | |
| IF | 1637.0 (±35.0)↓a | 2269.3 (±358.9) | |
| HFG | 1801.1 (±187.4) | 2251.8 (±313.5) | |
| 5HT | |||
| Control | 2009.7 (±118.4) | 1551.9 (±101.3) | |
| 20% CR | 1753.7 (±132.8) | 1740.6 (±94.6) | |
| 40% CR | 1870.8 (±40.0) | 2115.0 (±144.5)↑a | |
| IF | 1667.2 (±123.4) | 1977.3 (±236.3) | |
| HFG | 1531.0 (±66.6)↓a | 1728.5 (±308.6) | |
Arrows pointing upward denote an increase, whereas arrows pointing downward denote a decrease. Values are mean ± sem (n = 8–15 rats per group).
P < 0.05.
P < 0.01.
TABLE 7.
Monoamine neurotransmitter and metabolite levels in the brain stem of male and female rats with different dietary energy intakes
| Neurotransmitter | Male mean ± sem (pg/mg) |
Female mean ± sem (pg/mg) |
|
|---|---|---|---|
| NE | |||
| Control | 227.8 (±15.3) | 218.6 (±19.1) | |
| 20% CR | 208.7 (±16.0) | 266.9 (±27.9) | |
| 40% CR | 257.2 (±16.9) | 250.4 (±17.5) | |
| IF | 243.9 (±14.3) | 244.7 (±13.9) | |
| HFG | 244.9 (±15.2) | 215.5 (±24.6) | |
| DOPAC | |||
| Control | 239.7 (±21.9) | 357.6 (±40.1) | |
| 20% CR | 300.9 (±37.2) | 366.6 (±24.3) | |
| 40% CR | 266.2 (±38.9) | 379.7 (±24.1) | |
| IF | 271.5 (±12.7) | 290.9 (±27.5) | |
| HFG | 304.9 (±44.7) | 351.8 (±56.9) | |
| DA | |||
| Control | 16.5 (±4.4) | 14.6 (±2.58) | |
| 20% CR | 17.1 (±4.7) | 16.1 (±5.06) | |
| 40% CR | 14.5 (±3.2) | 18.9 (±2.28) | |
| IF | 10.7 (±3.1) | 16.2 (±1.13) | |
| HFG | 15.6 (±1.73) | 17.4 (±5.1) | |
| 5HIAA | |||
| Control | 167.5 (±19.1) | 179.6 (±16.9) | |
| 20% CR | 166.0 (±30.2) | 192.1 (±38.4) | |
| 40% CR | 127.2 (±17.6) | 211.0 (±25.8) | |
| IF | 119.9 (±14.0) | 173.6 (±16.4) | |
| HFG | 138.2 (±20.9) | 203.6 (±22.6) | |
| 5HT | |||
| Control | 279.6 (±13.0) | 315.3 (±31.3) | |
| 20% CR | 321.8 (±46.9) | 348.3 (±28.4) | |
| 40% CR | 273.2 (±19.8) | 338.4 (±14.5) | |
| IF | 259.4 (±5.1) | 329.6 (±21.2) | |
| HFG | 256.9 (±21.3) | 367.5 (±60.1) | |
Values are mean ± sem (n = 8–15 rats/group).
There were no significant effects of any diet on NE levels in the hippocampus. Significant changes in both sexes were seen for the other major transmitters assessed. There was a trend, however, toward elevation of NE levels in the hippocampus of rats (males and females) in the 40% CR group (Table 3).
DA levels in the cerebral cortex, striatum, and brainstem were unaffected by diet in rats of either sex. In rats on the control diet, hippocampal DA levels in females were 3-fold greater than the DA level in males (Table 3). DA levels in the hippocampus of females (but not males) on the 20% CR and 40% CR and IF diets were 50–70% lower than the DA levels in the hippocampus of females on the control diet (Table 3). Interestingly, DA levels were also significantly reduced in the hippocampus of females on the HFG diet. Levels of DA in the cerebellum were below the limit of detection of the assay. DOPAC levels in the cerebral cortex, striatum, and brainstem were not significantly affected by diet in rats of either sex. DOPAC levels in the hippocampus of male rats on the 40% CR and IF diets were significantly higher than that of the control diet group, whereas levels of DOPAC in the hippocampus of females were not significantly affected by diet. Diet had no significant effects on the levels of 5-HT in the cerebellum, striatum, or the brainstem in either sex of rat. 5-HT and 5-HIAA levels were disrupted though in the hippocampus, cerebral cortex, and the cerebellum. HVA levels in most brain regions or either sex were largely unaffected apart from the male striatum and cerebral cortex.
