Abstract
The ecology of the uncultured, but large and morphologically conspicuous, rumen bacterium Oscillospira spp. was studied. Oscillospira-specific 16S rRNA gene sequences were detected in North American domestic cattle, sheep from Australia and Japan, and Norwegian reindeer. Phylogenetic analysis of the sequences obtained allowed definition of three operational taxonomic units within the Oscillospira clade. Consistent with this genetic diversity, we observed atypical smaller morphotypes by using an Oscillospira-specific fluorescence in situ hybridization probe. Despite the visual disappearance of typical large Oscillospira morphotypes, the presence of Oscillospira spp. was still detected by Oscillospira-specific PCR in the rumen of cattle and sheep. These observations suggest the broad presence of Oscillospira species in various rumen ecosystems with the level, and most likely the morphological form, dependent on diet. An ecological analysis based on enumeration of the morphologically conspicuous, large-septate form confirms that the highest counts are associated with the feeding of fresh forage diets to cattle and sheep and in two different subspecies of reindeer investigated.
Morphologically unique, large bacteria of the genus Oscillospira, which have not been grown in pure culture yet, can be routinely detected microscopically in the rumen contents of cattle and sheep (11, 18). A number of Oscillospira spp. are responsive to the advent of green pastures and fluctuate seasonally (13, 17) (see also Table 1). The only species of Oscillospira described in Bergey's Manual of Systematic Bacteriology (8) is Oscillospira guilliermondii, and this entry refers to the original morphological description by Chatton and Perard in 1913. Warner (34) proposed that at least two strains or species be included in the Oscillospira group based on cell diameter and the tendency to form spores as the main characteristic differences. This implies that there are different morphological forms or species related to different diets or in different gut ecosystems.
TABLE 1.
Ecological analysis of O. guilliermondii-like organisms as determined by light microscopic counts
| Herbivore | Gut compartment | No. (106/g or ml) | Morphological description and/or diet | Geographic location | Source or reference |
|---|---|---|---|---|---|
| Guinea pig | Cecum | Original description | France | 8 | |
| Rabbit | Cecum | See Bergey's Manual | France | 8 | |
| Sheep | Rumen | Gram-negative, multicellular rods, 5 by 8- to 50-μm, motile, large circular spores (3.5-μm diam); most frequently formed on lush pasture | Australia | 18 | |
| Sheep | Rumen | 0.0003-0.047 | Counts were highest on clover, low on grass; no Oscillospira in pen-fed sheep supplemented with grain plus protein; responds to the advent of green pasture and fluctuates seasonally | Australia | 17 |
| Sheep | Rumen | 0.003-19.50 | Varies with season and individual; 50% wheat plus 50% lucerne chaff diet; required ca. 12 days for reappearance after starvation | Australia | 33 |
| Sheep | Rumen | 0.34 | 50% wheat plus 50% lucerne chaff | Australia | 34 |
| 0.09 | 50% lucerne plus 50% grain | Australia | 34 | ||
| 0.10 | 50% wheat plus 50% lucerne; interval feeding | Australia | 35 | ||
| 20 | Wheat chaff to appetite | Australia | 36 | ||
| 0.89 | Grazing animals | Australia | 36 | ||
| Water buffalo | Rumen | 0.67 | Grass, wheat straw plus concentrate supplement | India | 24 |
| Zebu cattle | Rumen | 0.044 | Grass, wheat straw plus concentrate supplement | India | 24 |
| Water buffalo | Rumen | 4.7 | Grass, wheat straw plus concentrate | India | 13 |
| Sheep | Rumen | 2.0 | Pasture | Orkney, Scotland | 22 |
| 25 | Seaweed | Orkney, Scotland | 22 | ||
| Cattle and sheep | Rumen | Attach to cuticular surface of white clover, lucerne, and rye grass; none on leaves prior to ingestion | New Zealand | 3 | |
| Reindeer | Rumen | 7.0 | Grasses and sedges (summer) | Svalbard, Norway | 21 |
| 0.8 | Mosses and fibrous plants (winter) | Svalbard, Norway | 21 | ||
| Reindeer | Rumen | 1-24 | Natural pasture (autumn) | Svalbard, Norway | This study |
| 1-68 | Natural pasture (winter) | Svalbard, Norway | This study | ||
| Reindeer | Rumen | 20-50 | Natural pasture (summer) | Northern Norway | This study |
| 7-50 | Natural pasture (autumn) | Northern Norway | This study | ||
| 0.4-24 | Natural pasture (winter) | Northern Norway | This study | ||
| 0.4-150 | Pelleted reindeer feed (summer) | Northern Norway | This study | ||
| 0.3-20 | Pelleted reindeer feed (winter) | Northern Norway | This study | ||
| Sheep | Rumen | 0.5-3.8 | Kikuyu pasture | Australia | This study |
| 2.1-2.3 | 10% grain inclusion | Australia | This study | ||
| 2.1 | 30% grain inclusion | Australia | This study | ||
| 0.8-0.9 | 50% grain inclusion | Australia | This study | ||
| 0.06-0.0005 | 70% grain inclusion | Australia | This study | ||
| Cattle | Rumen | 0.25-0.68 | Grass pasture (summer) | Illinois | This study |
| Indoor feeding (winter); hay plus grain—no visible form | Illinois | This study |
Detection and identification of microbial populations are the most basic prerequisites for microbial ecology studies. Over the last decade, several molecular techniques have been developed that when applied to the rumen microbiota have revealed enormous microbial diversity not recognized previously because of limitations and biases inherent in the cultivation approach (24, 30-32, 37). The cultivation-independent approach provides technology for detection and monitoring of microorganisms such as Oscillospira, for which growth requirements are unknown and undetermined. The only requirement for the development of molecular detection techniques is the availability of a marker molecule. Fortunately, several 16S rRNA gene sequences have recently been retrieved from this large bacterium isolated by flow cytometric sorting (39), and this enabled the design of various molecular probes for detection and monitoring. We designed and validate here PCR and PCR-denaturing gradient gel electrophoresis (DGGE) procedures for the detection of Oscillospira spp. and used these techniques to determine the occurrence of this bacterium in different ruminants and during diet shifts in cattle and sheep, as well as to estimate the genetic diversity of this unique group of bacteria.
