Abstract
When exponentially growing Vibrio cholerae cells were shifted from 37°C to various lower temperatures, it was found that the organism could adapt and grow at temperatures down to 15°C, below which the growth was completely arrested. There was no difference between the patterns of the cold shock responses in toxinogenic and nontoxinogenic strains of V. cholerae. Gel electrophoretic analyses of proteins of cold-exposed cells revealed significant induction of two major cold shock proteins (Csps), whose molecular masses were 7.7 kDa (CspAVC) and 7.5 kDa (CspV), and six other Csps, most of which were much larger. We cloned, sequenced, and analyzed the cspV gene encoding the CspV protein of V. cholerae O139 strain SG24. Although CspAVC and CspV have similar kinetics of synthesis and down-regulation, the corresponding genes, cspA and cspV, which are located in the small chromosome, are not located in the same operon. A comparative analysis of the kinetics of synthesis revealed that the CspV protein was synthesized de novo only during cold shock. Although both CspAVC and CspV were stable for several hours in the cold, the CspV protein was degraded rapidly when the culture was shifted back to 37°C, suggesting that this protein is probably necessary for adaptation at lower temperatures. Northern blot analysis confirmed that the cspV gene is cold shock inducible and is regulated tightly at the level of transcription. Interestingly, the cspV gene has a cold shock-inducible promoter which is only 12 nucleotides from the translational start site, and therefore, it appears that no unusually long 5′ untranslated region is present in its mRNA transcript. Thus, this promoter is an exception compared to other promoters of cold shock-inducible genes of different organisms, including Escherichia coli. Our results suggest that V. cholerae may use an alternative pathway for regulation of gene expression during cold shock.
Most human pathogens have two distinct phases of their life cycle, one when they are within their host and the other when they are in their natural environmental niche (9, 14, 25). Changes in environmental parameters lead to changes in gene expression by microbial pathogens. Thus, pathogens need an exquisite gene regulation network to survive in two such distinct environments. Vibrio cholerae, a gram-negative enteric pathogen whose natural environmental niche includes various aquatic bodies (6, 7, 8, 18, 22), is responsible for the severe diarrheal disease cholera and has developed a mechanism to switch gene expression in order to overcome various stressful barriers (16). For example, when V. cholerae cells are in the aquatic environment, they have to face fluctuations in various physicochemical parameters. When they are ingested by humans, they have to tolerate a low gastric pH, increased temperature, activities of various intestinal proteases, and other unknown intestinal factors, which profoundly influence the gene expression of the pathogen for combating such stressful stimuli. Currently, little information is available regarding the molecular mechanisms that enable V. cholerae to survive fluctuating physical and chemical environmental parameters, such as salinity, oxygen and nutritient availability, osmolarity, pH, atmospheric pressure, and temperature. Among all these parameters environmental temperature plays a very crucial role in growth and survival. It has recently been shown by using remote sensing data that the occurrence of cholera cases has a high correlation with the annual cycle of sea surface temperature (7, 18).
V. cholerae is remarkable for its pandemic potential, and it can spread globally. The pandemic nature of V. cholerae indicates that this organism can adapt and survive in diverse environmental conditions (14). During its spread the pathogen must face drastic changes in environmental temperatures. Moreover, V. cholerae often encounters a sudden temperature downshift (i.e., cold shock) as a result of excretion from a human whose body temperature is 37°C. Colwell and Huq (8) showed that among various changes in environmental conditions, a reduction in temperature is important in inducing V. cholerae cells to enter a dormant state, termed the viable but nonculturable (VBNC) state, in which metabolically active cells cannot be cultured on microbiological media. Microcosm studies showed that VBNC cells could remain viable in the environment for years and continue to be capable of causing disease (8). Unfortunately, detailed information regarding the molecular basis of the cold shock response in V. cholerae is not available. Such information is also important in understanding how this pathogen manages to survive during interepidemic periods.
In Escherichia coli a temperature downshift from 37 to 10°C causes transient inhibition of synthesis of most cellular proteins, resulting in a growth lag period called the acclimation phase. During this period at least 16 different cold shock proteins (Csps) are induced (29); one of these, CspA, whose molecular mass is 7.4 kDa, is dramatically induced and is considered essential for cold adaptation (11, 29). The amino acid sequence of CspA exhibits 43% identity to the cold shock domain of the eukaryotic Y-box proteins, which interact with RNA and DNA to regulate their functions (17, 26, 34). It has been proposed that CspA of E. coli functions as an RNA chaperone to prevent the formation of secondary structures in RNA molecules at low temperatures (29). In addition to CspA, eight other very similar proteins (CspB to -I), constituting the CspA family of proteins, have been identified (29). However, not all of these proteins are cold shock inducible (29). Another unique feature of the cold shock-inducible cspA homologues is the presence of an unusually long 5′ untranslated region (5′-UTR) in the mRNA transcript that plays an important role in the regulation of cold shock gene expression (29).
In this report, we show that V. cholerae has a cold shock response analogous to but distinct from that of E. coli. In V. cholerae two small Csps, whose molecular masses were 7.7 and 7.5 kDa (CspAVC and CspV, respectively), were induced simultaneously upon sudden exposure to cold. However, only CspV appeared to be a true Csp. The cspV gene, located in the small chromosome and encoding the CspV protein, was cloned from strain SG24 of V. cholerae O139 and sequenced. Analysis of the sequence indicated that its promoter region does not contain a 5′-UTR, a common feature found in homologous genes characterized from other organisms. Northern blot analysis indicated that expression of the cspV gene is tightly regulated at the level of transcription.
(Part of this work was presented at the 5th International Conference on Molecular Epidemiology and Evolutionary Genetics of Infectious Diseases, Hyderabad, India, 12 to 16 November 2000.)
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The experiments were performed with the well-characterized, cholera toxin-producing V. cholerae O139 Bengal strain SG24 (3, 15, 24). E. coli DH5α (Promega, Madison, Wis.) and plasmid pUC19 (New England Biolabs, Beverly, Mass.) were used for cloning. E. coli strain BL21(DE3) and plasmid pET20b(+) (Novagen, Madison, Wis.) were used for expression purposes.
