Abstract
A comprehensive assessment of bacterial diversity and community composition in arctic and antarctic pack ice was conducted through cultivation and cultivation-independent molecular techniques. We sequenced 16S rRNA genes from 115 and 87 pure cultures of bacteria isolated from arctic and antarctic pack ice, respectively. Most of the 33 arctic phylotypes were >97% identical to previously described antarctic species or to our own antarctic isolates. At both poles, the α- and γ-proteobacteria and the Cytophaga-Flavobacterium group were the dominant taxonomic bacterial groups identified by cultivation as well as by molecular methods. The analysis of 16S rRNA gene clone libraries from multiple arctic and antarctic pack ice samples revealed a high incidence of closely overlapping 16S rRNA gene clone and isolate sequences. Simultaneous analysis of environmental samples with fluorescence in situ hybridization (FISH) showed that ∼95% of 4′,6′-diamidino-2-phenylindole (DAPI)-stained cells hybridized with the general bacterial probe EUB338. More than 90% of those were further assignable. Approximately 50 and 36% were identified as γ-proteobacteria in arctic and antarctic samples,respectively. Approximately 25% were identified as α-proteobacteria, and 25% were identified as belonging to the Cytophaga-Flavobacterium group. For the quantification of specific members of the sea ice community, new oligonucleotide probes were developed which target the genera Octadecabacter, Glaciecola, Psychrobacter, Marinobacter, Shewanella, and Polaribacter. High FISH detection rates of these groups as well as high viable counts corroborated the overlap of clone and isolate sequences. A terrestrial influence on the arctic pack ice community was suggested by the presence of limnic phylotypes.
Pack ice in the Arctic and Antarctic, with its vast extension and high biological productivity (3, 34, 35, 36, 56),constitutes one of the most significant polar ecosystems. Several similarities exist between the sea ice regimes in the north and the south; however, there are also fundamental differences in formation, development, thickness, maturity, and ice crystal structure (57). Moreover, the Arctic Ocean, in contrast to the Southern Ocean, is strongly influenced by warm Atlantic waters and has a high terrestrial input due to its nearly complete enclosure by landmasses. Whether these differences influence the colonization of sea ice and the development of microbial sea ice communities is still an open question.
Phylogenetic diversity studies of sea ice bacterial communities have focused mainly on the Antarctic (8, 18). In particular, land-fast ice surrounding the McMurdo base and pack ice between the Casey and Davis bases has been investigated. Sampling was limited to spring and summer seasons. The few arctic sea ice samples considered came from Baffin Bay (18), the Chukchi Sea (41), and Barrow, Alaska (31). Cultivation approaches provided the initial view of diversity of sea ice bacteria, mainly in the Antarctic, and revealed several novel genera and species that appear to be specific to sea ice (8, 9, 10, 11, 12, 13, 14, 15, 30, 32, 33, 40, 46). Only recently, the first phylogenetic analyses of environmental DNA from antarctic sea ice including a single arctic sample was reported, indicating some overlap of antarctic and arctic sea ice 16S rRNA gene phylotypes (18).
The aim of this study was to compare the diversity and structure of bacterial communities in arctic versus antarctic pack ice by cultivation and cultivation-independent methods, making use of sea ice samples from yet-unconsidered geographical areas and seasons. We sequenced 16S rRNA genes from pure-culture isolates and from environmental samples derived from multiyear arctic pack ice north of Svalbard and the Fram Strait. The pack ice in this region is strongly influenced by terrestrial input from Siberian rivers (51). 16S rRNA genes from pure cultures isolated from first-year (annual) antarctic pack ice sampled in the Weddell Sea during midwinter (36) as well as environmental samples from the Lazarev Sea taken during autumn were also sequenced. To find out if community composition is affected by the starkly contrasting sea ice habitats at either pole, fluorescence in situ hybridization (FISH) was applied to the environmental samples. New oligonucleotide probes specific for several members of the sea ice bacterial community were developed and used in combination with previously published probes for the FISH analyses. We present a comprehensive survey of bacterial diversity in arctic and antarctic pack ice and report the first data for in situ distribution and abundance of bacteria in sea ice.
MATERIALS AND METHODS
Sampling, processing, total and viable counts, and isolation.
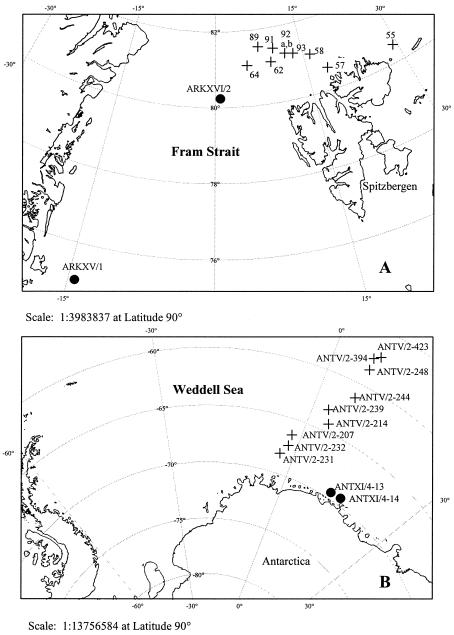
Multiyear arctic pack ice samples (thickness, 3 to 5 m) were collected during the R. V. Polarstern summer cruises ARKXIII/2 (June to July 1997), ARKXV/1 (June to July 1999), and ARKXVI/2 (July to August 2000) in the Fram Strait and northeast of Svalbard in the Arctic Ocean. First-year antarctic pack ice was sampled during the R. V. Polarstern midwinter cruise ANTV/2 (July to September 1986) in the open Weddell Sea and during the autumnal cruise ANTXI/4 (March to April 1994) in the area of the Lazarev Sea. Positions of all stations are shown in Fig. 1. Sea ice samples with and without algal accumulation were present among the arctic and the antarctic samples. The ARKXV/1 station ice core contained several large sediment inclusions. Samples were collected and processed as described previously by Helmke and Weyland (36). The arctic pack ice isolates were derived from the bottom sections of 10 different ice cores collected during cruise ARKXIII/2. The antarctic strains were isolated from the upper, middle, and bottom sections of seven ice cores as well as from grease ice and freshly formed pancake ice sampled during cruise ANTV/2 (36). Pure cultures were obtained from chitin agar (36), ZoBell agar 2216E, and a nutrient-poor agar medium containing 200 mg of yeast extract and 1 g of potassium nitrate in 1 liter of natural seawater. Total and viable counts were determined as previously described by Helmke and Weyland (36). Samples for clone libraries and FISH were collected during cruises ARKXV/1,ARKXVI/2, and ANTXI/4. Bacterioplankton of melted-ice samples (36) were collected onto polycarbonate filters (pore size, 0.2 μm) and stored at −80°C for later extraction of nucleic acids. Parallel samples were fixed with paraformaldehyde (final concentration, 2 to 4% [vol/vol]), immobilized on polycarbonate filters (pore size, 0.2 μm), and then rinsed with 3 ml each of phosphate-buffered saline and distilled water. Air-dried filters were stored at −20°C until analysis with FISH. Total count preservations were fixed with formalin (final concentration, 3% [vol/vol]) and stored at 2°C for up to 2 months before enumeration (38).
FIG. 1.
Sampling stations in the Arctic Ocean and Fram Strait (A) and the Weddell and Lazarev Seas in the Southern Ocean (B). Crosses represent sampling stations for isolate cultures and closed circles represent sampling stations for environmental samples. Maps were generated with PanMap software (http://www.pangaea.de).
Extraction of nucleic acids, amplification of 16S rRNA gene, and clone library construction.
Nucleic acids from pure cultures were extracted with a 3% cetyltrimethylammonium bromide procedure (22) For clone libraries, total community nucleic acids from filtered samples were extracted using the DNeasy tissue kit (Qiagen, Hilden, Germany) with additional lysozyme and lysostaphin preincubations. Efficiency of the preincubation was controlled by microscopic examination of filters before and after processing. Between 95 and 97% of observable 4′,6′-diamidino-2-phenylindole (DAPI)-stained bacterial cells were effectively lysed. An additional purification step carried out with the WIZARD DNA Cleanup System (Promega Corp., Madison, Wis.) was necessary to remove potential PCR inhibitors that were coextracted from samples.