MATERIALS AND METHODS
Sample collection.
Rumen samples were obtained from three species of ruminants in three different geographic regions. Whole rumen fluid was obtained from two rumen-cannulated Hereford steers maintained at the Beef Research Farm, Department of Animal Sciences, University of Illinois at Urbana—Champaign. In winter, steers were kept indoors and fed medium-quality grass-legume-hay ad libitum. During the remainder of the year, steers were allowed to graze green Timothy (Phleum pratense) pasture. Representative rumen content samples were collected through the rumen cannula by using a scoop, filtered through two layers of cheesecloth, placed on ice, and transported to laboratory. The numbers of Oscillospira in the filtered rumen fluid sample were determined by direct count under a phase-contrast microscope by using a hemocytometer chamber. Oscillospira organisms were identified by their large size and distinct morphology (11, 18). This sample set supplied DNA for PCR and PCR-DGGE analysis.
Rumen samples were collected from four healthy semidomesticated female adult reindeer (Rangifer tarandus tarandus) feeding on natural winter pasture dominated by lichens in northern Norway (28, 29). This sample set supplied DNA for PCR-DGGE analysis. A further sample collection of whole rumen contents for Oscillospira counts was obtained from free-ranging male reindeer calves on fresh coastal natural summer pasture (n = 3) and winter pastures (n = 5) in Northern Norway and from male reindeer calves fed pelleted reindeer feed (RF-80 with a chemical composition of 10.3% crude protein [CP], 8.2% water-soluble carbohydrates, 15.9% cellulose, and 27.9% hemicellulose) (25) in summer (n = 5) and winter (n = 5) maintained at the Department of Arctic Biology, University of Tromsø. Rumen samples were also obtained from adult female reindeer (n = 5) on natural autumn pasture (10 September 2001) and from adult female Svalbaard reindeer (Rangifer tarandus platyrhynchus) (n = 5) on natural autumn pasture (29 April to 5 May 2001). Reindeer were sacrificed, the gastrointestinal tracts were removed immediately, and samples of whole rumen content were stored in 70% ethanol at 4°C until counted.
Rumen samples were collected from adult cannulated sheep housed in indoor pens at Queensland Department of Primary Industries, Brisbane, Australia, in a balanced crossover design experiment with four sheep per group in two 26-day periods. Sheep were fed lucerne pellets to standardize rumen microbial populations prior to introduction of the experimental regimen. Sheep in group 1 were fed fresh-cut Kikuyu (Pennisetum clandestinum) grass for 26 days before being switched to a diet containing cracked barley grain and chaffed hay plus 1% urea-(NH4)2SO4. After the diet crossover, the grain was increased stepwise from 10 to 30 to 50 to 70% of the daily feed intake at 4-day intervals. Animals were kept on the 70% grain diet for last 14 days of the period. Sheep in group 2 were initially fed the grain diet increased stepwise at 4-day intervals from 0 to 10 to 30 to 50 to 70% levels before being switched to the fresh-cut Kikuyu grass diet for the last 26 days after the diet crossover. Rumen samples were collected by using a sampling tube, and a 4-ml aliquot added to 16 ml of formal-saline preservative for counting. Oscillospira organisms in rumen fluid samples were enumerated by using a counting chamber as described above for cattle (11, 18).
DNA extraction.
Total genomic DNA from 200-mg samples of rumen content from cattle, reindeer, and sheep was isolated by using the Ultraclean Soil DNA isolation kit (catalog no. 12800-100; MoBio Laboratories, Solana Beach, Calif.). The amount of DNA extracted was 5 to 10 μg/200 mg of wet sample. The same procedure was employed for extraction of DNA from soil and pasture samples.
Hybridization probe and PCR primers.
A PCR primer set, OSCI-FW (5′-AAGGAGTTTTCGGACAACGG) and OSCI-RV (5′-ATTCAAGGGGTACCGTCTTC), was designed based on retrieval of 16S rRNA gene sequences from Oscillospira organisms (39). A hybridization probe for fluorescent in situ hybridization (FISH) (5′-CCGCACCTAGTATTGATC) was as described earlier (39). A universal bacterial set of primers, 27f and 1525r (14), was used in control amplifications of total DNAs from rumen contents to verify the quality of DNA templates before amplification with the Oscillospira-specific primers. The specificity of Oscillospira-specific probe and primers was initially verified by using the GenBank (2) and RDPII (16) databases. At least two mismatches were allowed with nontarget sequences. All primers and probes were synthesized commercially (OPERON, Alameda, Calif.).
PCR and DGGE.