V. cholerae cells were grown in a gyratory shaker at 37°C in nutrient broth (Difco, Detroit, Mich.) containing 0.1 M NaCl (pH 8.0), and for growth at low temperatures, cells were grown aerobically in a rotary-action water bath shaker. Bacterial growth was monitored spectrophotometrically at a wavelength of 585 nm (optical density at 585 nm [OD585]). Viable plate counts were determined on nutrient agar plates (22) and were expressed in CFU per milliliter. Cells were maintained at −70°C in nutrient broth with 15% (vol/vol) glycerol. E. coli cells were grown in Luria-Bertani medium (1) at 37°C. When necessary, ampicillin was added at a final concentration of 100 μg ml−1.
Labeling of cellular proteins with [35S]methionine.
For labeling of cellular proteins with [35S]methionine (1,000 Ci mmol−1; Amersham, Little Chalfont, United Kingdom), the cells were grown in a modified M9 minimal medium, developed in this study and designated MM9 medium, whose composition was the same as the composition described previously (31) except that the concentration of ammonium chloride was 1.1% instead of 0.1%. V. cholerae cells showed optimal growth and efficient labeling when MM9 medium was used (data not shown). V. cholerae cells used for cold shock experiments were grown to the mid-exponential phase (OD585, 0.55) at 37°C in MM9 medium, and aliquots of the culture were shifted immediately to different low temperatures. The cultivability of a culture at a low temperature was assayed by plate counting (22) at different times after the temperature shift. To analyze Csps, 3 ml of the culture was labeled with [35S]methionine (10 μCi ml−1) for 10 min at 37°C, which served as a control, and for 30 min after different periods of exposure to 15°C (see Fig. 2). Sometimes cells were labeled with [35S]methionine for an extended period of time either at 37°C or at 15°C. For pulse-chase experiments cells were labeled with [35S]methionine for different periods of time at 15°C and then chased by adding nonradioactive methionine (Sigma-Aldrich, Bangalore, India) to a final concentration of 0.2 M (see Fig. 3). In some experiments (see Fig. 3), a V. cholerae culture was transferred from 37 to 15°C, labeled with [35S]methionine for 1 h, and then immediately shifted back to 37°C and chased with nonradioactive methionine as described above.
FIG. 2.
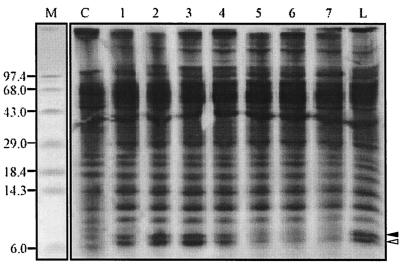
Autoradiogram showing the kinetics of induction of CspAVC and CspV. Pulse-labeling at 15°C was performed for the following periods: lane 1, 0 to 30 min; lane 2, 30 to 60 min; lane 3, 60 to 90 min; lane 4, 90 to 120 min; lane 5, 120 to 150 min; lane 6, 150 to 180 min; and lane 7, 270 to 300 min. Lane C contained cells that were labeled for 10 min at 37°C. Lane L contained cells that were labeled at 15°C for 5 h. The solid and open arrowheads indicate the positions of CspAVC and CspV, respectively. Lane M contained standard molecular mass markers, whose sizes (in kilodaltons) are indicated on the left.
FIG. 3.
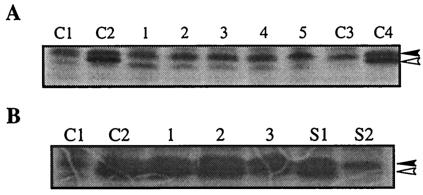
Stability of CspAVC and CspV. (A) Cells were pulse-labeled for the following periods after they were shifted back from 15 to 37°C: lane 1, 0 to 15 min; lane 2, 15 to 30 min; lane 3, 30 to 45 min; lane 4, 45 to 60 min; and lane 5, 0 to 3.5 h. Lanes C1 and C2 contained cells that were labeled for 10 min at 37°C and for 60 to 90 min at 15°C, respectively. Lanes C3 and C4 contained cells that were labeled for 3.5 h at 37°C and at 15°C, respectively. The solid and open arrowheads indicate the positions of CspAVC and CspV, respectively. (B) Lanes C1 and C2 contained cells that were labeled for 10 min at 37°C and for 60 to 90 min at 15°C, respectively. Cells were labeled for 2 h at 15°C and then chased with nonradioactive methionine (see Materials and Methods) for the following periods: lane 1, 1 h; lane 2, 3 h; and lane 3, 12 h. Lanes S1 and S2 contained cells that were labeled at 15°C for 1 h, shifted back to 37°C, and chased with nonradioactive methionine for 15 and 30 min, respectively. The solid and open arrowheads indicate the positions of CspAVC and CspV, respectively.
Protein analysis.
For better resolution of low-molecular-weight proteins we used the previously described 16.5% Tris-Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (T-SDS-PAGE) system (32). Prestained protein molecular mass standards, including high-molecular-mass standards (14.3 to 200 kDa) and low-molecular-mass standards (3 to 43 kDa) (Gibco-BRL, Gaithersburg, Md.) or SeeBlue prestained standards (210 to 4 kDa; Invitrogen, Carlsbad, Calif.), were used when they were needed. Concentrations of protein samples were determined by the Bradford method (1). For two-dimensional gel electrophoresis (2D-E), whole-cell protein samples were prepared from V. cholerae O139 strain SG24 cells by sonication (B. Braun Biotech, Allentown, Pa.), followed by suspension in lysis buffer as described previously (27, 28). Separation in the first dimension was performed by nonequilibrium pH gradient electrophoresis as described previously (27, 28) with a Bio-Rad Protean IIxi system by using ampholines (Sigma-Aldrich) in the pH range from 3 to 10. For the second dimension, 16.5% T-SDS-PAGE was performed as described above. For fluorography, gels were treated with the Amplify fluorographic reagent (Amersham) as directed by the manufacturer, and this was followed by drying and autoradiography.
Antibiotic treatment.
Chloramphenicol was used at a final concentration of 3 μg ml−1 as described previously (10), and V. cholerae cells were labeled with [35S]methionine before or after addition of chloramphenicol both at 37°C and after cells were shifted to 15°C.
PCR amplification, cloning, and sequencing of the cspV gene of V. cholerae.
