Abstract
Biomass samples from the Black Sea collected in 1988 were analyzed for SSU genes from Bacteria and Archaea after 10 years of storage at −80°C. Both clonal libraries and direct fingerprinting by terminal restriction fragment length polymorphism (T-RFLP) analyses were used to assess the microbial community. Uniform and discrete depth distributions of different SSU phylotypes were observed. However, most recombinant clones were not restricted to a specific depth in the water column, and many of the major T-RFLP peaks remain uncharacterized. Of the clones obtained, an ɛ-Proteobacteria and a Pseudoalteromonas-like clone accounted for major peaks in the fingerprint, while deeply branching lineages of α- and γ-Proteobacteria were associated with smaller peaks. Additionally, members were found among both the δ-Proteobacteria related to sulfate reducers and the Archaea related to phylotypes from the ANME groups that anaerobically oxidize methane.
The Black Sea is the largest surface-exposed, permanently anoxic basin on this planet. In this area, the high intensity of photosynthetic primary production in the surface waters, the associated flux of organic carbon, and the shallow sill depth has led to the development and maintenance of the largest, stable oxic/anoxic interface on the planet (3). This interface, or chemocline (defined by the first appearance of hydrogen sulfide in the water column) is located at 81 to 99 m depth (3, 16). A 20- to 30-m-deep suboxic layer depleted in both O2 and H2S overlies the sulfide zone (16). The stratified water column in the Black Sea is believed to host more active and diverse microbial assemblages than anywhere else in the pelagic ocean (14). As such, the Black Sea is an excellent model system for studying oxic/anoxic interfaces, essentially stretching a chemocline normally encountered on the submillimeter scale over tens of meters.
Although other oxic/anoxic regions exist and reports of molecular characterization of microbial communities from the Cariaco Trench (23) or sedimentary systems (12, 22, 40, 42) have been published, few systematic profiles of the transition between oxic and anoxic bacterial communities beyond a domain- or group-specific approach have been reported. The purpose of this study was to characterize the Bacteria and Archaea populations in the Black Sea at a species-specific level and to correlate the vertical distribution of the various prokaryotic plankton with the profiles of terminal electron acceptors that occur throughout the oxic/anoxic chemocline. To this end, we conducted culture-independent studies on samples collected from the Black Sea water column during the 1988 oceanographic expedition. Terminal restriction fragment length polymorphism (T-RFLP) analysis (1) was performed on samples between 10 and 500 m depth to characterize the microbial assemblages, using the 16S ribosomal RNA genes (20). Discrete bacterial communities were seen corresponding to the aerobic zone, the high-nitrate zone, the sulfate-reducing zone, and the anoxic deep waters. This research provides baseline profiling for examining the transition between oxic and anoxic environments in systems with much smaller interfaces spanning the millimeter to micron range, such as particles, biofilms, and sediments.
MATERIALS AND METHODS
Sample collection, DNA extraction, and library construction screening.
Microbial biomass was collected during the July 1988 Black Sea expedition (26). One-liter water samples were filtered onto 0.2-μm-pore-size Supor Gelman filters (Ann Arbor, Mich.) and stored between −20 and −80°C for over 10 years before extraction. Each filter was immersed in 225 μl of 50 mM glucose, 10 mM EDTA, 25 mM Tris-HCl (pH 8.0), 75 μl of 0.5 M EDTA, and subjected to three freeze-thaw cycles with liquid nitrogen. One hundred microliters of lysozyme solution (4 mg/ml) was added to the sample, and the filters were incubated at 37°C for 15 min with gentle shaking. A 50-μl volume of 10% sodium dodecyl sulfate (SDS) was added to each sample, followed by two extractions with an equal volume of phenol-chloroform-isoamyl alcohol (50:49:1). The aqueous phase was precipitated with 50 μl of 3.0 M sodium acetate and 1 ml of 100% ethanol O/N at −20°C. The DNA was collected by centrifugation, washed in cold 80% ethanol, dried, and resuspended in sterile distilled water or purified by cesium chloride gradient as described previously (18, 44). Target genes were selectively amplified from the genomic DNA by PCR as follows: archaeal16F (5′-CTGGTTGATCCTGCCAG-3′) and universal 1517R (5′-ACGGCTACCTTGTTACGACTT-3′) were used to selectively amplify the archaeal 16S rRNA genes, whereas the bacterial primer 8-27F (5′-AGAGTTTGATCCTGGCTCAG-3′) was used in combination with universal 1517R to selectively amplify the bacterial 16S rRNA genes. Template DNA was incubated in a thermal cycler in the presence of Taq DNA polymerase for 30 cycles under the following conditions: 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s. PCR products were gel purified using QIAquick spin columns (Qiagen, Inc., Chatsworth, Calif.) and resuspended in sterile distilled water. Amplified gene fragments were cloned in the pCR II plasmid vector (Invitrogen, Inc., Carlsbad, Calif.), and the resulting ligation products were used to transform competent Escherichia coli TOP10F′ cells. Ten environmental libraries were constructed from different depth samples, and 40 to 80 recombinant clones per library were randomly chosen and analyzed for insert-containing plasmids by direct PCR followed by gel electrophoresis of the amplified products.