Nearly full-length 16S rRNA gene sequences were amplified from isolate and environmental sample nucleic acid extracts (approximately 100 ng) by hot-start PCR with an automated thermal cycler (Eppendorf, Hamburg, Germany) by using the bacterium-specific primers 8f and 1542r (for isolates) or 1492r (for environmental samples) under the conditions described previously by Kopp et al. (42). PCR products were purified with the QIAquick purification kit (Qiagen). 16S rRNA gene clone libraries were constructed with the pGEM-T-Easy vector system (Promega Corp.).
ARDRA, sequencing, and phylogenetic analysis.
Amplified rDNA restriction analysis (ARDRA) (45) was used to characterize the 16S rRNA gene diversity within the culture collections and clone libraries. One, or in some cases, several representatives of the ARDRA pattern groups from each culture collection and clone library were selected for sequencing. Sequence data were analyzed with the ARB software package (http://www.mikro.biologie.tu-muenchen.de). Dendrograms (viewable at http://www.awi-bremerhaven.de/Pelagic/Sections/MarineChemistry/Helmke_seaice1-dohtml) were reconstructed for the phylogenetic analysis. The frequencies of 16S rRNA gene phylotypes determined by ARDRA and subsequent sequencing (i.e., those sharing >97% identity) were used for analysis of diversity. Shannon's index for diversity (H′) was calculated according to the method of Zar (65). Rarefaction curves were interpolated with the freeware program Analytic Rarefaction 1.3 (http://www.uga.edu/∼strata/software/index.html).Coverage of the clone libraries was estimated as described previously by Mullins et al. (48).
Probe design and FISH.
Oligonucleotide probes ranging in specificity from domain to species level (Table 1) were used with FISH to examine community structure of bacteria in arctic and antarctic pack ice. Probes for several bacterial groups characteristic of the sea ice community were developed and tested for hybridization specificity according to the method of Stahl and Amann (59) (Table 1). All of the probes have at least one strong mismatch (1.1 to 2.8 weighted mismatches); however, in some cases, a competitor was designed to enhance probe specificity (Table 1). FISH analysis with CY3-labeled oligonucleotide probes (final concentration, 5 ng/μl; Interactiva, Ulm, Germany) was conducted according to the previously described method of Glöckner et al. (27).
TABLE 1.
Oligonucleotide probes used in this study
| Probe | Specificity | Probe sequence (5′-3′) | Target site 16S or 23S rRNA position (nucleotide) | % FAa | Source or reference |
|---|---|---|---|---|---|
| NON338 | ACTCCTACGGGAGGCAGC | 16S (338-355) | 0 | 64 | |
| EUB338 | Bacteria | GCTGCCTCCCGTAGGAGT | 16S (338-355) | 35 | 2 |
| ALF968 | α-Proteobacteria | GGTAAGGTTCTGCGCGTT | 16S (968-985) | 20 | 49 |
| SPH120 | Sphingomonas spp. | GGGCAGATTCCCACGCGT | 16S (120-137) | 30 | 49 |
| ROSEO536 | Roseobacter clade | CAACGCTAACCCCCTCCG | 16S (536-553) | 20 | 16 |
| ODB1021 | Octadecabacter spp. | GCGTCCCCTAAGGGAACT | 16S (1021-1038) | 15 | This study |
| RSHP995 | Roseobacter sp. strain Shippagan group | CTCGGATTGTCCAGGCAT | 16S (995-1012) | 10 | This study |
| BET42a | β-Proteobacteria | GCCTTCCCACTTCGTTT | 23S (1027-1043) | 35 | 44 |
| GAM42a | γ-Proteobacteria | GCCTTCCCACATCGTTT | 23S (1027-1043) | 35 | 44 |
| ALT1413 | Alteromonas-Colwellia | TTTGCATCCCACTCCCAT | 16S (1413-1430) | 40 | 23 |
| PSA184 | Pseudoalteromonas-Colwellia | CCCCTTTGGTCCGTAGAC | 16S (184-210) | 30 | 23 |
| GPU622 | Glaciecola punicea | CTAAAAGGCCTTCCCACG | 16S (622-639) | 17 | This study |
| GVstr214b | Glaciecola sp. strain GVstr214.6 | CTAAATGCTATTCCCAGG | 16S (622-639) | 12 | This study |
| GVstr214c | Competitor for GVstr214 | CCAAATGCTATTCCCAGG | 16S (622-639) | 12 | This study |
| NOR2-1453 | Psychromonas spp. | GGTCATCGCCATCCCC | 16S (1453-1468) | 30 | 23 |
| SF825 | Shewanella frigidimarina | AAGTCACCAAACTCCGAG | 16S (825-842) | 10 | This study |
| PSYB476 | Psychrobacter spp. | CTGCAGCTAATGTCATCG | 16S (476-493) | 10 | This study |
| MB-IC022b | Marinobacter sp. strain IC022 group | GTTTCCGCCCGACTTGCA | 16S (55-72) | 25 | This study |
| MB-IC022c | Competitor for MB-IC022 | GTTTCCGCTCGACTTGCA | 16S (55-72) | 25 | This study |
| G V | Vibrio spp. | AGGCCACAACCTCCAAGTAG | 16S (822-841) | 30 | 26 |
| OCE232 | Oceanospirillum spp. | AGCTAATCTCACGCAGGC | 16S (232-249) | 40 | 23 |
| PS56a | True Pseudomonas spp. | GCTGGCCTAGCCTTC | 23S (1432-1446) | 0 | 55 |
| CF319a | CF group | TGGTCCGTGTCTCAGTAC | 16S (319-336) | 35 | 43 |
| PB223b | Polaribacter spp. | GGACGCATAGCCATCTTT | 16S (223-240) | 15 | This study |
| PB223c | Competitor for PB223 | GAACGCATAGCCATCTTT | 16S (223-240) | 15 | This study |
| HGC69a | Gram positive (Actinobacteria) | TATAGTTACCACCGCCGT | 23S (1901-1918) | 25 | 53 |
| PLA46 | Planctomycetales | GACTTGCATGCCTAATCC | 16S (46-63) | 30 | 50 |
| ARCH915 | Archaea | GTGCTCCCCCGCCAATTCCT | 16S (915-934) | 20 | 59 |
Values represent percent formamide in the hybridization buffer.
Probes GVstr214, MB-IC022, and PB223 were used with competitors GVstr214c, MBIC022c, and PB223c, respectively.
Nucleotide sequence accession numbers.
The almost full-length (>1,400 bases) 16S rRNA gene sequences from 196 isolates and 84 clones generated in this study were deposited in GenBank under the accession numbers AF468274 to AF468299, AF468301 to AF468321, AF468343 to AF468447, AY167251 to AY167341, AY165563 to AY165581, and AY165583 to AY165598.
RESULTS
Total counts and culturability.
Total counts of bacteria in arctic pack ice from bottom sections at 10 stations, collected during the R.V. Polarstern ARKXIII/2 cruise, ranged from 0.98 × 105 to 14.90 × 105 cells/ml (Table 2). Viable counts determined by CFU on chitin agar (36) at 1°C varied from 4.1 to as high as 26.5% (Table 2), which was within the range of viable/total count ratios for the middle and bottom sections of the diverse ANTV/2 winter pack ice samples (36). Total counts of bacteria in sea ice-adjacent water were comparable to densities in the ice floes; however, similar to the antarctic results (36), less than 1% of the bacteria were cultivatable (Table 2).
TABLE 2.