Dietary energy intake differentially affects brain and plasma levels of BDNF in males and females
BDNF is known to play important roles in synaptic plasticity and learning and memory (38), is also involved in the regulation of energy intake (39), and may mediate antidiabetic effects of exercise and dietary energy restriction (40-42). To elucidate a possible role for BDNF in differential responses of males and females to dietary energy restriction, we measured levels of BDNF in tissue samples from the hippocampus and cerebral cortex and plasma. BDNF levels were significantly reduced in the hippocampus and cortex of males on the IF and HFG diets (Fig. 9). In females, BDNF levels were significantly reduced by IF in the hippocampus and the HFG diet in the cerebral cortex. Plasma BDNF levels were significantly elevated in females on both CR diets and the IF diet, in contrast to males in which the diets did not alter plasma BDNF levels (Fig. 9).
Fig. 9.
Dietary energy restriction and excess differentially modify levels of brain-derived neurotrophic factor in the brains and plasma of male and female rats. BDNF levels in the hippocampus (panel A), cerebral cortex (panel B), and plasma (panel C) of male and female rats that had been maintained for 6 months on the indicated diets. Values are mean ± sem (n = 8–15 rats/group). C, Control. *, P < 0.05; **, P < 0.01, compared with the control value.
Discussion
In the present manuscript, we have elucidated the neuroendocrinological mechanisms by which animals respond to perturbations of their energy intake. We studied both caloric excess and deficiency applied to both male and female rats to assess whether both sexes responded in a similar manner. We noted that there was a strong sex-based difference in the animals' response to dietary disruption. The males' physiological adjustment to the alteration of diet was considerably less marked than that of the female rats. The females showed significant whole-body endocrinological realignment, especially in the case of mild and severe caloric restriction. The sex differences in physiological, neuroendocrine, and behavioral responses to dietary energy restriction characterized in this study may be suggestive of the presence of an evolutionarily conserved, sex-dependent dimorphism in responses to food scarcity. The profound response of female rats to caloric restriction, when compared with males, could represent a functional alteration in behavior and physiology to compete with other females as well as males for the limited food resources.
Female rats on the 40% CR diet increased their activity, despite an abnormally low body weight; exhibited elevations of corticosterone levels suggesting hyperactivation of the hypothalamic-pituitary-adrenal axis; ceased reproductive cycling and exhibited reduced gonad size and elevated testosterone levels; and enhanced their performance in cognitive tests. Each of these changes in physiology and behavior would be expected to increase the probability of survival of the individual and species in a natural setting in which food is scarce. The increased ambulatory activity, elevated corticosterone levels, and heightened cognitive ability of females on CR are consistent with a scenario in which food scarcity imposes a stress on the animal, motivating them to seek food elsewhere. This may be particularly important in females, in contrast to males, because they must obtain sufficient energy to support the survival and development of their offspring as well as themselves. Diverting energy from reproduction to brain and neuromuscular activity would be expected to increase the probability of survival. Compared with females, males subjected to energy restriction exhibited no change in gonad size, lesser increases in activity and corticosterone levels, and no enhancement of cognitive ability. Thus, energy restriction appears to impose less stress and behavioral activation in males. The preservation of reproductive function and fertility in males during times of food scarcity, which has been reported previously (43), would allow males to inseminate any available fertile females.
The cellular and molecular mechanisms that mediate sex-dependent physiological and behavioral responses to energy deprivation are unknown. We found that plasma levels of leptin were decreased and adiponectin increased in response to energy restriction in both males and females, whereas leptin levels were increased in response to the HFG diet in both sexes. However, ghrelin levels were decreased by 20% CR and IF diets in females only. The latter results would suggest that moderate energy restriction may decrease hunger in females but not males, although this interpretation would have to be confirmed with more direct measures of hunger. Nevertheless, the endocrine stress response and ambulatory activity levels were greater in females, compared with males, differences that are unlikely to be linked mechanistically to sex differences in ghrelin levels. We observed a striking sex difference in the effects of dietary energy intake on circulating GH levels; males exhibited large decreases in GH levels in response to CR and IF, whereas GH levels were unchanged (40% CR and IF) or increased (20% CR) in females. Previous studies have shown that CR reduces GH levels in young male rats (44) and that mice genetically deficient in GH exhibit some physiological changes that are similar to those induced by CR including improved glucose metabolism and extended longevity (45). However, GH levels increased in 6- to 11-yr-old girls during a 48-h fast (46). The reason GH levels were decreased in response to CR in males but increased in females is unknown, but this sex difference could contribute to the differential effects of CR on male and female reproductive physiology (47, 48).