T-RFLP.
A rapid fingerprinting technique that utilizes fluorescent end labeling of PCR product (target genes) and screening by T-RFLP (for a review, see reference 20) was used to characterize the various 16S rRNA genes in the samples. Twenty-five nanograms of labeled, PCR product was digested with MnlI for 6 h and precipitated using glycogen as a carrier. The digest was resuspended in 20 μl of formamide for 15 min and analyzed on an ABI 310 automated sequencer (Applied Biosystems, Foster City, Calif.).
RFLP, sequence, and phylogenetic analysis.
PCR-amplified 16S rRNA gene fragments were digested with the restriction endonuclease pair HaeIII and MspI (Promega, Inc., Madison, Wis.). The reaction products were visualized by electrophoresis on a 2.5% (wt/vol) agarose gel containing ethidium bromide (0.5 mg/liter). Representative clones for each library showing unique RFLP patterns were selected, and their sequences were determined for both strands on an ABI 310 automated sequencer. The average number of nucleotides of sequence determined was 900. Sequences were manually aligned to GenBank releases using the Genetic Data Environment (GDE) multiple sequence editor. Corrected evolutionary distances (17, 41) and a least-squares algorithm (6) were used to construct distance trees from a normal evolutionary distance matrix. Maximum-likelihood trees were constructed with fastDNAml (8, 9), which uses the generalized two-parameter model of evolution (19) and jumbled orders for the addition of taxa to avoid potential bias introduced by the order of sequence addition. The transition/transversion ratio was optimized, and bootstrap analysis was used to provide confidence estimates for phylogenetic tree topologies (8).
Nucleotide sequence accession numbers.
The Black Sea sequences used in the study have been deposited in GenBank under the accession numbers AY360476 to AY360524.
RESULTS
The Black Sea is characterized by a permanently stratified water column and represents the largest anoxic basin in the world. Despite its unique character, data on the distribution and function of microbial assemblages in the Black Sea water column and sediments are limited to a few studies (14, 16, 34, 36, 37). This study was designed to assess the diversity and distribution of bacterioplankton along and below the chemocline in the Black Sea and to correlate the occurrence of phylotypes with depth. Here, we have combined both clonal analysis and TRFLP fingerprinting to gain an understanding of the presence or absence of particular microorganisms in the water column.
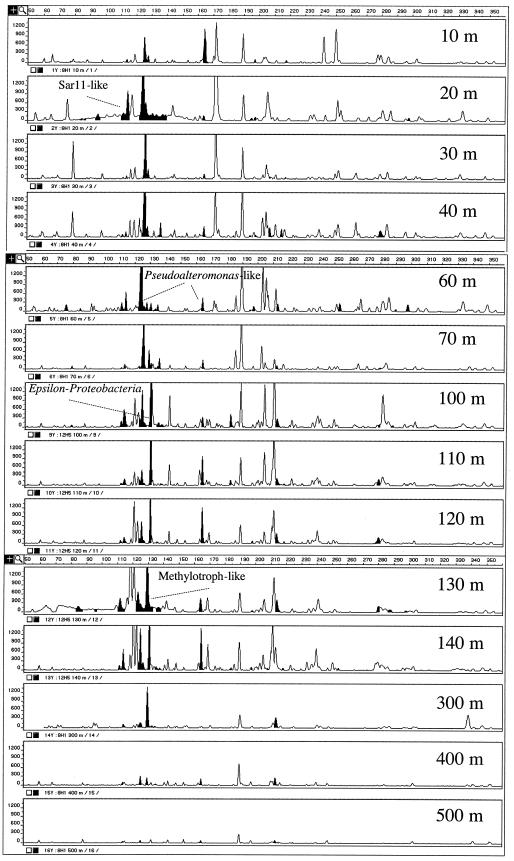
Previous reports on the chemical profiles in the Black Sea indicate that O2 approaches zero at 100 m, with subsurface NO3− and NO2− maxima between 50 and 100 m and H2S increasing below 100 m (3). Biomass samples collected at 10-m intervals showed a variety of bacterial SSU genes at different depth distributions throughout the water column (Fig. 1). Overall, T-RFLP analysis yielded between 50 and 90 peaks for most profiles. The largest TRF peaks (total area of from 1.8 × 105 to 6.2 × 105 fluorescence units) were mainly observed in the 60- to 220-bp size range and included a 172-bp peak at 20 m, a 126-bp peak restricted to 20 to 80 m, a 121- and a 131-bp peak seen between 60 and 140 m, and a large 119-bp peak detected at the 130 m depth range. The aerobic portion of the water column (0 to 60 m) harbored smaller TRFs (total area from 5 × 103 to 1.5 × 104 fluorescence units) occurring more than once, with sizes of 79, 98, 117, 128, 172, 250, 252, and 284 bp. Likewise, TRFs of 212, 282, and 356 bp were highest in the suboxic depth range (60 to 100 m), corresponding to lower oxygen levels and the highest nitrate concentrations. TRFs of 71, 73, and 163 bp had the highest peak areas between 100 and 140 m, where oxygen is absent and sulfide accumulates. All other TRFs were generally of low intensity (total area of <2 × 103 fluorescence units) or occurred in no visible pattern, while some TRFs, such as the 114-bp (area from 0.4 × 103 to 6× 103), 165-bp (area from 0.8 × 103 to 7 × 103), and 190-bp TRFs (area from 2 × 103 to 18 × 103) were present throughout the water column.