Cell counts and culturability of bacteria in pack ice and seawater sampled during cruise ARKXIII/2
| Station | Pack
ice
|
Seawater (adjacent to ice floe)
|
||
|---|---|---|---|---|
| Total countsa (105 cells ml−1) | CFU/total counts (%) | Total countsa (105 cells ml−1) | CFU/total counts (%) | |
| 44/055 | 4.18 ± 2.03 | 24.4 | ||
| 44/057 | 0.98 ± 0.48 | 4.1 | ||
| 44/058 | 1.06 ± 0.49 | 4.3 | ||
| 44/062 | 3.58 ± 0.67 | 14.0 | 2.26 ± 0.19 | 0.8 |
| 44/064 | 1.35 ± 0.88 | 26.5 | 17.9 ± 0.48 | 0.02 |
| 44/089 | 1.76 ± 0.89 | 5.6 | ||
| 44/091 | 1.35 ± 0.54 | 12.6 | 3.04 ± 1.28 | 0.2 |
| 44/092a | 13.50 ± 0.65 | 11.2 | ||
| 44/092b | 14.90 ± 0.44 | 13.0 | ||
| 44/093 | 3.76 ± 1.77 | 11.8 | 4.46 ± 1.35 | 0.2 |
Mean ± standard deviation.
Total counts of samples used for FISH and cloning were comparable to those used for isolation. Samples from arctic pack ice contained (3.61 ± 0.25) × 105 (ARKXV/1) and (4.56 ± 0.26) × 105 (ARKXVI/2) cells/ml. Cell counts for the autumnal antarctic ice samples with very high algae accumulations were almost an order of magnitude higher than those for the arctic samples, with (2.11 ± 0.51) × 106 (ANTXI/4_13) and (1.12 ± 0.72) × 106 (ANTXI/4_14) cells/ml.
Phylogenetic diversity of arctic isolates and 16S rRNA gene clones.
ARDRA screening of pure cultures of arctic pack ice and subsequent sequencing of representatives from each ARDRA pattern group revealed 33 phylotypes distributed among the γ- and α-proteobacteria, the Cytophaga-Flavobacterium (CF) group, and the Actinobacteria (Table 3). Thirteen phylotypes had >97% 16S rRNA gene sequence identity with species previously isolated from polar sea ice (8, 30, 32, 33) and other polar environments. Most of those sea ice isolates are recognizable in Table 3 and in the figures accessible online at htpp://www.awi-bremerhaven.de/Pelagic/Sections/MarineChemistry/Helmke_seaice1-d.html, htpp://www.awi-bremerhaven.de/Pelagic/Sections/MarineChemistry/Helmke_seaice2=d.html, and htpp://www.awi -bremerhaven.de/Pelagic/Sections/MarineChemistry/Helmke_seaice3-d.html by strain names beginning with ACAM or IC. Seven phylotypes shared <97% 16S rRNA gene sequence identity with previously described bacteria and can be considered new species (58). The remaining 13 phylotypes were related to isolates or 16S rRNA gene clones from nonpolar habitats and are therefore suspected to be allochthonous.
TABLE 3.
Phylogenetic affiliations and frequency of isolates and clone libraries
| Phylogenetic grouping | Arctic
|
Antarctic
|
Nearest phylogenetic relativea | % Identical 16S rRNAb | ||||
|---|---|---|---|---|---|---|---|---|
| No. of isolates | No. of
clones
|
No. of
isolates
|
No. of clones
|
|||||
| ARKXIII/2 | ARKXV/1 | ARKXVI/2 | ANTV/2 | ANTXI/4_13 | ANTXI/4_14 | |||
| Bacteria | 115 | 86 | 106 | 87 | 103 | 95 | ||
| α-Proteobacteria | ||||||||
| Roseobacter | 1 | 3 | 4 | Antarctic quartz stone sublithic isolate QSSC9-5 (AF170750) | 93.0-97.8 | |||
| 2 | 1 | Adriatic Sea isolate AS-26 (AJ391187) | 97.2-99.7 | |||||
| 5 | 6 | 4 | Roseobacter sp. strain Shippagan (AF100168) | 98.1-98.9 | ||||
| 2 | Roseobacter litoralis (X78312) | 98.0 | ||||||
| 3 | 1 | 4 | North Atlantic continental slope isolate EI-2 (AF254103) | 96.9-98.0 | ||||
| 29 | 7 | 5 | 8 | 1 | Octadecabacter arcticus (U73725) | 97.0-99.0 | ||
| 5 | Ligurian Sea isolate SRF3 (AJ002565) | 98.1 | ||||||
| 7 | Mediterranean Sea clone MED-6 (AF025549) | 97.0-98.3 | ||||||
| 1 | Nankai Trough clone NKB7 (AB013259) | 95.1 | ||||||
| Sphingomonas | 2 | Antarctic seawater isolate SW54 (U85838) | 96.5 | |||||
| 1 | Erythrobacter longus (M59062) | 97.8 | ||||||
| β-Proteobacteria | ||||||||
| 1 | Soil ultramicrobacterium strain ND5 (AB008506) | 97.8 | ||||||
| γ-Proteobacteria | ||||||||
| Shewanella | 1 | 8 | Shewanella frigidimarina ACAM584 (U85902) | 99.3-99.7 | ||||
| Psychromonas | 3 | 3 | Psychromonas antarctica (U14697) | 97.7-99.5 | ||||
| Colwellia | 5 | 9 | 1 | Colwellia psychroerythraea IC064 (U85842) | 98.2-98.9 | |||
| 15 | 21 | 6 | 37 | 40 | Colwellia sp. strain ANT5-9 (AF19350) | 97.4-99.6 | ||
| 9 | Colwellia rossensis ACAM608 (U14589) | 99.3-99.7 | ||||||
| 4 | 4 | 5 | 7 | 8 | Colwellia-like sp. strain IC169 (AF001376) | 98.0-98.2 | ||
| Glaciecola | 5 | 19 | 23 | 25 | 15 | Gas vacuolate sea ice bacterium strain 214.6 (GVstr214.6 [U73724]) | 97.6-98.7 | |
| 10 | 2 | 2 | 4 | Glaciecola punicea ACAM 611T (U85853) | 97.0-98.3 | |||
| 4 | 5 | 4 | Glaciecola pallidula ACAM 615T (U85854) | 98.6-98.7 | ||||
| Pseudoalteromonas | 2 | Pseudoalteromonas antarctica (X98336) | 99.7-100 | |||||
| Methylophaga | 1 | Methylophaga marina (X95459) | 94.5 | |||||
| Psychrobacter | 4 | 1 | 2 | 3 | 1 | 3 | Psychrobacter glacincola IC084 (U85876) | 98.1-99.7 |
| 1 | Psychrobacter marincola (PMA309941) | 99.6 | ||||||
| 3 | Psychrobacter sp. strain St1 (AF260715) | 98.0-99.5 | ||||||
| Acinetobacter | 1 | Acinetobacter johnsonii (X89775) | 99.5 | |||||
| 1 | Acinetobacter lwoffii (U10875) | 98.8 | ||||||
| Pseudomonas | 4 | Pseudomonas sp. strain ACAM213 (U85868) | 98.3-100 | |||||
| 2 | Arctic seawater bacterium R7366 (UBA293826) | 99.4 | ||||||
| Marinobacter | 8 | 13 | 1 | 1 | Marinobacter sp. strain IC022 (U85863) | 97.1-99.0 | ||
| 18 | Marinobacter sp. strain DS40M8 (AF199440) | 95.0-99.7 | ||||||
| 1 | Marinobacter hydrocarboniclasticus (AB021372) | 91.2 | ||||||
| Oceanospirillum | 2 | 11 | 2 | Oleispira antarctica (AJ426420) | 98.4-99.3 | |||
| 1 | 1 | Marinomonas protea (AJ238597) | 96.0-99.4 | |||||
| Halomonas | 2 | Halomonas variabilis strain SW04 (U85873) | 98.6-99.8 | |||||
| 1 | Gamma Proteobacterium MBIC3958 (AB020600) | 95.1 | ||||||
| 1 | Antarctic shelf clone MERTZ_2CM_20 (AF424059) | 97.0 | ||||||
| Terredinibacter | 2 | Arctic sea ice clone ARCTIC_ICE-12 (AF277547) | 98.1 | |||||
| NOR5c | 1 | North Sea clone KTc1119 (AF235120) | 95.1 | |||||
| CF | Polaribacter filamentus (U73726) | 98.9-99.5 | ||||||
| Polaribacter | 2 | 1 | Arctic Ocean clone Arctic 96B-11 (AF354621) | 93.7 | ||||
| 1 | 3 | 8 | Polaribacter franzmannii (U14586) | 97.9-98.1 | ||||
| 1 | 4 | 2 | 7 | Polaribacter irgensii (M61002) | 99.1-99.3 | |||
| Cellulophaga | 3 | 3 | Mesocosm diatom bloom isolate 5.3.10 (AF367849) | 98.0-98.3 | ||||
| Flavobacterium | 3 | Flavobacterium xanthum IC001 (U85889) | 98.7-99.1 | |||||
| Salegentibacter | 7 | Salegentibacter salegens (M92279) | 97.9-99.1 | |||||
| Psychroflexus | 2 | 2 | Psychroflexus torquis ACAM623 (U85881) | 98.1-98.7 | ||||
| 16 | North Atlantic abyssal isolate AIII4 (AF254114) | 92.9-96.2 | ||||||
| Psychroserpens | 6 | Psychroserpens burtonensis ACAM188 (U62913) | 94.0-95.8 | |||||
| Cytophaga | 2 | 5 | Arctic Ocean clone Arctic96AD-4 (AF354619) | 96.8-98.1 | ||||
| 1 | Japan Trench clone JTB132 (AB015260) | 98.8 | ||||||
| 1 | Antarctic sea ice clone SIC.42370 (AF277483) | 93.8 | ||||||
| Green nonsulfur bacteria | 1 | Arctic Ocean clone Arctic 95A (AF355054) | 87.3 | |||||