Our measurements of monoamine levels in different central nervous system regions suggest a possible role for changes in hippocampal dopaminergic signaling in behavioral and some hormonal responses of females and males to energy restriction. We found that DA levels in the hippocampus were reduced in response to CR and IF in female rats but not males. However, there were no sex differences in DA levels in the other brain regions examined (cerebral cortex, cerebellum, striatum, and brainstem). We did not observe a similar sex-specific pattern of disruption by the effects of differential dietary energy intake on levels of NE in the hippocampus. Past studies have provided evidence for changes in dopaminergic signaling in response to caloric restriction in rodents (49, 50), but sex differences have not been described previously. DA is known to play important roles in motivational and cognitive functions associated with the hippocampus (51-53). The hyperactivity and enhanced cognitive performance of female rats on the 40% CR diet may therefore involve alterations in dopaminergic signaling.
Another possible factor that could have attributed to the hyperactivity, heightened stress response, and enhanced cognitive function observed in the 40% CR female rats could be that the severely calorie restricted animals demonstrated differences in the states of their food-entrainable oscillators, compared with the ad libitum-fed control rats. In mammals, daily rhythms in physiology and behavior are driven by a circadian timing system, which is comprised of a master pacemaker in the suprachiasmatic nuclei (SCN) of the hypothalamus and peripheral oscillators in most body cells. The SCN clock is mainly entrained by light/dark cycles, whereas the peripheral oscillators can be strongly affected by daily feeding cycles, which have little effect on the phase of the SCN. When feeding schedules are coupled with a restriction in caloric intake, behavioral and physiological circadian rhythms, and gene expression in the SCN are shifted and/or entrained to mealtime. Several studies (for excellent reviews on this topic, see Refs. 54-56) have shown that rats and mice can anticipate a fixed daily meal by entrainment of a circadian oscillator or clock, which is distinct from the clock mechanism that generates the daily rhythms entrained to light/dark cycles. It has been demonstrated that scheduled food and/or water restriction causes activity in the hypothalamic-pituitary axis increases activity levels and causes an elevation in core body temperature around the time of feeding (57-64). Entrainment of the hypothalamic-pituitary axis and the anticipatory activity to food restriction have been shown to be determined by food intake (55, 64, 65); however, prolonged periods of access to food that allow for normal weight gain and normal food consumption have been shown to not alter circadian rhythms. Thus, there is a possibility that there was an alteration in the food entrained circadian oscillator that could have accounted for some of the alterations we observed in physiology and behavior in the severely calorie restricted animals, compared with the ad libitum-fed control rats. It would be important and informative to investigate the potential effects of food entrainment in calorie-restricted animals on the function of circadian oscillators in future experiments.
On the other hand, perhaps the female rats' robust responses to caloric perturbation may shed light on human disorders of caloric intake. AN is approximately 10 times more common in women, compared with men (11). It has been suggested that this sex difference is the result of the importance for women of maintaining a slender and therefore attractive body (66). However, our findings show that female rats (which are assumedly free of body image issues) subjected to 40% CR develop physiological and behavioral changes similar to those of human females with AN. The changes include emaciation, increased physical activity, reproductive shutdown, and hormonal masculinization, and heightened attention/cognitive ability. The fact that females subjected to a lower level of energy restriction (20% CR) did not develop AN-like physiological and behavioral alterations suggests that there is a threshold level of energy restriction to which females respond by suppressing reproductive function and increasing their motor and cognitive activities.
We have demonstrated that female rats possess a greater sensitivity to perturbation of their caloric intake. In part this may be due to the relative robustness and simplicity of the males' neuroendocrine axis, compared with the females' complex reproductive system and additional requirements of energy for nursing of offspring. The endocrinological, neurophysiological, and behavorial changes that the females demonstrate on food scarcity all could logically assist them in competing with males and other females for the remaining food in their environment. In the future a better understanding of the neurohormonal and neuropsychological mechanisms that sensitize females to reduced energy intake may lead to novel strategies or therapies for the easy maintenance of a healthy body weight.
TABLE 4.