FIG. 1.
T-RFLP fingerprints for different depths (in meters) in the Black Sea. The TRF peaks that are represented by SSU genes within a clonal library are indicated in black.
Phylogenetic characterization of bacteria.
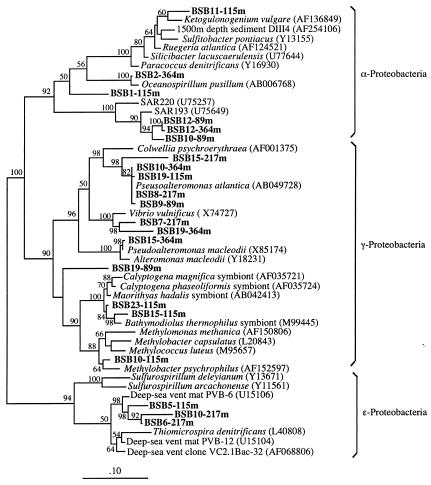
Bacterial 16S rRNA gene libraries were constructed from water samples collected above (20 to 40 m depth), along (89 and 115 m depth), and below (217 and 364 m depth) the chemocline. A total of 62 different SSU genes were identified in the various libraries by a preliminary RFLP screening procedure. Members of the γ-Proteobacteria were present at all depth intervals and represented the dominant phylotype based on clonal frequency in the 20- to 40- (44%), 115- (47.5%), 217- (42.8%), and 364-m (37.5%) libraries. A phylogenetic tree reconstruction of phylotypes in the 89-to-364-m depth range is shown in Fig. 2. Many γ-proteobacteria clones were affiliated with Pseudoalteromonas (>96% sequence identities; e.g., clone BSB19-115m), Vibrio (>95% sequence identities; e.g., clone BSB7-217m), and thiotrophic symbionts (e.g., clone BSB15-115m). One sequence from the 89-m depth interval was not related to any known organism (BSB19-89m), and a second unique sequence from the 115-m depth interval was related to the genus Methylobacter (98% sequence identities; clone BSB10-115m).
FIG. 2.
Phylogenetic analysis of the proteobacteria from the Black Sea using maximum likelihood. The scale bar represents the expected number of changes per sequence position. Black Sea bacteria (in boldface) are indicated by BSB followed by the phylotype number and the depth interval from which the specific phylotype was obtained. The numbers depict bootstrap values obtained for a bootstrap sampling of 100.
Members of the α-Proteobacteria were detected at 20 to 40, 89, 115, and 364 m depth, although they occurred most frequently in the 89- and 115-m-depth clonal libraries. Phylogenetic analysis placed most of the α-proteobacterial phylotypes in a cluster of sequences related to the SAR11 lineage, previously detected in the Sargasso Sea (e.g., clone BSB10-89m), while two phylotypes were related to Ketogulonogenium vulgare (41) (<95% sequence identities; clone BSB11-115m) and to the genus Oceanospirillum (98% sequence identities; clone BSB2-364m).
Phylotypes related to the ɛ-Proteobacteria were detected only in the 115-m and 217-m clonal libraries, accounting for 12.5 and 21.4% of the recovered clones, respectively. None of these phylotypes was related to cultured organisms. Representative ɛ-proteobacterial clones formed a cluster of sequences closely related to uncultured phylotypes previously detected at deep-sea hydrothermal vents (>94% sequence identities; e.g., clone BSB10-217m) (24, 32).