PCR amplifications were performed with a GeneAmp PCR system 2400 thermocycler (Perkin-Elmer, Norwalk, Conn.). A typical PCR mixture contained 125 ng of genomic DNA, 25 pmol of each primer, 1× ExTaq reaction buffer (PanVera Corp., Madison, Wis.), 100 μM concentrations of each deoxynucleoside triphosphate, and 1.0 U of ExTaq DNA polymerase (PanVera Corp.), adjusted to a total volume of 50 μl. PCR cycling was done with initial denaturation at 94°C for 5 min, followed by 30 cycles at 94°C for 45 s, 59°C for 45 s, and 72°C for 90 s, and with a final extension step at 72°C for 7 min. The PCR products were separated by gel electrophoresis in 3% (wt/vol) NuSieve agarose (FMC Bioproducts, Rockland, Maine) and visualized with ethidium bromide. For PCR-DGGE, the forward primer had a GC-clamp attached at the 5′ end (19). The optimized PCR protocol initially consisted of eight touchdown cycles, which were performed while decreasing the annealing temperature from 66 to 59°C (1°C/cycle), followed by 22 additional cycles as described above. Electrophoresis was performed at 60°C and 150 V for 2 h, followed by 200 V for 1 h, by using the D-Gene System (Bio-Rad Laboratories, Richmond, Calif.). After the run, gels were rinsed in double-distilled H2O, fixed in a solution of 10% ethanol and 0.5% acetic acid, and silver stained. Gel images were captured and digitized by using a Bio-Rad GS-710 calibrated imaging densitometer connected to a G3 Macintosh computer with Diversity Database fingerprinting software.
Cloning and sequencing.
DNA bands were excised from DGGE gels, crushed, and equilibrated in Tris-EDTA buffer at room temperature overnight. Eluted DNA solution (1 μl) was used for reamplification by using the same set of Oscillospira-specific primers but without the GC-clamp. PCR products were directly cloned by using the pGEM-T Easy Vector System II kit (Promega Corp., Madison, Wis.) according to the manufacturer's protocol. DNA sequence analysis of inserts in recombinant plasmids was performed on both strands at the University of Illinois Biotechnology Center. Online similarity search was performed by using the basic local alignment search tool (BLAST) family of programs in GenBank (15). The GenBank accession numbers for the eight 409-bp Oscillospira sequences generated here are AY244475 to AY244482.
FISH.
The FISH procedure essentially followed the method described by Amann (1) with our modifications (39). Rumen fluid and pure culture sample preparations were hybridized in 8 μl of the hybridization solution (Sigma, St. Louis, Mo.) containing 1 μl of probe (28 ng) at 48°C for 2 h. After hybridization, the slides were washed in hybridization buffer for 20 min at 48°C, rinsed with distilled water, and air dried. Slides were mounted by using the antifade mounting medium (SlowFade Antifade Kit; Molecular Probes, Eugene, Oreg.). In preliminary experiments, the slides were viewed with a Nikon epifluorescence EFD-3 microscope equipped with a suitable filter set (Nikon). Sequences of phylogenetically close, but nontarget, bacterial species exhibited at least two mismatches with the probe sequence and produced no FISH signal demonstrating high probe specificity. For subsequent confocal microscopy, a Fluoview FV300 laser-scanning biological microscope (version 3.00; Olympus, New York, N.Y.) was used. Transmission electron micrographs (TEMs) were obtained from rumen content of reindeer by using the methods described by Olsen and Mathiesen (20).
RESULTS AND DISCUSSION
PCR detection.
The sensitivity of light microscopic detection of Oscillospira in rumen fluid may be low, and one of the objectives of the present study was to develop a more sensitive PCR detection technique allowing detection at levels beyond the sensitivity of the light microscopic without relying on the specific morphology of cells for enumeration. For this purpose, we designed a set of PCR primers based on Oscillospira 16S ribosomal DNA (rDNA) sequence generated from flow cytometric sorting of large cells (39). Since there was no positive culture available for the specificity test, the specificity of the primer set was checked against the GenBank and RDPII databases and by amplification and sequencing of PCR products from total ruminal DNA. For all of the rumen samples with conspicuous Oscillospira morphotypes surveyed by the PCR detection assay, amplification yielded the expected 409-bp product. This finding was confirmed by cloning and sequencing the PCR amplicon. No amplification was observed when phylogenetically related ruminal bacterial species (Sporobacter termitidis, Clostridium perfringens 3624A, Ruminococcus albus 7, and Ruminococcus flavefaciens FD-1) were used as negative controls in verification experiments. In addition, sequences amplified with this primer set from total ruminal DNA templates formed a phylogenetically coherent group with the sequences isolated earlier by the cell-sorting technique (39) (Fig. 2).
FIG. 2.
Phylogenetic placement of partial 16S rDNA sequences, amplified with the Oscillospira-specific primer set OSCI, within the main rumen bacterial phyla. Previously cloned Oscillospira sequences are labeled OSC1 through OSC5. The sequences generated in this work are labeled A through H. The Aquifex pyrophilus sequence is used as the outgroup for rooting the tree. Numbers above each node are confidence levels generated from 1,000 bootstrap trees (5). The scale bar is in fixed nucleotide substitutions per sequence position.
Diet change in cattle.