The primers used to amplify the cspV gene were designed from the available genome sequence data for V. cholerae O1 strain N16961 (12). The cspV gene was PCR amplified from genomic DNA of V. cholerae O139 strain SG24 by using Taq DNA polymerase (Invitrogen) and primers VCL1 (5′-GCGAATTCATTAATTCTGAAAGA-3′) and VC2 (5′-AAGGATCCGTAAATAATGAGCGGGGAGCAA-3′), which introduced EcoRI and BamHI cleavage sites (underlined) for cloning a ∼665-bp amplicon into the EcoRI-BamH1-digested vector DNA pUC19. The resulting recombinant plasmid containing the cspV gene was designated pPD665. The PCR-amplified insert DNA was directly sequenced with an automated DNA sequencer (ABI Prism model 377). A 210-bp fragment spanning the predicted promoter region along with part of the N-terminal coding sequence of the cspV open reading frame (ORF) was amplified by PCR by using the 665-bp DNA fragment as the template with primers VCL2 (5′-TGAATTCTCGATACCCTTCGTTCAACC-3′) and VC1 (5′-ACGGTACCATCGTTGCCACCGTTGTCTT-3′) and was used as a probe.
Construction of pET20b(+)-cspV for overexpression of the cspV gene.
For overexpression of cspV, a 288-bp XbaI-BamHI fragment containing the complete ORF plus the intact Shine-Dalgarno sequence of the gene was obtained by digesting pPD665. The gel-purified fragment was ligated to XbaI- and BamHI-double-digested pET20b(+) overexpression vector, which contained a T7 promoter under control of the lac operator (Novagen). The ligation mixture was transformed into E. coli DH5α cells, and the recombinant plasmid was designated pET20b(+)-cspV. Then cspV expression plasmid pET20b(+)-cspV was transformed into E. coli strain BL21(DE3), which contained T7 RNA polymerase. The transformed cells were grown in Luri-Bertani medium supplemented with 0.1% glucose at 37°C with shaking to the mid-logarithmic phase (OD585, 0.55). At this cell density, isopropyl-β-d-thiogalactopyranoside (IPTG) (final concentration, 1.0 mM) was added for induction of expression of the cspV gene, and incubation was continued for another 2 h; this was followed by harvesting of the cells and analysis of the overexpressed protein by T-SDS-PAGE.
Western blot analysis.
Total cellular proteins were separated by T-SDS-PAGE and transferred to nitrocellulose in a transblot apparatus (Bio-Rad) as described previously (1). The blots were incubated for 1 h with rabbit antibody raised against the CspA protein of E. coli (kindly provided by M. Inouye) at a dilution of 1:500, and this was followed by treatment with anti-rabbit immunoglobulin G conjugated with alkaline phosphatase (Gibco-BRL). Color was developed by using standard methods described previously (1).
Northern blot procedure.
Total RNA of V. cholerae cells, which were grown at different temperatures for various times, was isolated by using an RNeasy kit (Qiagen GmbH, Hilden, Germany) and the protocol described by the manufacturer. For Northern analysis, the same amount (∼20 μg) of RNA sample was loaded in each lane and separated in a 1.2% agarose gel containing formaldehyde (31); this was followed by staining with ethidium bromide and transfer of the RNA from the gel to a Nytran membrane (Hybond N+; Amersham). The PCR-amplified 210-bp DNA segment of the cspV gene of V. cholerae (see above) was labeled with [α-32P]dCTP by using an NEBlot random priming kit (New England Biolabs) as instructed by the vendor and was used as a probe. Hybridization was carried out at 60°C, and this was followed by washing, exposure to X-ray film, and autoradiography.
Nucleotide sequence and other computer-based analyses.
Restriction enzyme recognition sites were determined with the DNASIS program (Hitachi Corporation). A nucleotide similarity search was performed by using the BLASTN program (www.ncbi.nlm.nih.gov). The promoter region was predicted by using the Neural Network Promoter Prediction program (www.fruitfly.org/seq_tools/promoter.html), and the ORF region was predicted by using the ORF Finder program (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). All the primers were designed by using the Primer3 program (http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi). Theoretical isoelectric points (pI) and molecular weights (Mw) of putative cold shock gene-encoded proteins of V. cholerae were determined by using the ExPASy compute pI/Mw tool (www.expasy.ch/tools/#align).
Nucleotide sequence accession number.
The cspV gene sequence determined in this study has been deposited in the GenBank database under accession number AF409091.
RESULTS AND DISCUSSION
Minimal growth temperature of V. cholerae.
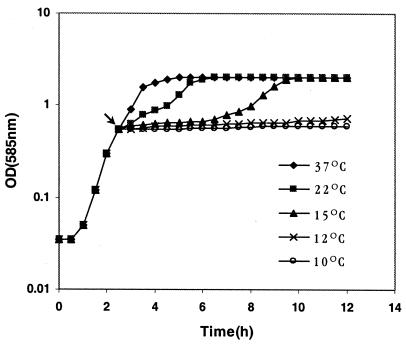
When exponentially growing V. cholerae O139 cells were transferred from 37 to 10 or 12°C, growth stopped immediately, and the cells exhibited negligible growth for more than 12 h, indicating that these low temperatures are growth nonpermissive for V. cholerae (Fig. 1). These results are similar to the results obtained for other V. cholerae strains (24) belonging to different serotypes and biotypes (data not shown). There was no significant difference in the cell counts (∼7 × 108 CFU ml−1) between the cultures before the temperature downshift and the cultures after the temperature downshift, indicating that at the lower temperatures the cells were unable to replicate. Our results are consistent with the microcosm study conducted by Singleton et al. (33). These authors suggested that prolonged survival at 10°C may ultimately induce the VBNC state in V. cholerae (33). However, when V. cholerae cells were shifted from 37 to 15°C, there was a growth lag of about 4 h, after which growth resumed, and within 6 h of the shift the culture reached the stationary phase (Fig. 1). These results suggest that 15°C is the minimal temperature for growth of V. cholerae and that below this temperature the cells are unable to grow. In contrast, E. coli cells exhibit a similar type of cold shock response (11) at a much lower temperature (10°C), indicating that V. cholerae is more sensitive to cold than E. coli. When V. cholerae cells were shifted from 37 to 22°C, the length of the acclimation phase was reduced drastically, to only 30 min (Fig. 1), indicating that under laboratory conditions a downshift to 22°C has a minor effect on the growth of V. cholerae.
FIG. 1.
Growth kinetics of V. cholerae O139 strain SG24 at various temperatures. The arrow indicates the OD585 at which cultures were shifted from 37°C to low temperatures.