| Actinobacteria | 2 | Nesterenkonia halobia (X80847) | 97.9 | |||||
| 1 | 1 | Soil ultramicrobacterium str. 121 (AB008511) | 97.8-98.0 | |||||
| 1 | Brachybacterium faecium (X91032) | 91.8 | ||||||
| 1 | Microbacterium sp. VKM Ac-2047 (AB042082) | 99.4 | ||||||
| 3 | Freshwater clone HTG6 (AF418963) | 91.7-92.0 | ||||||
| Total 16S rRNA phylotypes | 33 | 15 | 19 | 20 | 13 | 11 | ||
| Shannon index ± variance | 1.291 ± 0.002 | 1.065 ± 0.002 | 1.091 ± 0.001 | 1.127 ± 0.002 | 0.844 ± 0.002 | 0.812 ± 0.001 | ||
| Coverage (%) | 53.3 | 73.6 | 84.6 | 81.8 | ||||
In ARB database.
Estimated in ARB.
Reference 24.
The highest diversity of phylotypes was found within the γ-proteobacteria (see supplemental figure at http://www.awi-bremerhaven.de/Pelagic/Sections/MarineChemistry/Helmke_seaice1-d.html) with Marinobacter spp. as the dominant phylotype. Most of the phylotypes identified as α-proteobacteria were affiliated to the Roseobacter clade, with Octadecabacter spp. being the most frequently isolated phylotype (see supplemental figure at http://www.awi-bremerhaven.de/Pelagic/Sections/MarineChemistry/Helmke_seaice2-d.html). Additional phylotypes within the α-proteobacteria were associated with Sphingomonas spp. Seven phylotypes were detected within the CF group (see supplemental figure at http://www.awi-bremerhaven.de/Pelagic/Sections/MarineChemistry/Helmke_seaice3-d.html) with the numerically abundant Salegentibacter spp. and Psychroserpens spp. Four highly diverse gram-positive phylotypes representing potentially four genera (Clavibacter, Microbacterium, Brachybacterium, and Nesterenkonia) within the phylum Actinobacteria were also identified (Table 3).
PCR and cloning analysis of 16S rRNA genes from two discrete arctic samples revealed 28 different phylotypes (Table 3). Ten clone phylotypes were 95 to 100% identical to our arctic isolates, and seven were identical to previously cultivated antarctic bacteria. Only four clones clustered with sequences from isolates of nonpolar origin, and six clones clustered with sequences from not-yet-cultivated bacteria. Although clones and isolates derived from different sea ice samples, the same phylotypes (the Colwellia spp. and the Glaciecola spp. within the Colwellia assemblage [61], the Marinobacter spp., and the Octadecabacter spp.) were found to dominate. Within the CF group, only three different clone phylotypes were obtained. The most abundant CF clone phylotype had no counterpart among the sea ice isolates.
Phylogenetic diversity of antarctic isolates and 16S rRNA gene clones.
With only 20 phylotypes, the diversity of the antarctic sea ice isolates was less than that among our arctic isolates (Table 3). Most of the antarctic isolates were >98% identical to species previously isolated from the antarctic and/or arctic sea ice or other polar habitats (supplemental figures). Similar to the arctic isolates, the antarctic phylotypes were distributed among the γ- and α-proteobacteria as well as the CF group. The Actinobacteria were represented by only one isolate. Colwellia spp. and Glaciecola spp. were the most abundant γ-proteobacteria. In contrast to the arctic isolates, the Marinobacter spp. were rare. Similar to arctic sea ice, the α-proteobacteria were dominated by members of the Roseobacter clade, but Octadecabacter was not the prevalent phylotype in antarctic pack ice. Instead, three phylotypes that clustered with Roseobacter sp. strain Shippagan and other yet-undescribed isolates were quite numerous. In comparison to the arctic isolates, the diversity of the CF group was clearly reduced and concentrated within the Polaribacter group.
Like our arctic clone libraries, cloned 16S rRNA genes from antarctic pack ice samples (ANTXI/4_13 and ANTXI/4_14) revealed a strong overlap of phylotypes with cultivated isolates (Table 3), although these samples were derived from sea ice of a different season. However, unlike our arctic libraries, diversity of the antarctic clones was limited to typical sea ice phylotypes. Again, the Colwellia assemblage, the Roseobacter clade, and the CF group (in particular, the Polaribacter spp.) were the dominant phylogenetic groups.
Analysis of diversity.
Using a two-tailed t test (α = 0.05) (65), we tested Shannon's index (H′) of diversity for arctic versus antarctic isolates and clone libraries for significant differences. These results are included in Table 3 and show that diversity of arctic isolate phylotypes was significantly higher than that of antarctic isolates. Similarly, diversity in the arctic clone libraries was significantly higher than in the antarctic clone libraries. Coverage indicated that most of the actual diversity of antarctic pack ice (82 and 85%) had been revealed by cloning, whereas only 50 to 75% of the actual diversity in arctic pack ice had been detected. For rarefaction analysis, the expected number of ARDRA patterns was plotted at one-knot intervals (63) against the number of individuals (clones or isolates) screened. Curves calculated for the isolates and clones indicated that the number of 16S rRNA phylotypes in arctic and antarctic pack ice had reached saturation (data not shown).
Community structure.
Percent distributions of bacteria in samples from arctic and antarctic pack ice that hybridized with domains to species-specific probes are listed in Table 4. Target groups for most of the probes used in this study are bracketed in the online dendrograms. The target groups for probes and their percent frequencies (identified from ARDRA patterns) in the clone libraries and cultures are included in Table 4 for comparison. In most cases, phylogenetic groups detected by isolation and cloning were also detectable with FISH. Most of the bacteria visualized with DAPI staining (∼95%) were detectable with the EUB338 probe specific for Bacteria. The background signal of samples, observed with the probe NON338, was negligible (0 to 0.1%). For all samples, most of the DAPI-stained cells (>95%) could be assigned with probes targeting the larger phylogenetic groups within the domain Bacteria. In arctic samples, the highest percentage of bacterial cells (∼50%) was detected with the γ-proteobacteria-specific probe. The α-proteobacteria and the CF group accounted for ∼30% and ∼25% of the total bacteria, respectively. β-Proteobacteria were also detected in samples, making up ∼6% of the total bacteria. Abundances of gram-positive bacteria, Planctomycetales, and Archaea were below the detection limit of FISH. Similar to arctic samples, the γ-proteobacteria accounted for the highest fraction (∼36%) of the total in antarctic samples. The CF group made up the second-highest distribution of cells, with a fraction of ∼32%, followed by the α-proteobacteria, with a fraction of ∼25%. A few β-proteobacteria (∼2%) were detectable. Gram-positive bacteria, Planctomycetales, and Archaea were not observable in either antarctic sample.