Monoamine neurotransmitter and metabolite levels in the cerebral cortex of male and female rats with different dietary energy intakes
| Neurotransmitter | Male mean ± sem (pg/mg) |
Female mean ± sem (pg/mg) |
|
|---|---|---|---|
| NE | |||
| Control | 751.2 (±59.7) | 763.3 (±19.0) | |
| 20% CR | 776.9 (±27.7) | 813.3 (±29.1) | |
| 40% CR | 814.9 (±34.9)↑a | 879.2 (±34.6) | |
| IF | 844.3 (±61.8) | 798.0 (±38.9) | |
| HFG | 819.0 (±21.6)↑a | 866.6 (±29.2) | |
| DOPAC | |||
| Control | 141.4 (±31.9) | 135.8 (±25.0) | |
| 20% CR | 173.1 (±25.9) | 155.4 (±29.3) | |
| 40% CR | 145.7 (±26.2) | 151.8 (±28.6) | |
| IF | 174.2 (±34.3) | 191.3 (±31.6) | |
| HFG | 175.1 (±17.7) | 183.7 (±30.2) | |
| DA | |||
| Control | 598.1 (±42.9) | 623.1 (±94.3) | |
| 20% CR | 656.9 (±82.1) | 575.6 (±66.4) | |
| 40% CR | 604.4 (±89.6) | 727.9 (±68.2) | |
| IF | 595.6 (±81.5) | 601.9 (±58.9) | |
| HFG | 567.8 (±55.2) | 735.8 (±75.6) | |
| 5HIAA | |||
| Control | 237.4 (±16.9) | 226.8 (±17.3) | |
| 20% CR | 194.8 (±16.0)↓a | 226.1 (±11.5) | |
| 40% CR | 220.7 (±23.0) | 262.4 (±13.3) | |
| IF | 179.1 (±23.5)↓a | 173.6 (±13.8)↓a | |
| HFG | 185.6 (±24.2) | 187.8 (±11.5)↓a | |
| 5HT | |||
| Control | 400.4 (±12.7) | 380.6 (±22.1) | |
| 20% CR | 368.2 (±12.5) | 373.8 (±25.2) | |
| 40% CR | 358.7 (±18.8) | 406.9 (±18.5) | |
| IF | 343.3 (±29.5) | 354.7 (±28.9) | |
| HFG | 353.9 (±16.2)↓a | 375.6 (±14.1) | |
| HVA | |||
| Control | 47.9 (±5.8) | 46.6 (±3.7) | |
| 20% CR | 45.4 (±3.7) | 44.2 (±3.2) | |
| 40% CR | 45.2 (±5.8) | 57.0 (±6.4) | |
| IF | 37.6 (±4.4) | 41.7 (±5.8) | |
| HFG | 32.4 (±4.2)↓a | 45.0 (±5.7) | |
Arrows pointing upward denote an increase, whereas arrows pointing downward denote a decrease. Values are mean ± sem (n = 8–15 rats/group).
P < 0.05.
TABLE 5.
Monoamine neurotransmitter and metabolite levels in the cerebellum of male and female rats with different dietary energy intakes
| Neurotransmitter | Male mean ± sem (pg/mg) |
Female mean ± sem (pg/mg) |
|
|---|---|---|---|
| NE | |||
| Control | 238.2 (±15.8) | 218.1 (±12.5) | |
| 20% CR | 231.7 (±18.4) | 208.5 (±19.8) | |
| 40% CR | 249.7 (±21.8) | 197.1 (±14.6)↓a | |
| IF | 256.3 (±19.4) | 242.4 (±25.6) | |
| HFG | 284.4 (±21.7) | 255.0 (±22.7) | |
| DOPAC | |||
| Control | 165.2 (±18.2) | 188.6 (±32.5) | |
| 20% CR | 186.0 (±28.9) | 199.8 (±24.5) | |
| 40% CR | 190.6 (±6.9) | 192.9 (±13.7) | |
| IF | 231.0(±13.2)↑a | 281.9 (±15.8)↑a | |
| HFG | 217.5 (±12.7)↑a | 242.0 (±6.8) | |
| 5HIAA | |||
| Control | 17.3 (±3.4) | 20.7 (±2.6) | |
| 20% CR | 11.7 (±2.9) | 17.3 (±4.1) | |
| 40% CR | 19.0 (±2.1) | 27.1 (±5.2) | |
| IF | 11.3 (±3.6)↓a | 10.7 (±2.4)↓a | |
| HFG | 13.9 (±2.2) | 18.0 (±3.6) | |
| 5HT | |||
| Control | 35.7 (±2.8) | 35.8 (±2.0) | |
| 20% CR | 35.1 (±4.6) | 35.9 (±2.7) | |
| 40% CR | 32.0 (±3.4) | 38.8 (±2.9) | |
| IF | 35.2 (±2.1) | 38.3 (±3.7) | |
| HFG | 32.9 (±1.3) | 37.5 (±2.6) | |
Arrows pointing upward denote an increase, whereas arrows pointing downward denote a decrease. Values are mean ± sem (n = 8–15 rats/group).