Simultaneous induction of a 7.7-kDa Csp (CspAVC) and a 7.5-kDa Csp (CspV) occurs at 15°C.
To determine the cold shock-induced proteins of V. cholerae, cells were pulse-labeled with [35S]methionine at 15°C and then subjected to T-SDS-PAGE and autoradiography as described in Materials and Methods. The autoradiogram obtained revealed significant induction of two closely migrating protein bands at molecular masses of approximately 7.7 and 7.5 kDa (Fig. 2), which were designated CspAVC and CspV, respectively. This is in contrast to the findings obtained for E. coli, in which only one major protein, the 7.4-kDa CspA protein, was induced at a significant level (11). The dramatic increases in the intensities of CspAVC and CspV compared to the intensities of other cellular proteins of V. cholerae suggest that these two proteins are likely to be the major Csps of V. cholerae. Furthermore, it appeared from the autoradiogram that in addition to CspAVC and CspV another low-molecular-mass protein band in the 8.3-kDa region appeared to be slightly up-regulated at 15°C. However, small amounts of CspAVC were also detected at 37°C (Fig. 2). Thus, it is highly likely that CspV is induced only during exposure of V. cholerae cells to a low temperature and is truly a product of a cold shock-inducible gene.
Strains belonging to different serotypes and biotypes, as well as toxinogenic and nontoxinogenic groups (24), had cold shock-induced protein profiles similar to that of V. cholerae O139 strain SG24 (data not shown). In contrast, Carroll et al. (5) recently reported induction of only one 8-kDa protein in V. cholerae cells upon exposure to cold. The discrepancy between the results of these authors and our results was probably due to poor separation of low-molecular-weight proteins by conventional Tris-glycine SDS-PAGE. Simultaneous induction of significant amounts of two small Csps appears to be typical for V. cholerae cells and has not been reported for any other bacterium examined so far. Further analyses of the kinetics of induction revealed that synthesis of both proteins reached the maximum level within 30 to 90 min after the shift (Fig. 2, lanes 1 to 3). The maximal amount of each of the proteins was about 7% of the total cellular proteins, and thus, during cold shock these proteins accounted for about 14% of the total proteins within 1 to 1.5 h. Although the de novo synthesis of both the proteins decreased significantly just after 2 h after the shift, the synthesized proteins were found to be stable for at least 5 h at 15°C (Fig. 2).
Synthesis of CspV is more tightly regulated than synthesis of CspAVC.
Initial exposure of V. cholerae cells to 15°C for 1 h and then a shift of the culture to 37°C resulted in inhibition of synthesis of CspV within 15 min (Fig. 3A). In contrast, the synthesis of CspAVC decreased only slightly (Fig. 3), indicating that CspV is a true cold shock-induced protein and stable only at low temperatures. The stability of each protein after the cells were shifted from 15 to 37°C was also verified by pulse-chase experiments, as shown in Fig. 3B. These results also indicate that the synthesis of CspV is more tightly regulated than the synthesis of CspAVC.
Role of Csps in cold adaptation.
When an exponentially growing culture of V. cholerae was preincubated at 15°C for 2 h to allow sufficient expression of the cold stress proteins CspAVC and CspV (as shown in Fig. 2) and then exposed to 10°C, the cells with prior exposure to 15°C were able to grow at 10°C, whereas cells which were shifted directly from 37 to 10°C were not able to grow (Fig. 4A). Thus, priming of cells at 15°C probably helped the organism adapt and grow at 10°C, which otherwise is nonpermissive for growth. However, the possibility that other uncharacterized Csps induced during priming of V. cholerae cells at 15°C play a role in cold adaptation at 10°C cannot be ruled out.
FIG. 4.
Role of Csps in cold adaptation of V. cholerae and status of CspAVC and CspV in the presence of chloramphenicol. (A) Growth of V. cholerae SG24 at various temperatures was monitored as described in Materials and Methods. The solid and open arrowheads indicate the OD585 values at which V. cholerae cells were shifted from 37°C to low temperatures and from 15 to 10°C, respectively. The curved arrow indicates the enhanced growth rate at 10°C after preincubation at 15°C. (B) Lanes 1 and 2 contained V. cholerae cells grown at 37°C in absence and presence of chloramphenicol, respectively. [35S]methionine labeling of cellular proteins in the presence of chloramphenicol at 15°C was done for the following periods: lane 3, 0 to 30 min; lane 4, 30 to 60 min; lane 5, 60 to 90 min; lane 6, 150 to 180 min; and lane 7, 5 to 5.5 h. For lanes 8 and 9, chloramphenicol was added 4.5 h after the shift from 37 to 15°C and this was followed by labeling at 5 to 5.5 h and at 6 to 6.5 h, respectively. The solid and open arrowheads indicate the positions of CspAVC and CspV, respectively.
Status of CspAVC and CspV in the presence of chloramphenicol.
When the antibiotic chloramphenicol, an inhibitor of translation, was added at a concentration of 3 μg ml−1 to an actively growing culture of V. cholerae at 37°C, the growth of cells was arrested almost immediately. No growth was observed for several hours (data not shown), and there was no induction of any Csps (Fig. 4B, lane 2). These results indicate that inhibition of translation is not the stimulus for induction of Csps during cold shock. However, when chloramphenicol was added just after the shift from 37 to 15°C, although the cells failed to resume growth after a lag period, CspAVC and CspV were found to be synthesized constitutively (Fig. 4B, lanes 3 to 7). Furthermore, constitutive production of both of the proteins continued for several hours (Fig. 4B), which is significantly different from the results obtained with cells that were not treated with chloramphenicol, as shown in Fig. 2. It was also observed that in the presence of chloramphenicol the expression of CspV was more pronounced than the expression of CspAVC (Fig. 4B, lanes 6 and 7). These results further support the contention that in V. cholerae CspV plays a more vital role in cold adaptation than CspAVC plays. Prolonged treatment (>5 h) of cells with chloramphenicol at 15°C showed that there was no further increase in the synthesis of CspAVC and CspV (Fig. 4B, lane 7). Our data are consistent with the results obtained in similar experiments carried out with E. coli (29). However, in contrast to the results obtained with E. coli, addition of chloramphenicol at 4.5 h after the temperature shift resulted in no significant de novo synthesis of CspAVC or CspV (Fig. 4B, lanes 8 and 9). Together, the data suggest that there is a common mechanism for induction of Csps by chloramphenicol in these two heterogeneous organisms.