TABLE 4.
Frequency of bacterial phylotypes in Arctic and Antarctic pack ice observed by FISH in comparison to clone and isolate libraries
| Probe target group | Probe | Results for samples
collected in thea:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arctic
|
Antarctic
|
||||||||||
| FISHb
|
Clone
libraryc
|
Isolatesd
|
FISHb
|
Clone
libraryc
|
Isolatesd
|
||||||
| ARKXV/1 | ARKXVI/2 | ARKXV/1 | ARKXVI/2 | ARKXIII/2 | ANTXI/4_13 | ANTXI/4_14 | ANTXI/4_13 | ANTXI/4_14 | ANTV/2 | ||
| Bacteria | EUB338 | 94 ± 5 | 95 ± 5 | N/A | N/A | N/A | 93 ± 6 | 96 ± 4 | N/A | N/A | N/A |
| α-Proteobacteria | ALF968 | 28 ± 6 | 33 ± 7 | 9 | 13 | 42 | 26 ± 5 | 24 ± 4 | 7 | 4 | 24 |
| Roseobacter clade | ROSEO536 | 27 ± 8 | 28 ± 5 | 9 | 13 | 39 | 16 ± 5 | 7 ± 2 | 7 | 4 | 24 |
| Octadecabacter spp. | ODB1021 | 19 ± 5 | 23 ± 5 | 8 | 5 | 25 | <1 | <1 | 1 | 0 | 9 |
| Roseobacter sp. strain Shippagan group | RSHP995 | <1 | <1 | 0 | 0 | 1 | 3 ± 3 | 2 ± 1 | 6 | 15 | 5 |
| Sphingomonas spp. | SPH120 | <1 | <1 | 0 | 0 | 3 | 4 ± 3 | <1 | 0 | 0 | 0 |
| β-Proteobacteria | BET42a | 7 ± 6 | 5 ± 4 | 1 | 0 | 0 | 2 ± 2 | 2 ± 3 | 0 | 0 | 0 |
| γ-Proteobacteria | GAM42a | 49 ± 5 | 51 ± 9 | 71 | 78 | 30 | 34 ± 11 | 40 ± 9 | 88 | 80 | 65 |
| Alteromonas-Colwellia | ALT1413 | 30 ± 7 | 33 ± 9 | 22 | 20 | 4 | 19 ± 4 | 22 ± 4 | 42 | 46 | 17 |
| Pseudoalteromonas-Colwellia | PSA184 | 32 ± 11 | 37 ± 14 | 5 | 32 | 9 | 16 ± 5 | 19 ± 5 | 27 | 22 | 41 |
| Glaciecola sp. strain GVstr214.6 | GVstr214 | 4 ± 5 | <1 | 0 | 18 | 4 | 3 ± 4 | <1 | 25 | 18 | 28 |
| Glaciecola punicea | GPU622 | 5 ± 5 | 3 ± 4 | 0 | 10 | 0 | 3 ± 2 | 3 ± 3 | 2 | 4 | 1 |
| Marinobacter sp. strain IC022 group | MB-IC022 | 23 ± 13 | 22 ± 8 | 14 | 1 | 7 | <1 | <1 | 0 | 0 | 1 |
| Psychrobacter spp. | PSYB476 | 4 ± 3 | 9 ± 3 | 1 | 2 | 7 | 5 ± 1 | 7 ± 3 | 1 | 3 | 3 |
| Shewanella frigidimarina | SF825 | 3 ± 2 | 7 ± 4 | 0 | 5 | 1 | <1 | <1 | 0 | 0 | 0 |
| Psychromonas spp. | NOR2-1453 | <1 | 1 ± 1 | 0 | 3 | 0 | <1 | <1 | 0 | 0 | 3 |
| Vibrio spp. | GV | <1 | <1 | 0 | 0 | 0 | <1 | 3 ± 3 | 0 | 0 | 0 |
| Oceanospirillum spp. | OCE232 | 3 ± 5 | 1 ± 1 | 2 | 11 | 1 | 1 ± 1 | 5 ± 4 | 2 | 0 | 0 |
| Pseudomonas spp. | PS56a | <1 | <1 | 1 | 0 | 5 | <1 | <1 | 0 | 0 | 0 |
| CF group | CF319a | 22 ± 4 | 28 ± 6 | 18 | 6 | 23 | 31 ± 5 | 34 ± 4 | 8 | 17 | 9 |
| Polaribacter spp. | PB223 | 2 ± 1 | <1 | 0 | 0 | 3 | 28 ± 6 | 35 ± 7 | 5 | 17 | 6 |
| Actinobacteria | HGC69a | <1 | <1 | 0 | 3 | 4 | <1 | <1 | 0 | 0 | 1 |
| Planctomycetales | PLA46 | <1 | <1 | 0 | 0 | 0 | <1 | <1 | 0 | 0 | 0 |
| Archaea | ARCH915 | <1 | <1 | 0 | 0 | 0 | <1 | <1 | 0 | 0 | 0 |
Data present results obtained during the indicated research cruises; for more information, please refer to the text.
Values represent the mean percent total cells observed with DAPI staining ± standard deviation.
Values represent the percent total 16S rRNA gene clones. N/A, not applicable.
Values represent the percent total isolates. N/A, not applicable.
We took a closer look at the community composition using probes more specific for the most abundant group, the γ-proteobacteria. Application of two partially overlapping probes specific for the Alteromonas-Colwellia (ALT1413) and Pseudoalteromonas-Colwellia (PSA184) groups within the Colwellia assemblage resulted in very similar numbers. Probes ALT1413 and PSA184 hybridized with ∼31 and ∼34% of DAPI-stained cells in arctic samples, and each accounted for ∼20% in antarctic samples. The newly designed probes GVstr214 and GPU622, nesting within the Pseudoalteromonas-Colwellia group, detected only up to 5% of the total bacteria, respectively, although they were quite redundant in the clone libraries. Most of the remaining γ-proteobacteria could be assigned with probes targeting groups outside of the Colwellia assemblage. Marinobacter sp. strain IC022, which was frequent in our ARKXV/1 clone library and among our arctic isolates, was also found in situ with the probe MB-IC022 to make up ∼22% of the total bacteria in the arctic samples. Concurring with the clone library results, we detected no bacteria with this probe in the antarctic samples. Psychrobacter spp. were detectable in all samples with probe PSYB476 (∼6%). Shewanella frigidimarina (probe SF825) appeared to occur mainly in arctic pack ice (3 to 7%), and Oceanospirillum spp., Vibrio spp., Psychromonas spp., and Pseudomonas spp. were either low in abundance (1 to 3%) or not detectable.
The α-proteobacterial sequences in clone libraries and cultures were mostly, if not entirely, associated with members of the Roseobacter clade. Our probing results corroborated that this clade accounted for most of the α-proteobacteria in the samples. Octadecabacter spp., which predominated in number among Roseobacter clade isolates and clone sequences, also dominated the in situ community of arctic samples. In antarctic samples, similar to the clone results, very few cells of Octadecabacter spp. were detected. Probe RSHP995, which was specific for a group of sequences that were >97% identical to Roseobacter sp. strain Shippagan, detected only a small fraction (<1%) in arctic samples but was more abundant (∼2 to 3%) in antarctic samples. Probe PB223, which was specific for Polaribacter spp., detected only ∼1% of cells in arctic samples while, similar to our cultural and cloning results, Polaribacter spp. dominated the CF fraction of the antarctic samples.
DISCUSSION
Congruence of cultivation, 16S rRNA gene cloning, and FISH.