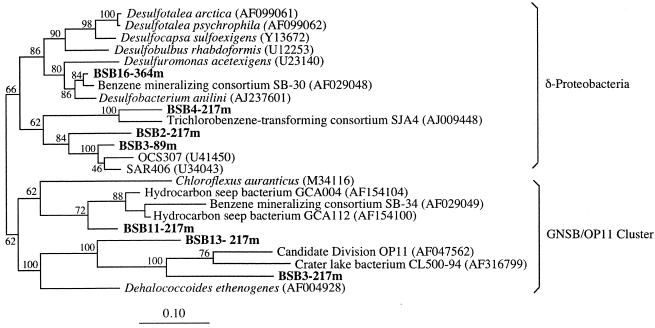
Phylotypes related to the δ-Proteobacteria were detected at 89, 217, and 364 m depth (Fig. 3). These phylotypes were related to members of hydrocarbon-degrading consortia (clones BSB16-364m and BSB4-217m) (29, 43) and to the SAR406 cluster (e.g., clone BSB3-89m) (11). Phylotype BSB16-364m was related to clone SB-30, a member of a sulfate-reducing consortium which mineralized benzene, to members of an aromatic hydrocarbon degrading sulfate-reducing enrichment, and to Desulfobacterium anilini, a bacterium that couples sulfate reduction to the oxidation of aliphatic hydrocarbons, aminobenzoates, aniline, and other aromatic compounds (29, 33). Close relatives to this group of microorganisms, designated as the Eel-1 group, were recently found to be associated with anoxic seep sediments, and they have been considered as potential syntrophic partners which couple sulfate reduction to the anaerobic oxidation of methane by the archaea (ANME group [27]). Phylotype BSB4-217m was related to clone SJA4, a member of a trichlorobenzene-transforming consortium (43).
FIG. 3.
Phylogenetic analysis of the δ-Proteobacteria and GNSB/OP11 cluster from the Black Sea using maximum likelihood (notation as for Fig. 2).
Three additional phylotypes recovered from the 217-m depth profile fell within the GNSB/OP11 cluster (7). Among these sequences, BSB11-217m was related both to clone SB-34, a deeply branching member of the benzene-mineralizing consortium (29), and to phylotypes recovered from deep-sea hydrocarbon seeps (GenBank accession numbers AF154100 and AF154104). Phylotypes BSB13-217m and BSB3-217m were related to the OP11 cluster, a group of environmental clones retrieved from a hydrocarbon-contaminated aquifer (7). The phylogenetic association of both the Black Sea δ-proteobacteria and GSNB/OP11-related clones with hydrocarbon-degrading bacteria was in all cases supported by high bootstrap values and may be indicative of the presence of SRB and related organisms involved in the anaerobic degradation of hydrocarbons or methane in the anoxic zone of the Black Sea water column.
The remaining phylotypes were related to the Cytophagales (2.5% of the total clones) and to the Planctomycetales (6.25% of the total clones). Members of the Cytophagales were only detected at 89 m depth, whereas members of the Planctomycetales were detected at 89, 217, and 364 m depth (data not shown).
Vertical profiling and phylogenetic characterization of archaea.
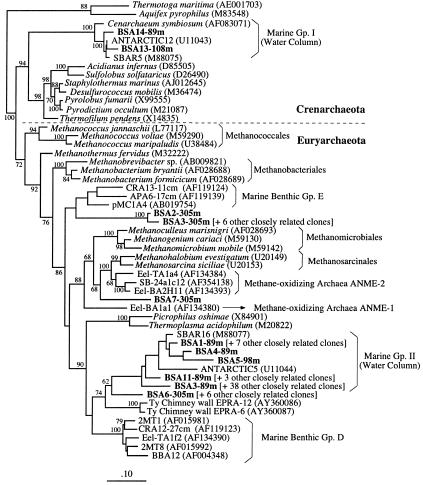
Archaeal 16S rRNA gene libraries were also constructed from water samples collected along (89, 98, and 108 m depth) and below (305 m depth) the chemocline. In order to estimate the depth-related diversity of the archaeal population, 75 recombinant clones were analyzed by RFLP. A total of 11 unique RFLP patterns were detected, and representative clones for each profile were sequenced. A phylogenetic tree reconstruction of the archaea is shown in Fig. 4. The 89-, 98-, and 108-m libraries were dominated by the planktonic Archaea group II (∼90% of the clones recovered from these three depth profiles), among which BSA3-89m represented the most commonly occurring phylotype (accounting for 52% of all archaeal clones). These uncultured archaea are phylogenetically related to the Thermoplasmales. About 10% of the clones from these three depth profiles were affiliated to the planktonic Archaea group I, a widely distributed group of Crenarchaeota commonly detected in marine pelagic environments (5, 10).
FIG. 4.
Phylogenetic analysis of the Black Sea Archaea using maximum likelihood (notation as for Fig. 2).
The 305-m, sulfide- and methane-rich depth interval below the chemocline (Fig. 4) was dominated by archaeal sequences unique to the anoxic region of the Black Sea water column. These sequences fell into three unique clusters: the first one (∼10% of the total clones; e.g., clone BSA2-305m) was placed between the Methanobacteriales and Methanosarcinales and was related to a group of environmental clones isolated from deep-sea sediments (42) (Fig. 4). The second cluster (BSA7-305m; 1.3% of the total clones) was placed between two groups of organisms involved in anaerobic methane oxidation, ANME-1 and ANME-2, previously detected in anoxic marine sediments (2, 13, 27, 35, 38). The third phylotype (clone BSA6-305; ∼10% of the total clones) was related to the order Thermoplasmales and was placed between the planktonic Archaea group II and a group of phylotypes previously detected in reducing marine environments (13, 25, 42).