2D-E analysis of cold-induced proteins.
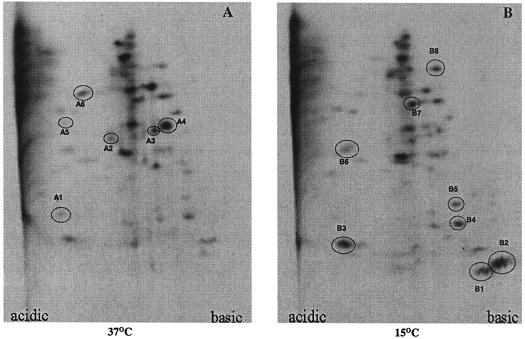
The number of proteins of V. cholerae that were differentially expressed due to cold shock was determined by 2D-E. Comparison of autoradiograms containing the protein spots of cells grown at 37°C and cells grown at 15°C revealed that while the levels of intensity of 40 proteins remained the same, there was down-regulation of at least six proteins (Fig. 5A, spots A1 to A6) and up-regulation of eight proteins of V. cholerae during cold shock adaptation (Fig. 5B, spots B1 to B6). Of the eight cold-induced proteins, two basic proteins at about 7.5 and 7.7 kDa were induced significantly (Fig. 5B, spots B1 and B2, respectively), and the sizes of these proteins were similar to those of CspV and CspAVC, respectively, as determined by one-dimensional T-SDS-PAGE (Fig. 2). These results are consistent with our one-dimensional T-SDS-PAGE results (Fig. 2). An 8.3-kDa small acidic protein was also up-regulated compared to the basal level at 37°C due to cold shock (Fig. 5B, spot B3). It is interesting that while the CspAVC and CspV proteins of V. cholerae are highly basic, the major cold-induced protein of E. coli, CspA, and its homologues in other organisms are acidic (2, 29).
FIG. 5.
Autoradiograms of 2D-E gels showing the major Csps of V. cholerae. Proteins were labeled and separated as described in Materials and Methods. The spots for proteins that were down-regulated due to a shift from 37 to 15°C and the spots for proteins which were induced due to cold shock are circled in panels A and B, respectively, and are numbered on the basis of size in ascending order in the autoradiograms. The same amount of protein was loaded in each gel.
Identification of genes coding for CspAVC and CspV.
By using the sequence data for the whole genome of V. cholerae (12) it was found that there are four cold shock-inducible genes coding for ∼7-kDa small proteins (Table 1). The cspD gene is present in the large chromosome, and the other three genes, cspA, a cold shock domain family protein gene, and a cold shock DNA binding domain protein gene (no specific designations have been assigned to the last two genes), are located in the small chromosome and are widely separated from each other (12). The Mw and pI of each of the four cold shock gene products were theoretically calculated by using the ExPaSy compute pI/Mw tool (see Materials and Methods) and are shown in Table 1. The analyses showed that of the four Csps of V. cholerae, the CspA protein (pI 9.22; Mw, 7.629) and the cold shock domain family protein (pI 8.12; Mw, 7.628) are basic, while CspD (pI 6.02; Mw, 8.328) and the cold shock DNA binding domain protein (pI 6.57; Mw, 7.531) are acidic (Table 1). From 2D-E analyses of total cellular proteins of V. cholerae, it was found that both the CspV and CspAVC proteins are basic (Fig. 5B, spots B1 and B2, respectively) and that the latter protein is more basic than the former since it migrated further towards the basic end of the gel. Therefore, it is most likely that the CspV protein is the product of the cold shock domain family protein gene. Since no specific designation was assigned to this gene in the study of Heidelberg et al. (12), we designated the gene cspV. Similarly, CspAVC is probably the product of the cspA gene (12). Furthermore, as determined by combining the proteomic and genomic data, the 8.3-kDa acidic protein of V. cholerae, which was up-regulated at 15°C (Fig. 5B, spot B3), is most probably the product of the cspD gene. In our 2D-E experiment we failed to detect the protein product of the putative cold shock DNA binding domain protein gene of V. cholerae (12), which should have produced a protein spot in the acidic region (pI 6.57; Mw, 7.531), as shown in Table 1. It is possible that this gene responds under different physiological stress conditions. This is analogous to the situation in E. coli, in which only four of the nine cold shock family genes (cspA, cspB, cspG, and cspI) are cold shock inducible and the rest (cspC, cspD, cspE, cspF, and cspH) are expressed under conditions other than cold shock (29). On the other hand, it may be possible that the cold shock DNA binding domain protein is a cold shock-inducible protein but is expressed only in the absence of CspV, CspAVC, or the 8.3-kDa protein or in the absence of all three of these proteins. Taken together, our results indicate the importance of the small chromosome of V. cholerae in the management of cold stress and survival in the environment.
TABLE 1.
Computed pI and Mw values of putative cold shock proteins encoded by the predicted cold shock genes of V. choleraea
| Gene (chromosome) | pI | Mw | TIGR accession no.b |
|---|---|---|---|
| cspA (small) | 9.22 | 7.629 | VCA0166 |
| Cold shock domain family protein coding gene (small)c | 8.12 | 7.628 | VCA0933 |
| Cold shock DNA binding domain protein coding gene (small) | 6.57 | 7.531 | VCA0184 |
| cspD (large) | 6.02 | 8.328 | VC1142 |
See reference 12.
TIGR, The Institute for Genome Research.
Designated cspV in this study.
Cloning, sequencing, and analysis of the cspV gene.
The nucleotide sequence of the PCR-amplified cspV gene region of V. cholerae SG24 was determined and analyzed. The nucleotide sequence similarity search (BLASTN) revealed that the amplified fragment contained sequences identical to those of the cold shock domain family protein gene of V. cholerae O1 El Tor strain N16961 (12). These results were not unexpected since several lines of evidence indicate that the O139 clone was derived from an El Tor strain (3, 4, 30, 36). When the cloned cspV gene was used as a probe in a Southern blot hybridization experiment with restriction enzyme EcoRI or HindIII, the autoradiogram revealed no restriction fragment length polymorphism among the genomes of various V. cholerae strains (24), and the gene was found to be located in the small chromosome in all of the strains examined (unpublished observations).