The strong agreement of the results obtained with the described methods emphasizes the exceptional nature of sea ice bacterial communities, which have highly active members despite the extreme conditions in sea ice. The high percentages of cultivatable bacteria observed in the viable/total count ratio data from arctic pack ice in this study and former studies (36, 41) indicated that the composition of phylotypes observed among our cultures potentially represent much of the natural community composition. Although the original samples used for cultivation were not available for molecular analyses, cloning of 16S rRNA genes recovered from sea ice samples collected during subsequent cruises detected many of the same phylotypes found among cultivated sea ice bacteria. During the course of our clone library analyses, Brown and Bowman (18) presented the first 16S rRNA environmental sequence data from sea ice, mainly in the antarctic, which showed that previously cultivated phylotypes (see the sequences designated SIC, McMurdo, or ARCTIC_ICE in the online dendrograms) are detectable with PCR. Their data agreed with our findings that the cultivatable fraction and the PCR-detected fraction of bacteria in sea ice are strongly overlapping. These findings are in strong contrast to those for most marine environments, for which cultivation and cloning analyses rarely agree.
The high FISH detection yield of the CY3-monolabeled probe EUB338, which was specific for the domain Bacteria with ∼95% of DAPI-stained cells and the bright probe signals (54), indicated that the sea ice microbial community, as a whole, was highly active at the time of sampling. With the exception of activated sludge (2), there are very few examples of microbial habitats with FISH detection rates as high as those in sea ice. Typically, only ∼50% of the bacterioplankton community can be detected with domain-specific probes (28), and much less (∼60%, i.e., sum of probes) of the bacterial fraction can usually be assigned to specific phylogenetic groups. Some trends in the marine environment are emerging. Glöckner et al. (28) reported 96% detectability of DAPI-stained cells with EUB338 and >87% assignability with phylogenetic group probes in a sample collected from a Phaeocystis sp. bloom in the Southern Ocean. Similarly, Eilers et al. (24) observed that FISH detection rates of bacterial communities increased dramatically from 30 to >90% as phytoplankton biomass peaked over the course of a seasonal bloom in the German Bight. Unlike these studies that showed highest assignability for the CF group, the γ-proteobacteria was the predominating group in our sea ice samples, and this finding appears to be a unique characteristic for sea ice bacterial communities (7, 28). FISH analysis of sea ice sampled throughout several seasons is needed to confirm this.
A reason for the different FISH detection yields in sea ice and in the water column could be the different substrate quantity and quality found in the two environments. High concentrations of dissolved organic matter (DOM) in sea ice have been reported for the Arctic Ocean (62) and the Southern Ocean (37),exceeding surface seawater concentration by a factor of 2 to 30. Additionally, DOM in sea ice appears to be very labile, providing a favorable growth substrate for bacteria (3). The high substrate quality leads to increased microbial activity in sea ice relative to surface seawater, which usually contains less fresh and labile (<10%) DOM (3, 4). The higher microbial activity in sea ice seems to be reflected in elevated FISH detection yields.
Arctic versus antarctic communities.
The higher diversity of phylotypes indicated by the Shannon's index for our arctic sea ice isolates and clone libraries as well as the detection of typical limnic phylotypes, i.e., β-proteobacteria and Actinobacteria (7, 29, 47), suggest a terrestrial influence on the sea ice bacterial communities found in the pack ice of the Fram Strait region. Seasonality also appears to influence the diversity of antarctic pack ice communities. Fewer taxonomic groups were detected in our winter and autumnal samples than in previous surveys of summer sea ice (8, 18).
Temperature plays an important role in shaping the composition of sea ice bacterial communities (18, 20, 36); however, the dominance of the Colwellia assemblage, the Roseobacter clade, and the CF group in both arctic as well as antarctic pack ice may also be explained by the strong association of these groups with surfaces (1, 19, 21, 24, 52). Studies have shown that the recruitment of bacteria into sea ice is primarily facilitated through their attachment to microalgae or particles (34, 35). The dominant groups in sea ice are also characteristic of the cultivatable fraction of bacteria in seawater (6, 23, 24). Although less than 1% of the bacteria in seawater adjacent to ice floes (Table 2) were cultivatable, most of these in the arctic were similar to phylotypes found in the sea ice (included in the online dendrograms).
The general similarity of bacterial phylotypes in arctic and antarctic pack ice implies that the same selective mechanisms occur at both poles. However, this alone cannot explain the extensive overlap of almost identical 16S rRNA phylotypes (25 out of 33) between the arctic and antarctic sea ice communities and makes a simple speciation model based on geographical isolation difficult to support, especially since phylogenetic surveys of the world's oceans and lakes (5, 17, 25, 29, 39, 48, 66) have strongly suggested the mixing of bacterial populations on a global scale. The physiological (i.e., psychrophily or high hydrostatic pressure intolerance) and geographic distance barriers (60) appear to be permeable. However, analysis at the conservative gene level of 16S rRNA is not sufficient to determine if the same species occur at both poles. Other analytical methods, e.g., DNA-DNA hybridization, could elucidate diversity that is not detectable by 16S rRNA gene sequencing.
Conclusions.
The strong overlap of the cultivatable, PCR-, and FISH-detected bacterial fractions in arctic as well as antarctic sea ice indicated exceptional bacterial communities whose major parts are metabolically active and cultivatable. Similar to nutrient-enriched samples, γ-proteobacteria were dominant and α-proteobacteria and members of the CF group were highly abundant. Although almost the same phylotypes were detected at both poles, differences in their quantitative contribution to the arctic and antarctic pack ice communities were revealed by FISH.
Acknowledgments
This study was supported by the German Ministry of Education and Science (grant no. BMBF 03F0233B), the Alfred Wegener Institute for Polar and Marine Research Foundation, and the Max Planck Society.
We thank the captains and crews of the R. V. Polarstern cruises ANTV/2, ANTXI/4, ARKXVIII/2, ARKXV/1, and ARKXVI/2 for their assistance. We are also grateful to our fellow scientists on these cruises who volunteered to assist with sample collection. In particular, we are indebted to the VICTOR6000 team of the IFREMER Institute and to K. Berlitz, M. Wanger, J. Jokiel, K. Darling, D. Kroon, S. Schmidt, K. Reuter, S. Klein, J. Langreder, S. Ackley, G. Dieckmann, K. Meiners, K. von Juterzenka, Y. Okolodkov, and U. Klauke for assistance with ice coring. Discussions with R. Amon regarding DOM were invaluable.