Depth distribution of bacterial SSU clones.
Using the sequence of the cloned SSU genes, it was possible to assign a terminal MnlI restriction site to identify various TRFs in the microbial fingerprints generated throughout the oxic/anoxic region of the Black Sea (Fig. 5). The clones are presented in groups that correspond to the depth of sample collection. It can be seen that many of the retrieved clones are not restricted to the depth of sampling. Some clones, such as BSB F7-20/40, are detectable in the upper water column. Other clones, such as BSB D9-20/40, are not detectable in the T-RFLP profiles but were retrieved from the clonal libraries. Many clones such as BSB 6-89 demonstrate a disjunct distribution with detection of an appropriately sized TRF in the upper water column separated from a lower water column signal by profiles lacking the TRF. Here, it seems likely that two distinct populations with the same MnlI site are occurring, and the BSB 6-89 clone corresponds to the deeper population. The libraries harbored few clones restricted to the specific depth where the sample was collected. For example, the 20-to-40-m library had 3 of 19 clones with distributions between 10 and 40 m. Each of the libraries generated from samples from 89, 115, 217, and 364 m did not contain a single clone with a depth distribution indicative of the actual sample depth.
FIG. 5.
Depth distribution of T-RFLP peaks associated with various SSU clones based on presence or absence of signal. The depth sample from which the SSU clonal library was created is indicated by the dashed line.
DISCUSSION
The combination of T-RFLP fingerprinting and clonal library construction of 16S rRNA genes from the Black Sea has documented the community transition between oxic and anoxic environments. Our analyses have recovered SSU clones which correspond to the highest T-RFLP peaks, e.g., the Pseudoalteromonas-like (124- and 126-bp) and ɛ-Proteobacteria (131-bp) clones (Fig. 1). The Pseudoalteromonas-like TRFs peaks were highest in the upper part of the water column. This finding is consistent with other studies on marine bacterioplankton, where members of the group were found to be widespread in clonal libraries. Likewise, the ɛ-proteobacteria T-RFLP peaks are highest in the 100- to 120-m depth range. Phylogenetic analysis of clones BSB5-115m, BSB 6-217m, and BSB10-217m suggest they are related to a group of sequences recovered from deep-sea hydrothermal vent microbial mats (Fig. 2) (24). The closest cultured relative to these phylotypes was Thiomicrospira denitrificans, a chemolithotroph capable of coupling sulfide oxidation to nitrate reduction (39). The nitrate-sulfide interface occurred at 100 m in 1988 (3), and the large ɛ-proteobacteria TRF peak in this depth range suggests that thiotrophic bacteria capable of utilizing alternative electron acceptors may be significant microorganisms in the chemocline of the Black Sea water column.
The second highest TRF corresponds to a γ-proteobacterial phylotype (BSB10-115) related to other organisms involved in methanotrophic metabolism. The 130-bp TRF was highest at the 130- and the 300-m depth range, with minor peaks at 70 and 400 m (Fig. 1). The 130 m depth corresponds approximately to the upper boundary of the H2S and CH4 zone of the chemocline (3, 31). The 300 m depth is a region where anaerobic methane oxidation rates ranged from 0.5 to 10 nM day−1 (31) and geochemical evidence of archaeal involvement in anaerobic methane oxidation was obtained (34). Additional lines of evidence supporting the occurrence of thiotrophic and/or methanotrophic bacteria at the upper boundary of the chemocline are the following: (i) the occurrence of high rates of sulfide oxidation in anoxic water at the top of the chemocline, concurrent with high rates of dark CO2 assimilation (16); (ii) the previously reported isolation of thiotrophic bacteria from the suboxic sulfide zone, between 83 and 130 m depth (15); (iii) the additional retrieval, in this study, of one phylotype closely related to Methylobacter psychrophilus from the 115-m depth profile (BSB10-115m; Fig. 3); and (iv) the observation that pmoA genes, indicative of aerobic methane oxidation, were only detected within the 108-to-115-m depth range (data not shown). Interestingly, most known sulfide- and methane-oxidizing bacteria use oxygen as an electron acceptor. Therefore, the presence of these organisms in the suboxic, lower boundary of the chemocline suggests two alternate hypotheses: (i) electron acceptors other than oxygen are available for microbial oxidations at the upper boundary of the Black Sea chemocline, although all the Black Sea thiotrophic isolates tested in one study used oxygen as the only electron acceptor (15); (ii) the aerobic sulfide- and methane-oxidizing bacteria may be responsible for the removal of oxygen down to suboxic levels.