Nucleotide sequence analysis of the upstream region of the cspV gene of V. cholerae O139 strain SG24 indicated that there are two probable promoter regions, P1 (nucleotide positions 59 to 93) and P2 (nucleotide positions 274 to 313). Extensive comparisons of this region in the V. cholerae genome with the corresponding promoter regions of the E. coli cspA gene and homologues from other organisms available in the GenBank database suggested that the P2 region is the probable promoter of the cspV gene. The P2 region exhibited high levels of sequence similarity in the −35 and −10 regions and had a highly conserved TGn (n = any nucleotide) motif adjacent to the −10 region (Fig. 6) when it was compared with the promoters of cspA homologues of other organisms (9, 17, 19, 23, 35, 38). While an unusually long 5′-UTR mRNA (about 156 to 256 bases) is transcribed in all cold shock-inducible cspA-like genes examined so far (13, 29), in the P2 region no such 5′-UTR is possible as there is a gap of only 12 nucleotides between the putative transcription start site and the translation start site (nucleotide positions 314 to 325). Thus, it appears that P2 is a canonical type of promoter. Furthermore, the presence of an AT-rich UP element (5′-ATATAATAG-3′; nucleotide positions 264 to 272) immediately upstream of the −35 region of P2, a common feature of the cspA-like genes (29), and the absence of such an element in the P1 promoter region also suggest that P2 is the promoter of cspV. The high promoter activity of the cspA gene of E. coli upon cold shock was reported to be due to the presence of this UP element (21). Thus, it appears most likely that the P2 region drives the cspV gene expression in V. cholerae upon exposure to cold. Interestingly, an 11-base common sequence motif, called the cold box, which is present in the 5′-UTRs of the cspA, cspB, cspG, cspI, and csdA mRNAs of E. coli (13, 37), was found to be absent in the upstream region of cspV. This analysis indirectly supports the absence of a 5′-UTR in the cspV mRNA. It has been shown that the cold box sequence in E. coli is able to prolong the production of CspA upon cold shock (21). If these analyses are considered together, it is highly probable that the regulation of expression of cspV is different than the cspA-like gene regulation in E. coli, and further investigation is warranted. On the other hand, the presence of a conserved sequence (5′-ATGACTGGTTCTGTA-3′) 12 bases downstream of the initiation codon (nucleotide positions 338 to 352), called the downstream box, which is a characteristic of all true (class I) cold shock-inducible genes examined so far (21, 37), indicates that cspV is probably induced during cold shock. This downstream box feature in the cold shock genes has been suggested to be important for the most effective formation of a translation initiation complex (21). Our assumption that the cspV gene of V. cholerae is a class I cold shock-inducible gene is strongly supported by the recent microarray-based study of Merrell et al. (20), in which they performed a human-shed whole-bacterial-cell transcriptional profiling analysis. These authors showed that it is indeed the cspV gene (The Institute for Genomic Research accession number VCA0933) which is induced significantly when the cells are exposed to the environment (apparently low temperatures compared to the human body temperature, 37°C).
FIG. 6.
Comparison of the promoter regions of cspA homologues. The promoter regions of the E. coli cspA (35), cspB (17), cspG (23), and cspI (37) genes, the Bacillus subtilis cspB gene (38), the Salmonella enterica serovar Typhimurium cspB gene (9), and the Lactobacillus plantarum cspL gene (19) and P2 of the cspV gene of V. cholerae (this study) are compared. The putative transcriptional start sites are indicated by lowercase type; the proposed −10 and −35 regions are underlined; and the highly conserved TGn motifs just upstream of the −10 regions in the homologues are indicated by boldface type.
Expression of cspV in V. cholerae is regulated at the transcription level.
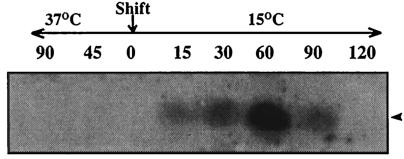
Northern blot analysis performed with the 210-bp cspV gene region as a probe revealed that transcription of the cspV gene begins within 15 min after transfer of a culture from 37 to 15°C and reaches a maximum at 60 min and that inhibition occurs within 120 min (Fig. 7). In sharp contrast, no cspV transcript was detected in cells maintained at 37°C for various lengths of time (Fig. 7). These results strongly suggest that the cspV gene of V. cholerae is induced only during cold shock and that its expression is very tightly regulated at the level of transcription. These results are also consistent with the findings of Merrell et al. (20), who showed that there was a high level of transcription of the cspV gene (accession number VCA0933) when V. cholerae cells were released from the human intestine into the environment.
FIG. 7.
Northern blot analysis of the cspV mRNA transcripts after cold shock. A 210-bp DNA fragment of the cspV gene was used as a probe. The time (in minutes) at which an aliquot was removed from a V. cholerae culture growing either at 37°C or at 15°C is indicated above each lane. The zero indicates the time of the shift from 37 to 15°C. The same amount of RNA was loaded in each lane. The arrowhead indicates the position of the hybridized band of cspV transcripts.
Overexpression of CspV and recognition of CspV by the CspA antiserum of E. coli.