P < 0.05.
TABLE 6.
Monoamine neurotransmitter and metabolite levels in the striatum of male and female rats with different dietary energy intakes
| Neurotransmitter | Male mean ± sem (pg/mg) |
Female mean ± sem (pg/mg) |
|
|---|---|---|---|
| NE | |||
| Control | 205.7 (±42.1) | 253.5 (±66.2) | |
| 20% CR | 220.5 (±10.7) | 242.5 (±29.8) | |
| 40% CR | 231.1 (±57.1)↑a | 242.1 (±52.9) | |
| IF | 157.6 (±25.2) | 177.2 (±46.8) | |
| HFG | 162.4 (±23.6) | 164.5 (±20.1) | |
| DOPAC | |||
| Control | 1657.3 (±98.9) | 1462.9 (±68.7) | |
| 20% CR | 1493.2 (±102.5) | 1595.1 (±49.0) | |
| 40% CR | 1615.4 (±118.8) | 1586.3 (±88.5) | |
| IF | 1635.2 (±82.0) | 1557.8 (±64.0) | |
| HFG | 1456.2 (±52.7) | 1472.2 (±86.7) | |
| DA | |||
| Control | 3771.1 (±196.9) | 3648.3 (±140.4) | |
| 20% CR | 3613.3 (±143.1) | 3897.7 (±114.9) | |
| 40% CR | 3968.1 (±242.0) | 3891.1 (±182.6) | |
| IF | 4135.6 (±86.7) | 3917.7 (±133.9) | |
| HFG | 4035.7 (±174.6) | 4060.6 (±164.6) | |
| 5HIAA | |||
| Control | 595.8 (±87.1) | 574.4 (±33.7) | |
| 20% CR | 438.1 (±33.8) | 557.4 (±26.2) | |
| 40% CR | 512.9 (±43.0) | 667.8 (±44.3) | |
| IF | 469.7 (±24.9) | 575.9 (±69.3) | |
| HFG | 487.7 (±19.2) | 559.2 (±44.3) | |
| 5HT | |||
| Control | 444.0 (±82.6) | 347.7 (±83.7) | |
| 20% CR | 307.7 (±61.7) | 345.1 (±81.8) | |
| 40% CR | 325.7 (±69.6) | 423.8 (±75.7) | |
| IF | 358.2 (±66.8) | 427.6 (±69.9) | |
| HFG | 359.2 (±64.0) | 365.1 (±49.8) | |
| HVA | |||
| Control | 554.2 (±88.1) | 629.9 (±49.5) | |
| 20% CR | 557.9 (±30.9) | 609.9 (±33.5) | |
| 40% CR | 609.0 (±65.2)↑a | 660.9 (±28.9) | |
| IF | 572.3 (±38.4) | 569.8 (±39.8) | |
| HFG | 467.9 (±17.7) | 638.2 (±65.2) | |
Arrows pointing upward denote an increase, whereas arrows pointing downward denote a decrease. Values are mean ± sem (n = 8–15 rats/group).
P < 0.05.
Acknowledgments
The authors thank Dr. D. Ingram, Dr. R. De Cabo, R. G. Cutler, Dr. M. Bauman, and T. Iyun for help and advice during the study. We also thank Dr. A. F. Parlow (National Hormone and Peptide Program, Torrance, CA) for excellently performing the assays for GH, prolactin, estradiol, testosterone, and corticosterone.
This work was supported by the Intramural Research Program of the National Institute on Aging.
Abbreviations
- AN
Anorexia nervosa
- BDNF
brain-derived neurotrophic factor
- CR
caloric restriction
- DA
dopamine
- DOPAC
dihydroxyphenylacetic acid
- HFG
high-fat/high-glucose
- 5HIAA
5-hydroxy indoleacetic acid
- 5-HT
serotonin
- HVA
homovanilic acid
- IF
intermittent fasting
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- NE
norepinephrine
- PRL
prolactin
- SCN
suprachiasmatic nuclei
Footnotes
The authors have nothing to disclose.