REFERENCES
- 1.Acinas, S. G., J. Antón, and F. Rodríguez-Valera. 1999. Diversity of free-living and attached bacteria in offshore western Mediterranean waters as depicted by analysis of genes encoding 16S rRNA. Appl. Environ. Microbiol. 65:514-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amon, R. M. W., H.-P. Fitznar, and R. Benner. 2001. Linkages among the bioreactivity, chemical composition, and diagenetic state of marine dissolved organic matter. Limnol. Oceanogr. 46:287-297. [Google Scholar]
- 4.Amon, R. M. W., and R. Benner. 2003. Combined neutral sugars as indicators of the diagenetic state of dissolved organic matter in the Arctic Ocean. Deep-Sea Res. Part I Oceanogr. Res. Pap. 50:151-169. [Google Scholar]
- 5.Bano, N., and J. T. Hollibaugh. 2002. Phylogenetic composition of bacterioplankton assemblages from the Arctic Ocean. Appl. Environ. Microbiol. 68:505-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benlloch, S., F. Rodriguez-Valera, and A. J. Martinez-Murcia. 1995. Bacterial diversity in two coastal lagoons deduced from 16S rDNA PCR amplification and partial sequencing. FEMS Microbiol. Ecol. 18:267-280. [Google Scholar]
- 7.Bouvier, T. C., and P. A. del Giorgio. 2002. Compositional changes in free-living bacterial communities along a salinity gradient in two temperate estuaries. Limnol. Oceanogr. 47:453-470. [Google Scholar]
- 8.Bowman, J. P., S. A. McCammon, M. V. Brown, D. S. Nichols, and T. A. McMeekin. 1997. Diversity and association of psychrophilic bacteria in antarctic sea ice. Appl. Environ. Microbiol. 63:3068-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowman, J. P., S. A. McCammon, J. L. Brown, P. D. Nichols, and T. A. McMeekin. 1997. Psychroserpens burtonensis gen. nov., sp. nov., and Gelidibacter algens gen. nov., sp. nov., psychrophilic bacteria isolated from antarctic lacustrine and sea ice habitats. Int. J. Syst. Bacteriol. 14:670-677. [DOI] [PubMed] [Google Scholar]
- 10.Bowman, J. P., D. S. Nichols, and T. A. McMeekin. 1997. Psychrobacter glacincola sp. nov., a halotolerant, psychrophilic bacterium isolated from antarctic sea ice. Syst. Appl. Microbiol. 20:209-215. [Google Scholar]
- 11.Bowman, J. P., S. A. McCammon, D. S. Nichols, J. H. Skerratt, S. M. Rea., P. D. Nichols, and, T. A. McMeekin. 1997. Shewanella gelidimarina sp. nov. and Shewanella frigidimarina sp. nov., novel antarctic species with the ability to produce eicosapentaenoic acid (20:5 Ω 3) and grow anaerobically by dissimilatory Fe(III) reduction. Int. J. Syst. Bacteriol. 47:1040-1047. [DOI] [PubMed] [Google Scholar]
- 12.Bowman, J. P. 1998. Pseudoalteromonas prydzensis sp. nov., a psychrotrophic, halotolerant bacterium from antarctic sea ice. Int. J. Syst. Bacteriol. 48:1037-1041. [DOI] [PubMed] [Google Scholar]
- 13.Bowman, J. P., S. A. McCammon, T. Lewis, J. H. Skerratt, J. L. Brown, D. S. Nichols, and T. A. McMeekin. 1998. Psychroflexus torquis gen. nov., sp. nov., a psychrophilic species from antarctic sea ice, and reclassification of Flavobacterium gondwanense (Dobson et al. 1993) as Psychroflexus gondwanense gen. nov., comb. nov. Microbiology 144:1601-1609. [DOI] [PubMed] [Google Scholar]
- 14.Bowman, J. P., S. A. McCammon, J. L. Brown, and T. A. McMeekin. 1998. Glaciecola punicea gen. nov., sp. nov., and Glaciecola pallidula gen. nov., sp. nov.: psychrophilic bacteria from antarctic sea-ice habitats. Int. J. Syst. Bacteriol. 48:1213-1222. [DOI] [PubMed] [Google Scholar]
- 15.Bowman, J. P., J. J. Gosink, S. A. McCammon, T. E. Lewis, D. S. Nichols, P. D. Nichols, J. H. Skerratt, J. T. Staley, and T. A. McMeekin. 1998. Colwellia demingiae sp. nov., Colwellia hornerae sp. nov., Colwellia rossensis sp. nov., and Colwellia psychrotropica sp. nov.: psychrophilic antarctic species with the ability to synthesize docosahexaenoic acid (22:6 ω 3) Int. J. Syst. Bacteriol. 48:1171-1180. [Google Scholar]
- 16.Brinkmeyer, R., M. Rappé, S. Gallacher, and L. Medlin. 2000. Development of clade (Roseobacter and Alteromonas)- and taxon-specific oligonucleotide probes to study interactions between toxic dinoflagellates and their associated bacteria. Eur. J. Phycol. 35:315-329. [Google Scholar]
- 17.Britschgi, T. B., and S. J. Giovannoni. 1991. Phylogenetic analysis of a natural marine bacterioplankton population by rRNA gene cloning and sequencing. Appl. Environ. Microbiol. 57:1707-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown, M. V., and J. P. Bowman. 2001. A molecular phylogenetic survey of sea-ice microbial communities (SIMCO). FEMS Microb. Ecol. 35:267-275. [DOI] [PubMed] [Google Scholar]
- 19.Dang, H., and C. R. Lovell. 2000. Bacterial primary colonization and early succession on surfaces in marine waters as determined by amplified rRNA gene restriction analysis and sequence analysis of 16S rRNA genes. Appl. Environ. Microbiol. 66:467-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delille, D. 1992. Marine bacterioplankton at the Weddell Sea ice edge, distribution of psychrophilic and psychrotrophic populations. Polar Biol. 11:449-456. [Google Scholar]
- 21.DeLong, E. F., D. G. Franks, and A. L. Alldredge. 1993. Phylogenetic diversity of aggregate-attached vs. free-living marine bacterial assemblages. Limnol. Oceanogr. 38:924-934. [Google Scholar]
- 22.Doyle, J. J., and J. L. Doyle. 1990. Isolation of plant DNA from fresh tissue. Focus 12:13-15. [Google Scholar]
- 23.Eilers, H., J. Pernthaler, F. O. Glöckner, and R. Amann. 2000. Culturability and in situ abundance of pelagic bacteria from the North Sea. Appl. Environ. Microbiol. 66:3044-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eilers, H., J. Pernthaler, J. Peplies, F. O. Glöckner, G. Gerdts, and R. Amann. 2001. Isolation of novel pelagic bacteria from the German Bight and their seasonal contributions to surface picoplankton. Appl. Environ. Microbiol. 67:5134-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuhrman, J. A., K. McCallum, and A. A. Davis. 1993. Phylogenetic diversity of subsurface marine microbial communities from the Atlantic and Pacific oceans. Appl. Environ. Microbiol. 59:1294-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuliano, L., E. DeDomenico, M. G. Höfle, and M. M. Yakimov. 1999. Identification of culturable oligotrophic bacteria within naturally occurring bacterioplankton communities of the Ligurian Sea by 16S rRNA sequencing and probing. Microb. Ecol. 37:77-85. [DOI] [PubMed] [Google Scholar]
- 27.Glöckner, F. O., R. Amann, A. Alfreider, J. Pernthaler, R. Psenner, K. Trebesius, and K.-H. Schleifer. 1996. An in situ hybridization protocol for detection and identification of planktonic bacteria. Syst. Appl. Microbiol. 19:403-406. [Google Scholar]
- 28.Glöckner, F. O., B. M. Fuchs, and R. Amann. 1999. Bacterioplankton compositions of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Appl. Environ. Microbiol. 65:3721-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glöckner, F. O., E. Zaichikov, N. Belkova, L. Denissova, J. Pernthaler, A. Pernthaler, and R. Amann. 2000. Comparative 16S rRNA analysis of lake bacterioplankton reveals globally distributed phylogenetic clusters including an abundant group of Actinobacteria. Appl. Environ. Microbiol. 66:5053-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gosink, J. J., R. P. Herwig, and J. T. Staley. 1997. Octadecabacter arcticus gen. nov., sp. nov., and O. antarcticus sp. nov., nonpigmented, psychrophilic gas vacuolate bacteria from polar sea ice and water. Syst. Appl. Microbiol. 20:356-365. [Google Scholar]
- 31.Gosink, J. J., R. L. Irgens, and J. T. Staley. 1993. Vertical distribution of bacteria in arctic sea ice. FEMS Microb. Ecol. 102:85-90. [Google Scholar]