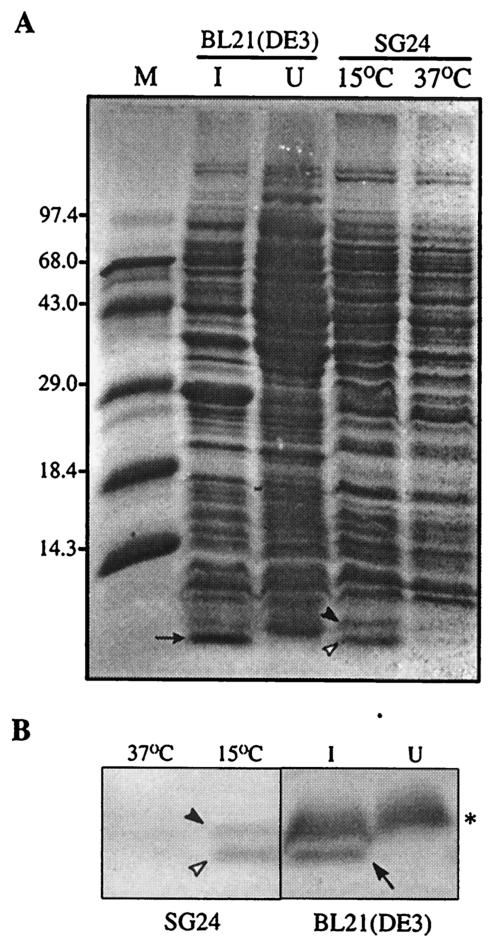
To further substantiate that the cspV gene does encode a 7.5-kDa protein, we constructed a cspV expression plasmid, pET20b(+)-cspV, as described in Materials and Methods. When the expression of CspV was induced by IPTG in E. coli, there was an additional overexpressed band at ∼7.5 kDa in the sample compared to the bands obtained with the cells that were not induced by IPTG (Fig. 8A). To determine the size of the overexpressed protein unequivocally, a protein sample prepared from V. cholerae cells exposed to 15°C was also electrophoresed side by side on a gel (Fig. 8A). The migration of the IPTG-induced protein band expressed in E. coli was found to be identical to that of the cold-induced CspV protein of wild-type V. cholerae (Fig. 8). The results strongly suggested that the cspV gene present in plasmid pPD665 coded for the protein CspV. This conclusion was confirmed by an immunoblot experiment in which we used rabbit polyclonal antiserum against the CspA protein of E. coli (kindly provided by M. Inouye, University of Medicine and Dentistry, Newark, N.J.). The anti-CspA antiserum reacted with the 7.5-kDa overexpressed protein of V. cholerae in E. coli, as well as cold-induced CspAVC and CspV of V. cholerae (Fig. 8B and C). These results confirmed that the insert in recombinant plasmid pPD665 indeed coded for the CspV protein of V. cholerae.
FIG. 8.
(A) Overexpression of the CspV protein in E. coli. Lanes I and U contained cells grown under IPTG-induced and uninduced conditions, respectively. The arrow indicates the position of the overexpressed CspV protein. The solid and open arrowheads indicate the positions of CspAVC and CspV, respectively. Lane M contained protein molecular mass standards, whose sizes (in kilodaltons) are indicated on the left. (B) Immunoblot detection of cold shock-induced CspAVC (solid arrowhead), CspV (open arrowhead), and overexpressed CspV (arrow) by using rabbit polyclonal antiserum against the CspA protein of E. coli. Lanes I and U contained cells grown under IPTG-induced and uninduced conditions, respectively. The asterisk indicates the position of a nonspecific protein band of E. coli that cross-reacted with the antiserum.
Conclusion.
In this study, we demonstrated the cold shock response in V. cholerae and showed that there is significant induction of two major cold shock-related proteins, CspAVC and CspV. It was found that CspV is a class I cold shock-induced protein which is stable only at low temperatures. Our in vitro experimental results are consistent with the results of the recently conducted in vivo study of Merrell et al. (20), in which transcriptional profiling of human-shed V. cholerae cells revealed a high level of expression of a cold shock-inducible gene (accession number VCA0933) (Table 1) which is actually the cspV gene identified and characterized in the present study. Further research based on these results may contribute to our understanding of the mechanisms of environmental persistence of V. cholerae for maintenance of its pathogenic cycle. It is noteworthy that the genes coding for CspAVC and CspV are both located in the small chromosome. Although the evolution and function of the small chromosome in Vibrio are currently not known, it has been suggested that the small chromosome might be involved in unique but poorly understood biological properties of V. cholerae (12). One such property is the ability of V. cholerae cells to enter into the VBNC state, which can be induced by environmental stresses, such as a suboptimal temperature or nutrient starvation (8, 33). Since the molecular basis of initiation and maintenance of the VBNC state has not been determined, it should be interesting to investigate whether the products of the cspA and/or cspV gene(s) have any role in the transition of V. cholerae cells to a dormant phase that leads to survival of the cells in the environment, which appears to be very crucial in maintaining the pathogenic cycle of the organism.
Acknowledgments
We are indebted to S. Bhattacharya for his constant support of this work. We are grateful to U. Dasgupta for critically reading the manuscript. We thank G. B. Nair for providing V. cholerae strains. We are grateful to M. Inouye (University of Medicine and Dentistry, Newark, N.J.) for his generous gift of E. coli anti-CspA antiserum.
This work was supported in part by the Department of Biotechnology (grant BT/PRO801/MED/09/154/97), Government of India. P.P.D is grateful to the Council of Scientific and Industrial Research for a research fellowship.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 2.Bae, W., P. G. Jones, and M. Inouye. 1997. CspA, the major cold shock protein of Escherichia coli, negatively regulates its own gene expression. J. Bacteriol. 179:7081-7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhadra, R. K., S. Roychoudhury, and J. Das. 1994. Vibrio cholerae O139 biotype El Tor. Lancet 343:728. [DOI] [PubMed] [Google Scholar]
- 4.Bik, E. M., A. E. Bunschoten, R. D. Gouw, and F. Mooi. 1995. Genesis of the novel epidemic Vibrio cholerae O139 strain: evidence for horizontal transfer of genes involved in polysaccharide synthesis. EMBO J. 14:209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll, W. J., M. C. Mateescu, K. Chava, R. R. Colwell, and A. K. Bej. 2001. Response and tolerance of toxigenic Vibrio cholerae O1 to cold temperatures. Antonie Leeuwenhoek 79:377-384. [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty, S., A. K. Mukhopadhyay, R. K. Bhadra, A. N. Ghosh, R. Mitra, T. Shimada, S. Yamasaki, S. M. Faruque, Y. Takeda, R. R. Colwell, and G. B. Nair. 2000. Virulence genes in environmental strains of Vibrio cholerae. Appl. Environ. Microbiol. 66:4022-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colwell, R. R. 1996. Global climate change and infectious diseases: the cholera paradigm. Science 274:2025-2203. [DOI] [PubMed] [Google Scholar]
- 8.Colwell, R. R., and A. Huq. 1994. Vibrios in the environment: viable but nonculturable Vibrio cholerae, p. 117-133. In I. K. Wachsmuth, P. A. Blake, and Ø. Olsvik (ed.), Vibrio cholerae and cholera. Vibrio cholerae and cholera: molecular to global perspectives. ASM Press, Washington, D.C.