Finally, a suite of small TRF peaks were identified which correspond to known sulfate reducers, sulfide oxidizers, and the SAR11 cluster. Although δ-proteobacteria were detected in the 89-, 217-, and 364-m depth clone libraries (Fig. 4), only the clone (BSB 2-217) was observed in the T-RFLP fingerprint at 130 m. Although a few dominant peaks were discovered by screening of clonal libraries (e.g., 126, 124, and 131 bp), many of the cloned SSU genes are from minor peaks, reiterating a clonal bias in the E. coli recombinant libraries as has been suggested previously (4).
Diversity and depth-related distribution of Archaea.
The diversity of the Archaea in the Black Sea water column appeared to be quite low compared to the bacterial diversity. The upper depth profiles, located above the chemocline, were largely colonized by members of the marine planktonic group II (related to the order Thermoplasmales), whereas only two phylotypes fell into the planktonic group I (Crenarchaeota). These groups designate two main clusters of Archaea that are ubiquitous in marine pelagic and, to some extent, benthic environments and that so far have resisted cultivation (Fig. 5) (5, 10, 25, 42). In contrast to the relative homogeneity of the archaea found in the oxic upper water column, the 305-m depth profile revealed a more complex structure. This distribution pattern is consistent with a previous comparative analysis of archaea in oxic and anoxic deep-sea sediments (42). Of the three discrete groups, unique to the Black Sea anoxic waters, identified at the 305-m depth profile, phylotype BSA7-305m is of particular interest, as it is related to a group of archaeal sequences designated ANME-2 (Fig. 4). This archaeal group was recently detected in several anoxic benthic marine environments, including sediments from deep-sea seeps (2, 13, 27), coastal sediments (38), gas hydrates (21), and geothermally heated sediments (35). These studies suggest that ANME-2, together with a related archaeal group, designated ANME-1, is involved in the anaerobic oxidation of methane. Preliminary data based on fluorescent in situ hybridization suggested that anaerobic methane oxidation could be mediated by a syntrophic consortium of ANME-2 archaea and SRB of the genus Desulfosarcina (2, 27). More recently, experiments that combined fluorescent in situ hybridization and ion mass spectrometry analyses suggested that single archaeal cells of the AMNE-1 group, as well as monospecific cell aggregates of the same group, could also oxidize methane anaerobically (28). So far, detection of the archaeal groups ANME-1 and -2 has been limited to sedimentary environments, including an early study that reported the presence of a carbonate-associated microbial mat in the Black Sea. The organisms present in this mat were both morphologically similar to the ANME-1 archaea and capable of anaerobic methane oxidation (30). Therefore, the recovery of phylotype BSA7-305m from the anoxic zone of the Black Sea water column is the first report of a member of the archaeal group ANME-2 in planktonic environments and suggests its involvement in the anaerobic oxidation of methane. In conclusion, the Black Sea is a good model system for observing how microbial communities are structured in the transition between oxic and anoxic environments. In the upper water column, Pseudoalteromonas-like groups appear to predominate, while ɛ-Proteobacteria are significant in the oxic/anoxic transition zone. Additionally, the presence of δ-Proteobacteria and ANME-2-like archaeal clones deeper in the water column implies that anaerobic oxidation processes may be an important part of the biogeochemistry of the Black Sea.
Acknowledgments
This research was supported in part by grants from of the U.S. Department of Energy Biotechnological Investigations—Ocean Margin Program (BIOMP; DE-FG02-00ER62978) and the National Science Foundation (NSF; OCE 98-72024) to L.J.K. and by partial funding from Rutgers, the State University of New Jersey, and the New Jersey Agricultural Experimental Station to C.V. Field sampling was supported by an NSF grant to Bess B. Ward (OCE 86-14470).
We thank Jim Murray for leadership of the Black Sea expeditions in 1998, Louis Codispoti and Gernot Friederich for use of a submersible pump in water sampling, and Lora McGuinness for assistance in sample processing.