References
- 1.Levine A, Billington C. Why do we eat? A neural systems approach. Annu Rev Nutr. 1997;17:597–619. doi: 10.1146/annurev.nutr.17.1.597. [DOI] [PubMed] [Google Scholar]
- 2.Badman M, Flier J. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307:1909–1914. doi: 10.1126/science.1109951. [DOI] [PubMed] [Google Scholar]
- 3.Volkow N, Wise R. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 4.Haslam D, James W. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 5.Sohal R, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffy P, Leakey J, Pipkin J, Turturro A, Hart R. The physiologic, neurologic, and behavioral effects of caloric restriction related to aging, disease, and environmental factors. Environ Res. 1997;73:242–248. doi: 10.1006/enrs.1997.3714. [DOI] [PubMed] [Google Scholar]
- 7.Anson R, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram D, Lane M, Mattson M. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci USA. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heilbronn L, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 9.Gull W. Anorexia nervosa (apepsia hysterica, anorexia hysterica) Trans Clin Soc Lond. 1874;7:22–28. [Google Scholar]
- 10.Chan J, Mantzoros C. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet. 2005;366:74–85. doi: 10.1016/S0140-6736(05)66830-4. [DOI] [PubMed] [Google Scholar]
- 11.Yager J, Andersen A. Clinical practice. Anorexia nervosa. N Engl J Med. 2005;353:1481–1488. doi: 10.1056/NEJMcp050187. [DOI] [PubMed] [Google Scholar]
- 12.Humphries L, Wrobel S, Wiegert H. Anorexia nervosa. Am Fam Physician. 1982;26:199–204. [PubMed] [Google Scholar]
- 13.Masoro E. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Knuth U, Friesen H. Starvation induced anoestrus: effect of chronic food restriction on body weight, its influence on oestrous cycle and gonadotrophin secretion in rats. Acta Endocrinol. 1983;104:402–409. doi: 10.1530/acta.0.1040402. [DOI] [PubMed] [Google Scholar]
- 15.Nelson J, Karelus K, Bergman M, Felicio L. Neuroendocrine involvement in aging: evidence from studies of reproductive aging and caloric restriction. Neurobiol Aging. 1995;16:837–843. doi: 10.1016/0197-4580(95)00072-m. [DOI] [PubMed] [Google Scholar]
- 16.McShane T, Wise P. Life-long moderate caloric restriction prolongs reproductive life span in rats without interrupting estrous cyclicity: effects on the gonadotropin-releasing hormone/luteinizing hormone axis. Biol Reprod. 1996;54:70–75. doi: 10.1095/biolreprod54.1.70. [DOI] [PubMed] [Google Scholar]
- 17.Munoz M, Argente J. Anorexia nervosa in female adolescents: endocrine and bone mineral density disturbances. Eur J Endocrinol. 2002;147:275–286. doi: 10.1530/eje.0.1470275. [DOI] [PubMed] [Google Scholar]
- 18.Misra M, Aggarwal A, Miller K, Almazan C, Worley M, Soyka L, Herzog D, Klibanski A. Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics. 2004;114:1574–1583. doi: 10.1542/peds.2004-0540. [DOI] [PubMed] [Google Scholar]
- 19.Maxwell S. Women and heart disease. Basic Res Cardiol. 1998;93:S79–S84. doi: 10.1007/s003950050225. [DOI] [PubMed] [Google Scholar]
- 20.Wilding J. Neuropeptides and appetite control. Diabet Med. 2002;19:619–627. doi: 10.1046/j.1464-5491.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- 21.Mattson M, Duan W, Maswood N. How does the brain control lifespan? Ageing Res Rev. 2002;1:155–165. doi: 10.1016/s1568-1637(01)00003-4. [DOI] [PubMed] [Google Scholar]
- 22.Diano S, Farr S, Benoit S, McNay E, da Silva I, Horvath B, Gaskin F, Nonaka N, Jaeger L, Banks W, Morley J, Pinto S, Sherwin R, Xu L, Yamada K, Sleeman M, Tschop M, Horvath T. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 23.Elias M, Elias P, Sullivan L, Wolf P, D'Agostino R. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 24.Greenwood C, Winocur G. High-fat diets, insulin resistance and declining cognitive function. Neurobiol Aging. 2005;26:S42–S45. doi: 10.1016/j.neurobiolaging.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Strupp B, Weingartner H, Kaye W, Gwirtsman H. Cognitive processing in anorexia nervosa. A disturbance in automatic information processing. Neuropsychobiology. 1986;15:89–94. doi: 10.1159/000118248. [DOI] [PubMed] [Google Scholar]