- 32.Gosink, J. J., and J. T. Staley. 1995. Biodiversity of gas vacuolate bacteria from antarctic sea ice and water. Appl. Environ. Microbiol. 61:3486-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gosink, J. J., C. R. Woese, and J. T. Staley. 1998. Polaribacter gen. nov., with three new species, P. irgensii sp. nov., P. franzmannii sp. nov., and P. filamentus sp. nov., gas vacuolate polar marine bacteria of the Cytophaga-Flavobacterium-Bacteroides group and reclassification of “Flectobacillus glomeratus” as Polaribacter glomeratus comb. nov. Int. J. Syst. Bacteriol. 48:223-235. [DOI] [PubMed] [Google Scholar]
- 34.Grossmann, S. 1994. Bacterial activity in sea ice and open water of the Weddell Sea, Antarctica: a microautoradiographic study. Microb. Ecol. 28:1-18. [DOI] [PubMed] [Google Scholar]
- 35.Grossmann, S., and G. Dieckmann. 1994. Bacterial standing stock, activity, and carbon production during formation and growth of sea ice in the Weddell Sea, Antarctica. Appl. Environ. Microbiol. 60:2746-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helmke, E., and H. Weyland. 1995. Bacteria in sea ice and underlying water of the eastern Weddell Sea in midwinter. Mar. Ecol. Prog. Ser. 117:269-287. [Google Scholar]
- 37.Herborg, L.-M., D. N. Thomas, H. Kennedy, C. Haas, and G. S. Dieckmann. 2001. Dissolved carbohydrates in antarctic sea ice. Antarct. Sci. 13:119-125. [Google Scholar]
- 38.Hobbie, J. E., R. J. Daley, and S. Jasper. 1977. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollibaugh, J. T., N. Bano, and H. W. Ducklow. 2002. Widespread distribution in polar oceans of 16S rRNA gene sequence with affinity to Nitrosospira-like ammonia-oxidizing bacteria. Appl. Environ. Microbiol. 68:1478-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Junge, K., J. J. Gosink, H.-G. Hoppe, and J. T. Staley. 1998. Arthrobacter, Brachybacterium, and Planococcus isolates identified from antarctic sea ice brine. Description of Planococcus mcmeekinii, sp. nov. Syst. Appl. Microbiol. 21:306-314. [DOI] [PubMed] [Google Scholar]
- 41.Junge, K., F. Imhoff, J. T. Staley, and J. W. Deming. 2002. Phylogenetic diversity of numerically important arctic sea-ice bacteria cultured at subzero temperature. Microb. Ecol. 43:315-328. [DOI] [PubMed] [Google Scholar]
- 42.Kopp, M., G. J. Doucette, M. Kodama, G. Gerdts, C. Schütt, and L. K. Medlin. 1997. Phylogenetic analysis of selected toxic and non-toxic bacterial strains isolated from the toxic dinoflagellate Alexandrium tamarense. FEMS Microbiol. Ecol. 24:251-257. [Google Scholar]
- 43.Manz, W., R. Amann, W. Ludwig, M. Vancanneyt, and K.-H. Schleifer. 1996. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum Cytophaga-Flavobacter-Bacteroides in the natural environment. Microbiology 142:1097-1106. [DOI] [PubMed] [Google Scholar]
- 44.Manz, W., R. Amann, W. Ludwig, M. Wagner, and K.-H. Schleifer. 1992. Phylogenetic oligodeoxynucleotide probes for the major subclasses of Proteobacteria: problems and solutions. Syst. Appl. Microbiol. 15:593-600. [Google Scholar]
- 45.Massol-Deya, A. A., D. A. Odelson, R. F. Hickey, and J. M. Tiedje. 1999. Bacterial community fingerprinting of amplified 16S and 16-23S ribosomal DNA gene sequences and restriction endonuclease analysis (ARDRA), p. 3.3.2/1-8. In A. D. L. Akkermans, J. D. Van Elsas, and F. J. De Bruijn (ed.), Molecular microbial ecology manual. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 46.McCammon, S. A., and J. P. Bowman. 2000. Taxonomy of the antarctic Flavobacterium species: description of Flavobacterium gillisiae sp nov., Flavobacterium tegetincola sp. nov., and Flavobacterium xanthum sp. nov., nom., rev. and reclassification of [Flavobacterium] salegens as Salegentibacter salegens gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 50:1055-1063. [DOI] [PubMed] [Google Scholar]
- 47.Methé, B. A., W. D. Hiorns, and J. P. Zehr. 1998. Contrasts between marine and freshwater bacterial community composition: analyses of communities in Lake George and six other Adirondack lakes. Limnol. Oceanogr. 43:368-374. [Google Scholar]
- 48.Mullins, T. D., T. B. Britschgi, R. L. Krest, and S. J. Giovannoni. 1995. Genetic comparisons reveal the same unknown bacterial lineages in Atlantic and Pacific bacterioplankton communities. Limnol. Oceanogr. 40:148-158. [Google Scholar]
- 49.Neef, A. 1997. Anwendung der in situ-Einzelzell-Identifizierung von Bakterien zur Populationsanalyse in komplexen mikrobiellen Biozönosen. Ph.D. thesis. Technische Universität München, Munich, Germany.
- 50.Neef, A., R. Amann, H. Schlesner, and K.-H. Schleifer. 1998. Monitoring a widespread bacterial group: in situ detection of Planctomycetes with 16S rRNA-targeted probes. Microbiology 144:3257-3266. [DOI] [PubMed] [Google Scholar]
- 51.Nürnberg, D., I. Wollenburg, D. Dethleff, H. Eiken, H. Kassens, T. Letzig, E. Reimnitz, and J. Thiede. 1994. Sediments in arctic sea ice: implications for entrainment, transport and release. Mar. Geol. 119:179-184. [Google Scholar]
- 52.Rath, J., K. Y. Wu, G. J. Herndl, and E. F. DeLong. 1998. High phylogenetic diversity in a marine-snow-associated bacterial assemblage. Aquat. Microb. Ecol. 14:261-269. [Google Scholar]
- 53.Roller, C., M. Wagner, R. Amann, W. Ludwig, and K.-H. Schleifer. 1994. In situ probing of gram-positive bacteria with high DNA G+C content using 23S rRNA-targeted oligonucleotides. Microbiology 140:2849-2858. [DOI] [PubMed] [Google Scholar]
- 54.Ruimy, R., V. Breittmayer, V. Boivin, and R. Christen. 1994. Assessment of the state of activity of individual bacterial cells by hybridization with a ribosomal oligonucleotide probe. FEMS Microbiol. Ecol. 15:207-214. [Google Scholar]
- 55.Schleifer, K.-H., R. Amann, W. Ludwig, C. Rothemund, N. Springer, and S. Dorn. 1992. Nucleic acid probes for the identification and in situ detection of pseudomonads, p. 127-134. In E. Galli, S. Silver, and B. Witholt (ed.), Pseudomonas: molecular biology and biotechnology. American Society for Microbiology, Washington, D.C.
- 56.Smith, R. E., and P. Clement. 1990. Heterotrophic activity and bacterial productivity in assemblages of microbes from sea ice in the high arctic. Polar Biol. 10:351-357. [Google Scholar]
- 57.Spindler, M. 1990. A comparison of arctic and antarctic sea ice and the effects of different properties on sea ice biota, p. 173-183. In U. Bleil and J. Thiede (ed.), Geological history of the polar oceans: arctic versus antarctic. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 58.Stackebrandt, E., and B. M. Goebel. 1994. A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846-849. [Google Scholar]
- 59.Stahl, D. A., and R. Amann. 1991. Development and application of nucleic acid probes, p. 205-248. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 60.Staley, J. T., and J. J. Gosink. 1999. Poles apart: biodiversity and biogeography of sea ice bacteria. Annu. Rev. Microbiol. 53:189-215. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki, M. T., and E. F. DeLong. 2002. Marine prokaryotic diversity, p. 209-234. In J. T. Staley and A.-L. Reysenbach (ed.), Biodiversity of microbial life: foundations of Earth's biosphere. Wiley-Liss, New York, N.Y.
- 62.Thomas, D. N., R. J. Lara, H. Eiken, G. Kattner, and A. Skoog. 1995. Dissolved organic matter in arctic multi-year sea ice during winter: major components and relationship to ice characteristics. Polar Biol. 15:477-483. [Google Scholar]
- 63.Tipper, J. C. 1979. Rarefaction and rarefiction—the use and abuse of a method in paleoecology. Paleobiology 5:423-434. [Google Scholar]
- 64.Wallner, G., R. Amann, and W. Beisker. 1993. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry 14:136-143. [DOI] [PubMed] [Google Scholar]
- 65.Zar, J. H. 1984. Biostatistical analysis. Prentice Hall, Englewood Cliffs, N.J.
- 66.Zwart, G., W. D. Hiorns, B. A. Methé, M. P. van Agterveld, R. Huismans, S. C. Nold, J. P. Zehr, and H. J. Lannbroek. 1998. Nearly identical 16S rRNA sequences recovered from lakes in North America and Europe indicate the existence of clades of globally distributed freshwater bacteria. Syst. Appl. Microbiol. 21:546-556. [DOI] [PubMed] [Google Scholar]