Although not quantified, the Oscillospira-specific PCR signals from the rumen fluid of cattle feeding on Timothy pasture were more prominent than those of animals kept indoors. Dilutions of equal amounts of total rumen genomic DNA from the two diets, used in amplifications with the OSCI primer set, demonstrated that the DNA from the pasture-fed animals still produced PCR signal at a 10−2 dilution, whereas samples from animals kept indoors showed no amplification at a 10−1dilution. Longitudinal microscopic detection and enumeration of Oscillospira and PCR detection performed on two rumen-cannulated steers kept at the University Research Farm showed that distinct Oscillospira morphotypes were consistently observed in all samples from both animals when fed on green pasture. However, two weeks after the transition from pasture fed to indoor fed, no typical Oscillospira morphotypes could be detected microscopically. These morphotypes reappeared again 18 days after the advent of pasture feeding. It is interesting that more than 2 weeks were needed for the reappearance of Oscillospira but quickly reached the level characteristic of pasture-fed animals (1.2 × 105) within just 1 week after the first detection. However, all samples produced the specific 409-bp PCR amplicon, irrespective of diet or the presence or absence of the specific Oscillospira morphotype evaluated microscopically. To exclude the possibility of Oscillospira entering the rumen with feed, total DNA from the pasture grass and soil samples was isolated and subjected to amplification with the OSCI primer set, with negative results. Clarke (3) also reported that Oscillospira was not found on leaves prior to ingestion based on examination of material by light microscopy.
Enumeration of Oscillospira in reindeer.
An ecological analysis (Table 1) confirmed that the high counts of the large morphologically conspicuous form of Oscillospira, termed O. guilliermondii, were recorded in Orkney sheep consuming seaweed diets (2.5 × 107 per g of ingesta) and in Svalbard reindeer on summer pasture (7 × 106 per g of ingesta) (20, 21). The results from the present study show that consistently high counts were obtained from the rumen of reindeer in northern Norway and Svalbard (1 × 107 to 5 × 107 per g of ingesta).
Diet shift from grass to grain diets and vice versa in sheep.
In order to evaluate the effect of diet change from lush forage to grain-containing diets, a changeover experiment was performed with sheep. Microscopic counts showed that on the lucerne pellet standard diet numbers of Oscillospira were 1.3 × 105 to 1.7 × 105 per g of ingesta. However, after the changeover Oscillospira numbers decreased on the 50% grain diet. After the animals were on a 70% grain diet for 10 days, the numbers of Oscillospira decreased to 50 per g of ingesta. For group 2, the numbers of Oscillospira decreased to 5 × 103 for animals on the 70% cracked barley diet. However, 8 days after the shift to fresh Kikuyu grass, the numbers of Oscillospira had increased to 1.1 × 105 to 1.9 × 105 per g of ingesta. Importantly, all samples produced the specific 409-bp PCR amplicon for Oscillospira regardless of diet (data not shown).
PCR-DGGE and phylogeny.
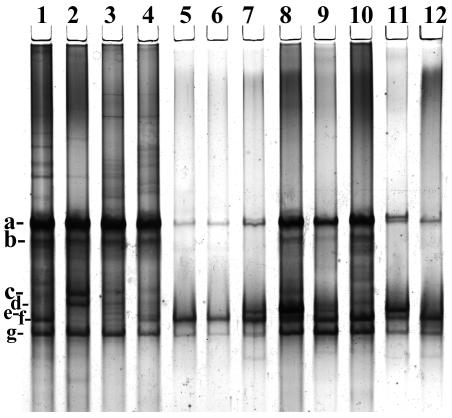
In order to access the diversity of Oscillospira in different ruminant species, the Oscillospira-specific primers were used in conjunction with PCR-DGGE (Fig. 1). This analysis demonstrated the presence of at least seven different phylotypes in these animals based on gel banding patterns, with the most diverse group detected in the Norwegian reindeer (six phylotypes), with four phylotypes in the Australian sheep and three phylotypes in U.S. domestic cattle. Phylotypes a, f, and g were universally detected in all animal species, whereas phylotypes c and d were unique to reindeer, they were detected only in two animals. Phylotype e was encountered only in sheep from Australia. Eight DGGE bands were cloned, sequenced, and incorporated into the DNA similarity analysis, together with the five previously cloned sequences obtained from sheep in Japan (39). This analysis suggests the existence of at least three subgroups (possibly species) of Oscillospira in the rumen. In the subsequent phylogenetic analysis, these 13 cloned sequences were incorporated into the rumen bacterial phylogenetic tree (Fig. 2). All Oscillospira sequences formed a tight phylogenetic group within the cluster IV of the low-G+C gram-positive bacterial (LGCGPB) phylum with 78% bootstrap support. The nearest cultivated neighbors of the Oscillospira group were the human colonic isolate Clostridium orbiscindens (91% 16S rDNA similarity) (26, 38) and the ASF500 strain (Clostridium sp.) component of the murine altered Schaedler flora (92% similarity) (4). The sequence of Quinella ovalis, another large, morphologically conspicuous, rumen bacterium (12), was located within the cluster IX of the LGCGPB phylum. Despite the phylogenetic clustering in cluster IV of the LGCGPB phylum, the sequences of Oscillospira are quite heterogeneous and are represented by at least three groups, with the first group being represented by clones A, F, and H, the second group being represented by clones D, E, and G, and the third, most numerous group, being represented by clones OSC1 to OSC5 and B and C (Fig. 2). It is noteworthy that the sequences of Oscillospira cells, which were cloned after cell sorting (39), form a separate cluster with close DNA similarity values, suggesting possible large morphotype selection during the cell size sorting procedure (Fig. 2). With the cell sorting procedure, other less morphologically prominent or smaller forms may have escaped detection, collection, and subsequent 16S rRNA gene analysis. However, in PCR-generated libraries, these sequences were successfully retrieved and analyzed, confirming the existence of several Oscillospira species in the rumen. The sequence data are limited to an analysis of the 409-bp 16S rRNA gene and do not support any host species-specific affiliation of Oscillospira. For example, the genetically coherent group II is represented by sequences from American cattle, Australian sheep, and Norwegian reindeer (Fig. 2).