REFERENCES
- 1.Avaniss-Aghajani, E., K. Jones, D. Chapman, and C. Brunk. 1994. A molecular technique for identification of bacteria using small subunit ribosomal RNA sequences. BioTechniques 17:144-146. [PubMed] [Google Scholar]
- 2.Boetius, A., K. Ravenschlag, C. J. Schubert, D. Rickert, F. Widdel, A. Gleseke, R. Amann, B. B. Jorgensen, U. Witte, and O. Pfannkuche. 2000. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407:623-626. [DOI] [PubMed] [Google Scholar]
- 3.Codispoti, L. A., G. E. Friederich, J. W. Murray, and C. M. Sakamoto. 1991. Chemical variability in the Black Sea: implications of continuous vertical profiles that penetrated the oxic/anoxic interface. Deep Sea Res. 38(Suppl. 2):S691-S710. [Google Scholar]
- 4.Cottrell, M. T., and D. L. Kirchman. 2000. Community composition of marine bacterioplankton determined by 16S rRNA gene clone libraries and fluorescence in situ hybridization. Appl. Environ. Microbiol. 66:5116-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLong, E. F. 1992. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 89:5685-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeSoete, G. 1983. A least squares algorithm for fitting additive trees to proximity data. Psychometrica 48:621-626. [Google Scholar]
- 7.Dojka, M. A., P. Hugenholtz, S. K. Haack, and N. R. Pace. 1998. Microbial diversity in a hydrocarbon- and chlorinated-solvent- contaminated aquifer undergoing intrinsic bioremediation. Appl. Environ. Microbiol. 64:3869-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 19:783-791. [DOI] [PubMed] [Google Scholar]
- 9.Felsenstein, J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 17:368-376. [DOI] [PubMed] [Google Scholar]
- 10.Fuhrman, J. A., K. McCallum, and A. A. Davis. 1992. Novel major archaebacterial group from marine plankton. Nature 356:148-149. [DOI] [PubMed] [Google Scholar]
- 11.Gordon, D. A., and S. J. Giovannoni. 1996. Detection of stratified microbial populations related to Chlorobium and Fibrobacter species in the Atlantic and Pacific Oceans. Appl. Environ. Microbiol. 62:1171-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hines, M. E., R. S. Evans, B. R. S. Genthner, S. G. Willis, S. Friedman, J. N. Rooney-Varga, and R. Devereux. 1999. Molecular phylogenetic and biogeochemical studies of sulfate-reducing bacteria in the rhizosphere of Spartina alterniflora. Appl. Environ. Microbiol. 65:2209-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinrichs, K. U., J. M. Hayes, S. P. Sylva, P. G. Brewer, and E. F. DeLong. 1999. Methane-consuming archaebacteria in marine sediments. Nature 398:802-805. [DOI] [PubMed] [Google Scholar]
- 14.Jannasch, H. W. 1991. Microbial processes in the Black Sea water column and top sediment: an overview, p. 271-286. In E. Izdar and J. W. Murray (ed.), Black Sea oceanography. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 15.Jannasch, H. W., C. O. Wirsen, and S. J. Molyneaux. 1991. Chemolithotrophic sulfur-oxiding bacteria from the Black Sea. Deep-Sea Res. 38:S1105-S1120. [Google Scholar]
- 16.Joergensen, B. B., H. Fossing, C. O. Wirsen, and H. W. Jannasch. 1991. Sulfide oxidation in the anoxic Black Sea chemocline. Deep-Sea Res. 38:S1083-S1103. [Google Scholar]
- 17.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules. Academic Press, New York, N.Y.
- 18.Kerkhof, L. J., and P. F. Kemp. 1999. Small ribosomal RNA content in marine Proteobacteria during non-steady state growth. FEMS Microb. Ecol. 30:253-260. [DOI] [PubMed] [Google Scholar]
- 19.Kishino, H., and M. Hasegawa. 1989. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in Hominoidea. J. Mol. Evol. 29:170-179. [DOI] [PubMed] [Google Scholar]
- 20.Kitts, C. L. 2001. Terminal restriction fragment patterns: a tool for comparing microbial communities and assessing community dynamics. Curr. Issues Intest. Microbiol. 2:17-25. [PubMed] [Google Scholar]
- 21.Lanoil, B. D., R. Sassen, M. T. La Duc, S. T. Sweet, and K. H. Nealson. 2001. Bacteria and Archaea physically associated with Gulf of Mexico gas hydrates. Appl. Environ. Microbiol. 67:5143-5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacGregor, B. J., D. P. Moser, B. J. Baker, E. W. Alm, M. Maurer, K. H. Nealson, and D. A. Stahl. 2001. Seasonal and spatial variability in Lake Michigan sediment small-subunit rRNA concentrations. Appl. Environ. Microbiol. 67:3908-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madrid, V. M., G. T. Taylor, M. I. Scranton, and A. Y. Chistoserdov. 2001. Phylogenetic diversity of bacterial and archaeal communities in the anoxic zone of the Cariaco Basin. Appl. Environ. Microbiol. 67:1663-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moyer, C. L., F. C. Dobbs, and D. M. Karl. 1995. Phylogenetic diversity of the bacterial community from a microbial mat at an active, hydrothermal vent system, Loihi Seamount, Hawaii. Appl. Environ. Microbiol. 61:1555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munson, M. A., D. B. Nedwell, and T. M. Embley. 1997. Phylogenetic diversity of archaea in sediment samples from a coastal salt marsh. Appl. Environ. Microbiol. 63:4729-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray, J. W., H. W. Jannasch, S. Honjo, R. F. Anderson, W. S. Reeburgh, Z. Top, G. E. Friederich, L. A. Codispoti, and E. Izdar. 1989. Unexpected changes in the oxic/anoxic interface in the Black Sea. Nature 338:411-413. [Google Scholar]