- 26.Connan F, Murphy F, Connor S, Rich P, Murphy T, Bara-Carill N, Landau S, Krljes S, Ng V, Williams S, Morris R, Campbell I, Treasure J. Hippocampal volume and cognitive function in anorexia nervosa. Psychiatry Res. 2006;146:117–125. doi: 10.1016/j.pscychresns.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Winocur G, Greenwood C, Piroli G, Grillo C, Reznikov L, Reagan L, McEwen B. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- 28.Spangler E, Chachich M, Ingram D. Scopolamine in rats impairs acquisition but not retention in a 14-unit T-maze. Pharmacol Biochem Behav. 1988;30:949–955. doi: 10.1016/0091-3057(88)90125-6. [DOI] [PubMed] [Google Scholar]
- 29.Maudsley S, Pierce K, Zamah A, Miller W, Ahn S, Daaka Y, Lefkowitz R, Luttrell L. The β(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem. 2000;275:9572–9580. doi: 10.1074/jbc.275.13.9572. [DOI] [PubMed] [Google Scholar]
- 30.Andrews A, Ladenheim B, Epstein C, Cadet J, Murphy D. Transgenic mice with high levels of superoxide dismutase activity are protected from the neurotoxic effects of 2′-NH2-MPTP on serotonergic and noradrenergic nerve terminals. Mol Pharrmacol. 1996;50:1511–1519. [PubMed] [Google Scholar]
- 31.Howard B, Ruotolo G, Robbins D. Obesity and dyslipidemia. Endocrinol Metab Clin North Am. 2003;32:855–867. doi: 10.1016/s0889-8529(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Varela S, Chacon F, Cano P, Arce A, Esquifino A. Differential responses of circulating prolactin, GH, and ACTH levels and distribution and activity of submaxillary lymph node lymphocytes to calorie restriction in male Lewis and Wistar rats. Neuroimmunomodulation. 2004;11:247–251. doi: 10.1159/000078443. [DOI] [PubMed] [Google Scholar]
- 33.Lamberts S, Visser T, Wilson J. The influence of caloric restriction on serum prolactin. Int J Obes. 1979;3:75–81. [PubMed] [Google Scholar]
- 34.Means L, Higgins J, Fernandez T. Mid-life onset of dietary restriction extends life and prolongs cognitive functioning. Physiol Behav. 1993;54:503–508. doi: 10.1016/0031-9384(93)90243-9. [DOI] [PubMed] [Google Scholar]
- 35.Clifton P, Kennett G. Monoamine receptors in the regulation of feeding behaviour and energy balance. CNS Neurol Disord Drug Targets. 2006;5:293–312. doi: 10.2174/187152706777452254. [DOI] [PubMed] [Google Scholar]
- 36.Vermetten E, Bremner J. Circuits and systems in stress. II. Applications to neurobiology and treatment in posttraumatic stress disorder. Depress Anxiety. 2002;15:126–147. doi: 10.1002/da.10017. [DOI] [PubMed] [Google Scholar]
- 37.Myhrer T. Neurotransmitter systems involved in learning and memory in the rat: a meta-analysis based on studies of four behavioral tasks. Brain Res Rev. 2003;41:268–287. doi: 10.1016/s0165-0173(02)00268-0. [DOI] [PubMed] [Google Scholar]
- 38.Pang P, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Xu B, Goulding E, Zang K, Cepoi D, Cone R, Jones K, Tecott L, Reichardt L. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagawa T, Tsuchida A, Itakura Y, Nonomura T, Ono M, Hirota F, Inoue T, Nakayama C, Taiji M, Noguchi H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes. 2000;49:436–444. doi: 10.2337/diabetes.49.3.436. [DOI] [PubMed] [Google Scholar]
- 41.Duan W, Guo Z, Jiang H, Ware M, Mattson M. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003;144:2446–2453. doi: 10.1210/en.2002-0113. [DOI] [PubMed] [Google Scholar]
- 42.Vaynman S, Ying Z, Wu A, Gomez-Pinilla F. Coupling energy metabolism with a mechanism to support brain-derived neurotrophic factor-mediated synaptic plasticity. Neuroscience. 2006;139:1221–1234. doi: 10.1016/j.neuroscience.2006.01.062. [DOI] [PubMed] [Google Scholar]
- 43.Johnson L, May M, Busbee D, Williams J. Effect of age and dietary restriction on daily sperm production and number and transit time of epididymal spermatozoa in the mouse. AGE. 1992;15:65–71. [Google Scholar]
- 44.Sonntag W, Xu X, Ingram R, D'Costa A. Moderate caloric restriction alters the subcellular distribution of somatostatin mRNA and increases growth hormone pulse amplitude in aged animals. Neuroendocrinology. 1995;61:601–608. doi: 10.1159/000126885. [DOI] [PubMed] [Google Scholar]