FIG. 1.
DGGE analysis PCR-amplified 16S rRNA fragment of Oscillospira with the OSCI primers with an attached GC-clamp. The figure shows the DGGE separation pattern of PCR fragments obtained from Norwegian reindeer (lanes 1 to 4), cattle (lanes 5 and 6), and sheep (lanes 7 to 12). The arrows indicate the representatives of DNA bands, which were excised and subjected to cloning and sequencing.
Thus, we failed to detect any host species-specific association of these operational taxonomic units, suggesting the widespread presence of the same operational taxonomic units in geographically distant and diverse ruminant species consuming a variety of diets. Although a limited number of Oscillospira sequences were obtained and analyzed in the present preliminary study, more representative analyses may uncover broader diversity and the presence of these fascinating organisms in other ruminants as well as in the cecum and colon of a wide range of herbivores, including humans. In fact, the nearest cultivated neighbors of the Oscillospira group included the human colonic isolate C. orbiscindens capable of cleaving the flavonoid C-ring (26, 38) and the AFS 500 (Clostridium sp.) component of the murine altered Schaedler flora. Obviously, higher-scoring BLAST hits were observed with uncultivated representatives of the rumen, pig, human, chicken, and rodent gastrointestinal microbiota. Sporobacter termitidis, a bacterial species isolated from the termite gut (10) that grows exclusively on a limited range of methoxylated aromatic compounds with ring cleavage, has a similarity of 88.1%. In order to perform this analysis adequately, we require full-length 16S rRNA gene sequence or another molecule other than SSU rDNA, which may be too highly conserved, to provide sufficient resolution of speciation and biogeographical principles initiated in the present study (23, 27).
Cell morphology and electron microscopy.
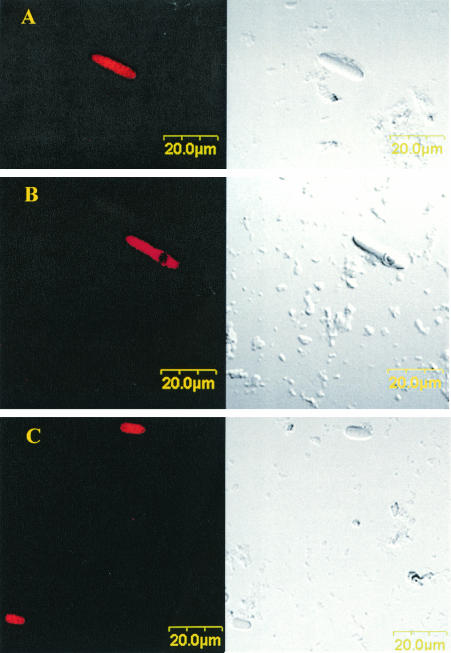
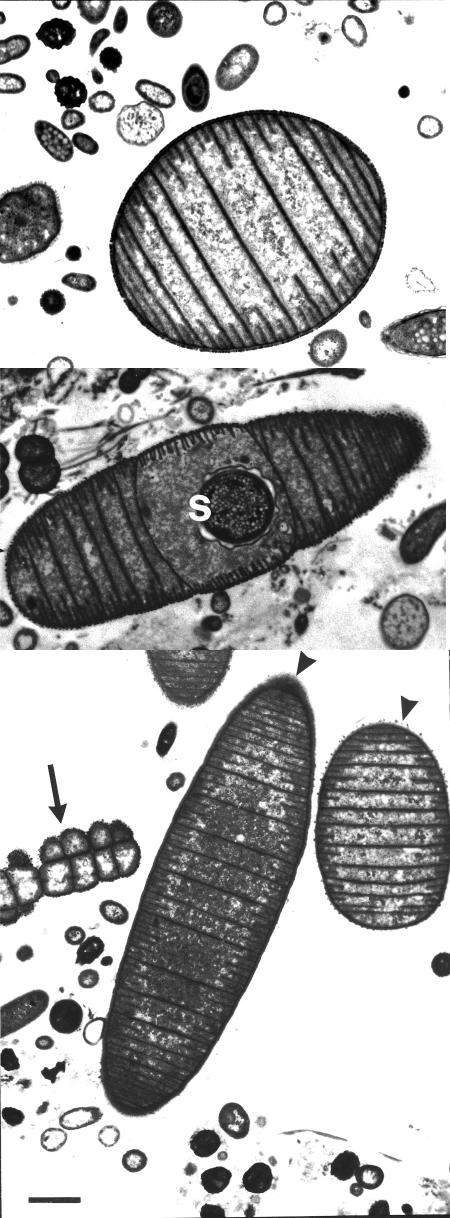
Rumen samples were examined in an attempt to determine whether there were other unidentified morphological forms of Oscillospira in the rumen. As shown in Fig. 3, bacteria with the characteristic morphology of Oscillospira were observed by FISH, although these bacteria differed significantly in size (ranging from 40 to 50 μm to 5 to 8 μm in length). Some cells contain an intracellular circular region that binds probe poorly and are presumably spores (Fig. 3B). Specific treatment may be needed for FISH probe penetration of spores (6). Sporulation events during the life cycle of this bacterium have been well documented (9, 34). Using this probe, we were also able to detect another ruminal morphotype in cattle, which is unusual for Oscillospira (Fig. 3C). No obvious Oscillospira-specific septations were present, and the general morphology differed from the more typical multicellular morphotype shown in Fig. 3A and B. The ultrastructure of characteristic Oscillospira cells has been described by Grain and Senaud (9), but we present here TEMs as further evidence of two distinct morphological forms: a large rod divided by multiple, closely spaced cross-walls (10 μm) (Fig. 4, bottom panel), and a small oval form divided by three to five cross walls (4 to 5 μm) (Fig. 4, top and bottom panels). Thus, in addition to genetic diversity, these observations add further evidence for the morphological heterogeneity of Oscillospira populations in the rumen and for the possibility of a life cycle that includes elongation by septation and a spore stage (Fig. 3B; Fig. 4, middle panel).