- 27.Orphan, V. J., K. U. Hinrichs, W. Ussler, C. K. Paull, L. T. Taylor, S. P. Sylva, J. M. Hayes, and E. F. Delong. 2001. Comparative analysis of methane-oxidizing archaea and sulfate-reducing bacteria in anoxic marine sediments. Appl. Environ. Microbiol. 67:1922-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orphan, V. J., C. H. House, K. U. Hinrichs, K. D. McKeegan, and E. F. DeLong. 2002. Multiple archaeal groups mediate methane oxidation in anoxic cold seep sediments. Proc. Natl. Acad. Sci. USA 99:7663-7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phelps, C. D., L. J. Kerkhof, and L. Y. Young. 1998. Molecular characterization of a sulfate-reducing consortium which mineralizes benzene. FEMS Microbiol. Ecol. 27:269-279. [Google Scholar]
- 30.Pimenov, N. V., I. I. Rusanov, M. N. Poglazova, L. L. Mityushina, D. Y. Sorokin, V. N. Khmelenina, and Y. A. Trotsenko. 1997. Bacterial mats on coral-like structures at methane seeps in the Black Sea. Microbiology 66:354-360. [Google Scholar]
- 31.Reeburgh, W. S., B. B. Ward, S. C. Whalen, K. A. Sanbeck, K. A. Kilpatrick, and L. J. Kerkhof. 1991. Black Sea methane geochemistry. Deep-Sea Res. 38:S1189-S1210. [Google Scholar]
- 32.Reysenbach, A. L., K. Longnecker, and J. Kirshtein. 2000. Novel bacterial and archaeal lineages from an in situ growth chamber deployed at a Mid-Atlantic Ridge hydrothermal vent. Appl. Environ. Microbiol. 66:3798-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnell, S., F. Bak, and N. Pfennig. 1989. Anaerobic degradation of aniline and dihydroxybenzenes by newly isolated sulfate-reducing bacteria and description of Desulfobacterium anilini. Arch. Microbiol. 152:556-563. [DOI] [PubMed] [Google Scholar]
- 34.Schouten, S., S. G. Wakeham, and J. S. S. Damste. 2001. Evidence for anaerobic methane oxidation by archaea in euxinic waters of the Black Sea. Org. Geochem. 32:1277-1281. [Google Scholar]
- 35.Teske, A., K. U. Hinrichs, V. Edgcomb, A. D. Gomez, D. Kysela, S. P. Sylva, M. L. Sogin, and H. W. Jannasch. 2002. Microbial diversity of hydrothermal sediments in the Guaymas Basin: evidence for anaerobic methanotrophic communities. Appl. Environ. Microbiol. 68:1994-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thamdrup, B., R. Rossello-Mora, and R. Amann. 2000. Microbial manganese and sulfate reduction in Black Sea shelf sediments. Appl. Environ. Microbiol. 66:2888-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thiel, V., J. Peckmann, H. H. Richnow, U. Luth, J. Reitner, and W. Michaelis. 2001. Molecular signals for anaerobic methane oxidation in Black Sea seep carbonates and a microbial mat. Mar. Chem. 73:97-112. [Google Scholar]
- 38.Thomsen, T. R., K. Finster, and N. B. Ramsing. 2001. Biogeochemical and molecular signatures of anaerobic methane oxidation in a marine sediment. Appl. Environ. Microbiol. 67:1646-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Timmer-ten Hoor, A. 1975. A new type of thiosulfate oxidizing, nitrate reducing microorganism: Thiomicrospira denitrificans sp. nov. Neth. J. Sea Res. 9:344-350. [Google Scholar]
- 40.Urakawa, H., K. Kita-Tsukamoto, and K. Ohwada. 1999. Microbial diversity in marine sediments from Sagami Bay and Tokyo Bay, Japan, as determined by 16S rRNA gene analysis. Microbiology 145:3305-3315. [DOI] [PubMed] [Google Scholar]
- 41.Urbance, J. W., B. J. Bratina, S. F. Stoddard, and T. M. Schmidt. 2001. Taxonomic characterization of Ketogulonigenium vulgare gen. nov., sp. nov. and Ketogulonigenium robustum sp. nov., which oxidize l-sorbose to 2-keto-l-gulonic acid. Int. J. Syst. Evol. Microbiol. 51:1059-1070. [DOI] [PubMed] [Google Scholar]
- 42.Vetriani, C., H. W. Jannasch, B. J. MacGregor, D. A. Stahl, and A. L. Reysenbach. 1999. Population structure and phylogenetic characterization of marine benthic archaea in deep-sea sediments. Appl. Environ. Microbiol. 65:4375-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Wintzingerode, F., B. Selent, W. Hegemann, and U. B. Gobel. 1999. Phylogenetic analysis of an anaerobic, trichlorobenzene-transforming microbial consortium. Appl. Environ. Microbiol. 65:283-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weeks, D. P., N. Beerman, and O. M. Griffith. 1986. A small-scale five-hour procedure for isolating multiple samples of CsCl-purified DNA: application to isolations from mammalian, insect, higher plant, algal, yeast, and bacterial sources. Anal. Biochem. 152:376-385. [DOI] [PubMed] [Google Scholar]