- 45.Bonkowski M, Rocha J, Masternak M, Al R, egaley K, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci USA. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kasa-Vubu J, Barkan A, Olton P, Meckmongkol T, Carlson N, Foster C. Incomplete modified fast in obese early pubertal girls leads to an increase in 24-hour growth hormone concentration and a lessening of the circadian pattern in leptin. J Clin Endocrinol Metab. 2002;87:1885–1893. doi: 10.1210/jcem.87.4.8250. [DOI] [PubMed] [Google Scholar]
- 47.Jansson J, Eden S, Isaksson O. Sexual dimorphism in the control of growth hormone secretion. Endocr Rev. 1985;6:128–150. doi: 10.1210/edrv-6-2-128. [DOI] [PubMed] [Google Scholar]
- 48.Jorgensen K, Svendsen O, Agergaard N, Skydsgaard K. Effect of human growth hormone on the reproduction of female rats. Pharmacol Toxicol. 1991;68:14–20. doi: 10.1111/j.1600-0773.1991.tb01201.x. [DOI] [PubMed] [Google Scholar]
- 49.Diao L, Bickford P, Stevens J, Cline E, Gerhardt G. Caloric restriction enhances evoked DA overflow in striatum and nucleus accumbens of aged Fischer 344 rats. Brain Res. 1997;763:276–280. doi: 10.1016/s0006-8993(97)00494-0. [DOI] [PubMed] [Google Scholar]
- 50.Carr K, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience. 2003;119:1157–1167. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- 51.O'Carroll C, Martin S, Sandin J, Frenuelli B, Morris R. Dopaminergic modulation of the persistence of one-trial hippocampus-dependent memory. Learn Mem. 2006;13:760–769. doi: 10.1101/lm.321006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci. 2006;26:7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adcock R, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli J. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 54.Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 55.Stephan FK, Becker G. Entrainment of anticipatory activity to various durations of food access. Physiol Behav. 1989;46:731–741. doi: 10.1016/0031-9384(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 56.Mendoza J. Circadian clocks: setting time by food. J Neuroendocrinol. 2006;19:127–137. doi: 10.1111/j.1365-2826.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- 57.Bolles RC, Stokes LW. Rat's anticipation of diurnal and a-diurnal feeding. J Comp Physiol Psychol. 1965;60:290–294. doi: 10.1037/h0022308. [DOI] [PubMed] [Google Scholar]
- 58.Mouret JR, Bobillier P. Diurnal rhythms of sleep in the rat: augmentation of paradoxical sleep following alterations of the feeding schedule. Int J Neurosci. 1971;2:265–270. doi: 10.3109/00207457109147009. [DOI] [PubMed] [Google Scholar]
- 59.Johnson JT, Levine S. Influence of water deprivation on adrenocortical rhythms. Neuroendocrinology. 1973;11:268–273. doi: 10.1159/000122139. [DOI] [PubMed] [Google Scholar]
- 60.Krieger DT. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology. 1974;95:1195–1201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- 61.Moberg GP, Bellinger LL, Mendel VE. Effect of meal feeding on daily rhythms of plasma corticosterone and growth hormone in the rat. Neuroendocrinology. 1975;19:160–169. doi: 10.1159/000122436. [DOI] [PubMed] [Google Scholar]
- 62.Gray GD, Bergfors AM, Levin R, Levine S. Comparison of the effects of restricted morning or evening water intake on adrenocortical activity in female rats. Neuroendocrinology. 1978;25:236–246. doi: 10.1159/000122745. [DOI] [PubMed] [Google Scholar]
- 63.Wilkinson CW, Shinsako J, Dallman MF. Daily rhythms in adrenal responsiveness to adrenocorticotropin are determined primarily by the time of feeding in the rat. Endocrinology. 1979;104:350–358. doi: 10.1210/endo-104-2-350. [DOI] [PubMed] [Google Scholar]
- 64.Honma K, vonGoetz C, Aschoff J. Effects of restricted daily feeding on free-running circadian rhythms in rats. Physiol Behav. 1983;30:905–913. doi: 10.1016/0031-9384(83)90256-1. [DOI] [PubMed] [Google Scholar]
- 65.Honma K, Honma S, Hiroshige T. Critical role of food amount for prefeeding corticosterone peak in rats. Am J Physiol. 1983;245:R339–R344. doi: 10.1152/ajpregu.1983.245.3.R339. [DOI] [PubMed] [Google Scholar]
- 66.Skrzypek S, Wehmeier P, Remschmidt H. Body image assessment using body size estimation in recent studies on anorexia nervosa. A brief review. Eur Child Adolesc Psychiatry. 2001;10:215–221. doi: 10.1007/s007870170010. [DOI] [PubMed] [Google Scholar]