FIG. 3.
Whole-cell FISH analysis of rumen fluid from cattle in Illinois. The cells were hybridized with Cy3-labeled oligonucleotide probe specific for Oscillospira. Images were obtained by confocal laser-scanning microscopy, with differential interference contrast (left) and fluorescence (right) images displayed for each identical field, showing the morphologically identified forms of Oscillospira. Panel A demonstrates characteristic morphology of Oscillospira, i.e., large, septate rod. Note the lack of probe penetration and staining of the spore (B) and the smaller, atypical septate form (C) that nevertheless gives a positive FISH signal. Scale bar, 20 μm.
FIG. 4.
TEMs of O. guilliermondii obtained from rumen content of Norwegian reindeer documenting an atypical, smaller, oval form (length, 5 μm) with septation (top panel); a morphologically conspicuous septate rod with round central spore (s) (middle panel); and both a morphologically conspicuous large, septate form (ca. 10 μm) and a smaller oval form (4 μm) (bottom panel). The two arrowheads indicate the long and oval forms, respectively. Animals, diet, and sampling of reindeer were as described by Olsen and Mathiesen (20). Scale bar, 1 μm.
The rapid association or colonization of the cuticular surface of plant material by Oscillospira suggests that this might constitute a specific habitat for this bacterium (3). It is likely that nutrients or other chemoattractents are released during mastication of freshly ingested forage, attracting these and other motile bacteria to this material. However, with no pure culture available, these observations remain anecdotal and unproven. Nevertheless, the conspicuous morphology of this bacterium enabled enumeration based on direct microscopic counts of rumen contents during various dietary regimens such as the transfer of cattle from indoor housing to green pasture and during a diet crossover experiment in sheep. Microscopic count data showed the highest numbers of this specific morphotype were recorded during green pasture feeding and ranged from 2.5 × 105 to 6.8 × 105 per ml of rumen fluid from cattle (Table 1). Direct microscopic counts of Oscillospira-like bacteria in reindeer are high (generally 107 but as high as 1.5 × 108; Table 1) and are associated not only with a diet of fresh summer pasture but also with winter diets containing large quantities of lichens and subarctic plants, including graminoids, shrubs, and heathers (28, 29). Unusually high numbers of Oscillospira were reported in the rumen of sheep on the island of North Ronaldsey, Orkney, Scotland, fed seaweed (2.5 × 107) (28). Seaweed is a rich source of mannitol that can constitute up to 35% of kelp dry weight (7). Thus, the abundance of Oscillospira in these sheep was linked to utilization of this six-carbon sugar alcohol by Orpin et al. (22), who observed that in mixed culture O. guilliermondii survived for extended periods with mannitol as a carbon source. Similar in vitro cultivation experiments in our laboratory, using centrifugation to concentrate Oscillospira as an inoculum and based on direct microscopic counts, did not demonstrate improved survival on mannitol compared to the same concentration of mixed carbohydrates (glucose-cellobiose-maltose-starch) generally added to ruminal, habitat-simulating media.
The advent of microbial molecular ecology techniques during the last decade has revolutionized the approaches used in detection, enumeration, and classification of previously uncultivated microorganisms. Our Oscillospira-specific PCR assay suggests that, despite the visual disappearance of this bacterium during indoor feeding on a mixed hay diet of cattle and on high levels of grain feeding in sheep, the specific signal was still detected in ruminal samples. This discrepancy may be either due to low sensitivity of microscopic examination or because of the presence of other Oscillospira species or forms without the conspicuous morphology. Indeed, during FISH analysis we encountered atypical forms, which hybridized at high stringency with the Oscillospira probe (Fig. 3). This was supported by the observation of small oval, septate forms 4 to 5 μm in length (Fig. 4) using transmission electron microscopy. We postulate that this atypical form is responsible for the PCR signal in the absence of typical Oscillospira morphotypes. At present, it is not clear whether this is another species of Oscillospira or if it is a different morphological form of the same bacterium that undergoes morphological changes during its life cycle induced by the diet shift. Therefore, only the large, morphologically conspicuous, visually obvious form is the one detected and counted by light microscopy. Since we detected a considerable level of genetic diversity within this group of bacteria based on cloning and sequencing of bands separated by PCR-DGGE, the most plausible explanation for the observed morphological heterogeneity could be the underlying genetic diversity of Oscillospira in the rumen. This idea is supported by the observation that the morphologically based flow cytometric cell sorting also selected the sequences that phylogenetically cluster within this group (Fig. 2) (39). It is likely that the genetically distant phylogenetic groups I and II may be represented by different, atypical forms of Oscillospira dominant on the mixed hay diet.