- 9.Craig, J. E., D. Boyle, K. P. Francis, and M. P. Gallagher. 1998. Expression of the cold-shock gene cspB in Salmonella typhimurium occurs below a threshold temperature. Microbiology 144:697-704. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta, U., R. K. Bhadra, D. Panda, A. Deb, and J. Das. 1994. Recombinant derivative of a naturally occurring non-toxinogenic Vibrio cholerae O1 expressing the B subunit of cholera toxin: a potential oral vaccine strain. Vaccine 12:359-364. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein, J., N. S. Pollitt, and M. Inouye. 1990. Major cold-shock protein of Escherichia coli. Proc. Natl. Acad. Sci. USA 87:283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heidelberg, J., J. A. Eisen, W. C. Nelson, R. J. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, O. White, S. L. Saizberg, H. O. Smith, R. R. Colwell, J. J. Mekalanos, J. C. Venter, and C. M. Fraser. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 403:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang, W., L. Fang, and M. Inouye. 1996. The role of the 5′-end untranslated region of the mRNA for CspA, the major cold-shock protein of Escherichia coli. J. Bacteriol. 178:4919-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaper, J. B., J. G. Morris, Jr., and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khetawat, G., R. K. Bhadra, S. Nandi, and J. Das. 1999. Resurgent Vibrio cholerae O139: rearrangement of cholera toxin genetic elements and amplification of rrn operon Infect. Immun. 67:148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, S. H., D. L. Havada, M. K. Waldor, and A. Camilli. 1999. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell 99:625-634. [DOI] [PubMed] [Google Scholar]
- 17.Lee, S. J., A. Xie, W. Jiang, J. P. Etchegaray, P. G. Jones, and M. Inouye. 1994. Family of the major cold-shock protein, CspA (CS7.4) of Escherichia coli, whose members show a high sequence similarity with the eukaryotic Y-box binding proteins. Mol. Microbiol. 11:833-839. [DOI] [PubMed] [Google Scholar]
- 18.Lobitz, B., L. Beck, A. Huq, B. Wood, G. Fuchs, A. S. G. Faruque, and R. R. Colwell. 2000. Climate and infectious disease: use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc. Natl. Acad. Sci. USA 97:1438-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayo, B., S. Derzelle, M. Fernandez, C. Leonard, T. Ferain, P. Hols, J. E. Suarez, and J. Decour. 1997. Cloning and charaterization of cspL and cspP, two cold-inducible genes from Lactobacillus plantarum. J. Bacteriol. 179:3039-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merrell, D. S., S. M. Butler, F. Qadri, N. A. Dolganov, A. Alam, M. B. Cohen, S. B. Calderwood, G. K. Shoolnik, A. Camilli. 2002. Host-induced epidemic spread of the cholera bacterium. Nature 417:642-645. [DOI] [PMC free article] [PubMed]
- 21.Mitta, M., L. Fang, and M. Inouye. 1997. Deletion analysis of cspA of Escherichia coli: requirement of the AT-rich UP element for cspA transcription and the downstream box in the coding region for its cold shock induction. Mol. Microbiol. 26:321-335. [DOI] [PubMed] [Google Scholar]
- 22.Nair, G. B., B. L. Sarkar, S. P. De, M. K. Chakrabarti, R. K. Bhadra, and S. C. Pal. 1988. Ecology of Vibrio cholerae in the fresh water environs of Calcutta, India. Microb. Ecol. 15:203-215. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima, K., K. Kanamaru, T. Mizuno, and K. Horikoshi. 1996. A novel member of the cspA family of genes that is induced by cold shock in Escherichia coli. J. Bacteriol. 178:2994-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nandi, S., G. Khetawat, S. Sengupta, R. Majumder, S. Kar, R. K. Bhadra, S. Roychoudhury, and J. Das. 1997. Rearrangements in the genomes of Vibrio cholerae strains belonging to different serovars and biovars. Int. J. Syst. Bacteriol. 47:858-862. [DOI] [PubMed] [Google Scholar]
- 25.Neuhaus, K., P. K. Francis, S. Rapposch, A. Georg, and S. Scherer. 1999. Pathogenic Yersinia species carry a novel, cold-inducible major cold shock protein tandem gene duplication producing both bicistronic and monocistronic mRNA. J. Bacteriol. 181:6449-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newkirk, K., W. Feng, W. Jiang, R. Tejero, S. D. Emerson, M. Inouye, and G. Montelione. 1994. Solution NMR structure of the major cold-shock proteins (CspA) from Escherichia coli: identification of a binding epitope for DNA. Proc. Natl. Acad. Sci. USA 91:5114-5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Farrell, P. H. 1975. High-resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250:4007-4021. [PMC free article] [PubMed] [Google Scholar]
- 28.O'Farrell, P. Z., H. M. Goodman, and P. H. O'Farrell. 1977. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell 12:1133-1142. [DOI] [PubMed] [Google Scholar]
- 29.Phadtare, S., J. Alsina, and M. Inouye. 1999. Cold-shock response and cold-shock proteins. Curr. Opin. Microbiol. 2:175-180. [DOI] [PubMed] [Google Scholar]
- 30.Ramamurthy, T., S. Garg, R. Sharma, S. K. Bhattacharya, G. B. Nair, T. Shimada, T. Takeda, T. Karaswa, H. Kurazono, A. Pal, and Y. Takeda. 1993. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet 341:703-704. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fristsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Schagger, H., and G. VonJagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 33.Singleton, F. L., R. Attwell, S. Jangi, and R. R. Colwell. 1982. Effects of temperature and salinity on Vibrio cholerae growth. Appl. Environ. Microbiol. 44:1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommerville, J., and M. Ladomery. 1996. Masking of mRNA by Y-box proteins. FASEB J. 10:435-443. [DOI] [PubMed] [Google Scholar]
- 35.Tanabe, H., J. Goldstein, and M. Inouye. 1992. Identification of the promoter region of the Escherichia coli major cold shock gene, cspA. J. Bacteriol. 174:3867-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waldor, M. K., and J. J. Mekalanos. 1994. Emergence of a new cholera pandemic: molecular analysis of virulence determinants in Vibrio cholerae O139 and development of a vaccine prototype. J. Infect. Dis. 170:278-283. [DOI] [PubMed] [Google Scholar]
- 37.Wang, N., K. Yamanaka, and M. Inouye. 1999. CspI, the ninth member of the CspA family of Escherichia coli, is induced upon cold shock. J. Bacteriol. 181:1603-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willimsky, G., H. Bang, G. Fischer, and M. A. Marahiel. 1992. Characterization of cspB, a Bacillus subtilis inducible cold shock gene affecting cell viability at low temperatures. J. Bacteriol. 174:6326-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]