In conclusion, although Oscillospira has been widely observed for almost 90 years, it remains uncultured. Modern molecular microbial ecology techniques have enabled us to place this large, morphologically conspicuous bacterium with the group IV LGCGP bacterial group. Combined evidence from PCR, DGGE, and FISH analyses strongly supports genetic diversity and morphological heterogeneity with the possibility of three (sub)species and the possibility of a life cycle that includes elongation by septation, as well as a spore stage.
Acknowledgments
We thank Andrea Turner, Luke Burrow, and the farm staff at the Animal Research Institute, Yerongpilly, Queensland, Australia, for technical assistance during the sheep trial partially funded by the Australian Research Council. We are grateful to Svein Disch Mathiesen and Pål Vegar Storeheier for their assistance in sampling the reindeer and to the Departments of Electron Microscopy and Morphology at the Faculty of Medicine, University of Tromsø, Tromsø, Norway, for assistance with the TEMs of reindeer Oscillospira.
The reindeer project was supported by the Reindeer Husbandry Research Fund, the University of Tromsø, and the Norwegian Research Council. Research at the University of Illinois was supported by the Agricultural Research Station, University of Illinois at Urbana-Champaign, and by USDA-NRI Competitive Grants Program 42.0 (award 99-35206-7950).
REFERENCES
- 1.Amann, R. I. 1995. In situ identification of microorganisms by whole cell hybridization with rRNA-targeted nucleic acid probes, p. 1-15. In A. D. L. Akkermans, J. D. van Elsas, and F. J. de Bruijn (ed.), Molecular microbial ecology manual. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 2.Benson, D. A., M. S. Boguski, D. J. Lipman, J. Ostell, B. F. Ouellette, B. A. Rapp, and D. L. Wheeler.1999. GenBank. Nucleic Acids Res. 27:12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke, R. T. J. 1979. Niche in pasture-fed ruminants for the large rumen bacteria Oscillospira, Lampropedia, and Quin's and Eadie's ovals. Appl. Environ. Microbiol. 37:654-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dewhirst, F. E., M. S. Boguski, D. J. Lipman, J. Ostell, B. F. Oullette, B. A. Rapp, and D. L. Wheeler. 1999. Phylogeny of the defined murine microbiota: altered Schaedler flora. Appl. Environ. Microbiol. 65:3287-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 6.Fischer, K., D. Hahn, W. Honerlage, and F. Schonholzer. 1995. In situ detection of spores and vegetative cells of Bacillus megaterium in soil by whole cell hybridization. Syst. Appl. Microbiol. 18:265-273. [Google Scholar]
- 7.Gerard, V. A. 1989. Seaweeds, p. 205-212. In O. Kitami and C. W. Hall (ed.), Biomass handbook. Gordon and Breach Scientific Publications, New York, N.Y.
- 8.Gibson, T. 1986. Genus Oscillospira Chatton and Perard 1913, p. 1207. In P. H. A Sneath et al. (ed.), Bergey's manual of systematic bacteriology, vol. 2. The Williams & Wilkins Co., Baltimore, Md.
- 9.Grain, J., and J. Senaud. 1976. Oscillospira guilliermondii, a rumen bacteria: ultrastructural study of the trichome and of sporulation. J. Ultrastruct. Res. 55:228-244. [DOI] [PubMed] [Google Scholar]
- 10.Grech-Mora, I., M. L. Fardeau, B. K. C. Patel, B. Ollivier, A. Rimbault, G. Prensier, J. L. Garcia, J. L., and E. Garnier-Sillam. 1996. Isolation and characterization of Sporobacter termitidis gen. nov., sp. nov., from the digestive tract of the wood-feeding termite Nasutitermes lujae. Int. J. Syst. Bacteriol. 46:512-518. [Google Scholar]
- 11.Hungate, R. E. 1966. The rumen bacteria, p. 8-90. In R. E. Hungate (ed.), The rumen and its microbes. Academic Press, Inc., New York, N.Y.
- 12.Krumholz, L. R., M. P. Bryant, W. J. Brulla, J. L. Vicini, J. H. Clark, and D. A. Stahl. 1993. Proposal of Quinella ovalis gen. nov., sp. nov., based on phylogenetic analysis. Int. J. Syst. Bacteriol. 43:293-296. [DOI] [PubMed] [Google Scholar]
- 13.Kulkerni, V. D., H. C. Pant, G. S. Rai, and J. S. Rawat. 1971. The seasonal variation in the concentration of protozoa and Oscillospira guilliermondii organisms in the rumen fluid of grazing buffalo. Indian Vet. J. 48:137-142. [PubMed] [Google Scholar]
- 14.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Inc., New York, N.Y.
- 15.Madden, T. L., R. L. Tatusov, and J. Zhang. 1996. Application of network BLAST server. Methods Enzymol. 266:131-141. [DOI] [PubMed] [Google Scholar]
- 16.Maidak, B. L., J. R. Cole, C. T. Parker, Jr., G. M. Garrity, N. Larsen, T. G. Li, B. Lilburm, M. J. McCaughey, G. J. Olsen, R. Overbeek, S. Pramanik, T. M. Schmidt, J. M. Tiedje, and C. R. Woese. 1999. A new version of the RDP (ribosomal database project). Nucleic Acids Res. 27:171-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moir, R. J. 1951. The seasonal variation in the ruminal microorganisms of grazing sheep. Austr. J. Agric. Res. 2:322. [Google Scholar]
- 18.Moir, R. J., and M. J. Masson. 1952. An illustrated scheme for the microscopic identification of the rumen microorganisms of sheep. J. Pathol. Bacteriol. 64:343-350. [DOI] [PubMed] [Google Scholar]
- 19.Muyzer, G., E. C. De Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen, M. A., and S. D. Mathiesen. 1998. The bacterial population adherent to plant particles in the rumen of reindeer fed lichen, timothy hay or silage. Rangifer 18:55-64. [Google Scholar]
- 21.Orpin, C. G., S. D. Mathiesen, Y. Greenwood, and A. S. Blix. 1985. Seasonal changes in the ruminal microflora of the high-arctic Svalbard reindeer (Rangifer tarandus platyrhynchus). Appl. Environ. Microbiol. 50:144-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orpin, C. G., Y. Greenwood, F. J. Hall, and I. W. Paterson. 1985. The rumen microbiology of seaweed digestion in Orkney sheep. J. Appl. Bacteriol. 59:585-596. [DOI] [PubMed] [Google Scholar]
- 23.Palys, T., K. Nakamura, and F. M. Cohan. 1997. Discovery and classification of ecological diversity in the bacterial world: the role of DNA sequence data. Int. J. Syst. Bacteriol. 47:1145-1156. [DOI] [PubMed] [Google Scholar]
- 24.Pant, H. C. 1966. A note on concentration of “Oscillospira guilliermondii-type” organisms in the rumen ingesta of buffalo and zebu cattle. Indian Vet. J. 43:111-113. [PubMed] [Google Scholar]
- 25.Sletten, H., and K. Hove. 1990. Digestive studies with a feed developed for realimentation of starving reindeer. Rangifer 10:31-38. [Google Scholar]
- 26.Stackebrandt, E., I. Kramer, J. Swiderski, and H. Hippe. 1999. Phylogenetic basis for a taxonomic dissection of the genus Clostridium. FEMS Immunol. Med. Microbiol. 24:253-258. [DOI] [PubMed] [Google Scholar]
- 27.Staley, J. T. 1999. Bacterial biodiversity: a time for place. ASM News 65:681-687. [Google Scholar]
- 28.Storeheier, P. V., S. D. Mathiesen, N. J. C. Tyler, and M. A. Olsen. 2002. Nutritive value of terricolous lichens for reindeer in winter. Lichenologist 34:247-257. [Google Scholar]
- 29.Storeheier, P. V., S. D. Mathiesen, N. J. C. Tyler, I. Schjelderup, and M. A. Olsen. 2002. Utilization of nitrogen and mineral-rich vascular forage plants by reindeer in winter. J. Agric. Sci. 139:1-10. [Google Scholar]
- 30.Tajima, K., R. I. Aminov, T. Nagamine, K. Ogata, M. Nakamura, H. Matsui, and Y. Benno. 1999. Rumen bacterial diversity as determined by sequence analysis of 16S rDNA libraries. FEMS Microbiol. Ecol. 29:159-169. [Google Scholar]
- 31.Tajima, K., S. Arai, K. Ogata, T. Nagamine, H. Matsui, M. Nakamura, R. I. Aminov, and Y. Benno. 2000. Rumen bacterial community transition during adaptation to high-grain diet. Anaerobe 6:273-284. [Google Scholar]
- 32.Tajima, K., T. Nagamine, H. Matsui, M. Nakamura, and R. I. Aminov. 2001. Phylogenetic analysis of archaeal 16S rRNA libraries from the rumen suggests the existence of a novel group of archaea not associated with known methanogens. FEMS Microbiol. Lett. 200:67-72. [DOI] [PubMed] [Google Scholar]
- 33.Warner, A. C. I. 1962. Enumeration of rumen microorganisms. J. Gen. Microbiol. 28:129-146.14005007 [Google Scholar]
- 34.Warner, A. C. I. 1966. Diurnal changes in the concentrations of microorganisms in the rumens of sheep fed to appetite in pens or at pasture. J. Gen. Microbiol. 45:243-251. [DOI] [PubMed] [Google Scholar]
- 35.Warner, A. C. I. 1966. Periodic changes in the concentrations of microorganisms in the rumen of sheep fed limited diets once daily. J. Gen. Microbiol. 45:213-245. [DOI] [PubMed] [Google Scholar]
- 36.Warner, A. C. I. 1966. Diurnal changes in the concentrations of microorganisms in the rumen of a sheep fed limited ration every 3 h. J. Gen. Microbiol. 45:237-242. [DOI] [PubMed] [Google Scholar]
- 37.Whitford, M. F., R. J. Foster, C. E. Beard, J. Gong, and R. M. Teather. 1998. Phylogenetic analysis of rumen bacteria by comparative sequence analysis of cloned 16S rRNA genes. Anaerobe 4:153-163. [DOI] [PubMed] [Google Scholar]
- 38.Winter, J., M. R. Popoff, P. Grimont, and V. D. Boekkenheuser. 1991. Clostridium orbiscindens sp. nov., a human intestinal bacterium capable of cleaving the flavonoid C-ring. Int. J. Syst. Bacteriol. 41:355-357. [DOI] [PubMed] [Google Scholar]
- 39.Yanagita, K., A. Manome, X.-Y. Meng, T. Kanagawa, H. Tsuchida, R. Mackie, and Y. Kamagata. 2003. Flow cytometric sorting, phylogenetic analysis and in situ detection of Oscillospira guilliermondii, a large morphologically conspicuous but uncultured ruminal bacterium. Int. J. Syst. Evol. Microbiol. 53:1609-1614. [DOI] [PubMed]