Abstract
A total of 31 bacterial isolates that have potential Alexandrium cyst formation-promoting activity (Alex-CFPB) were isolated from Hiroshima Bay (Japan), which is characterized by seasonal blooms of the toxic dinoflagellate Alexandrium tamarense. The population structure of Alex-CFPB was analyzed by means of restriction fragment length polymorphism analysis of the 16S rRNA genes (16S rDNA). Fourteen ribotypes, A to N, were observed among the 31 isolates of Alex-CFPB by using four restriction enzymes, MboI, HhaI, RsaI and BstUI. Among them, seven isolates, which were obtained from the seawater samples taken during the peak and termination periods of the A. tamarense bloom in 1998, belonged to ribotype A. This result suggests that bacterial strains of ribotype A may be dominant in the Alex-CFPB assemblages during these periods. The partial 16S rDNA-based phylogenetic tree of 10 ribotypes studied showed that nine of them fell into the Rhodobacter group of the α subclass of the Proteobacteria. Eight of nine ribotypes of the Rhodobacter group fell into the lineage of the Roseobacter subgroup, and one fell into the Rhodobacter subgroup. The non-Rhodobacter group type fell into the Marinobacterium-Neptunomonas-Pseudomonas group of the γ-Proteobacteria. Isolates of Alex-CFPB ribotypes A and C do not have clear growth-promoting activities but have strong cyst formation-promoting activities (CFPAs) under our laboratory conditions. These results show that the Alex-CFPB assemblage may consist of various bacteria that belong mainly to the Roseobacter group and have strong CFPAs. These results suggest that not only the Alexandrium cyst formation-inhibiting bacteria (Alex-CFIB) reported previously but also Alex-CFPB, especially bacteria of ribotype A, may play significant roles in the process of encystment and bloom dynamics of Alexandrium in the natural environment.
Associations between marine bacteria and microalgae, especially harmful algae, have been reported in many marine ecosystems (13, 15, 16), which suggest that bacterial assemblages may play significant roles in the processes of algal bloom dynamics. Fukami et al. (18) reported on the bacterial assemblage that specifically promoted the growth of the red tide causative species Gymnodinium (Krenia) mikimotoi (Dinophyceae) in succession from a diatom bloom to the G. mikimotoi bloom. They also reported on the subsequent assemblage that inhibited the growth of G. mikimotoi in succession from the G. mikimotoi bloom to a Chattonella sp. (Raphidophyceae) bloom in Uranouchi Inlet, Kochi, Japan. Yoshinaga et al. (58) also found a negative correlation between the abundance of G. mikimotoi and G. mikimotoi growth-inhibiting bacteria enumerated by the most probable number (MPN) method during the development and decay processes of G. mikimotoi red tides in Tanabe Bay, Japan. These reports suggest that the natural bacterial assemblages may play important roles in the processes that affect the growth of various marine phytoplankton including harmful species as well as the succession of phytoplankton communities in coastal waters.
The harmful dinoflagellates Alexandrium catenella and Alexandrium tamarense commonly occur in temperate coastal waters throughout the world, resulting in recurrent outbreaks of paralytic shellfish poisoning (25, 49). The life cycle of these organisms is extremely important for their ability to form blooms. The dominant mode of reproduction is by simple asexual fission, but a transition to sexual reproduction can occur whereby motile mating types, plus and minus, are formed that fuse to produce a motile diploid zygote (planozygote) (53, 57). Planozygotes swim for several days before losing motility and becoming thick-walled resting cysts (hypnozygotes) (38). When favorable growth conditions return, cysts germinate and reinoculate the water with vegetative swimming cells (9, 14, 50). The roles suggested for these cysts include “seed populations” for bloom initiation, short- and long-term survival through adverse conditions, species dispersal, and preservation of genetic variation (38).
Several studies of factors controlling encystment of Alexandrium have considered physical and/or chemical aspects. Anderson et al. (6) and Anderson and Lindquist (7) suggested that sexuality of A. tamarense is induced by nutrient (nitrogen and phosphorus) depletion in laboratory cultures. There are, however, field reports of encystment under nutrient conditions seemingly favorable for growth (5, 8, 36). Despite the emphasis on nutrient depletion in experimental studies, the precise set of environmental cues that trigger or stimulate encystment in the field has not been clearly defined.
Recently, we have focused on bacteria and have analyzed the effect of natural bacterial assemblages on the encystment of toxic dinoflagellates of the genus Alexandrium, since some close relationships between microalgae and bacteria have been reported as described above. We found activities of the Alexandrium cyst formation-promoting bacteria (Alex-CFPB) in water samples collected from Hiroshima Bay during 1997 to 1998 and a clear positive correlation between the abundance of A. tamarense and that of Alex-CFPB determined by the MPN method during blooms, which suggested that Alex-CFPB may play a significant role in the process of encystment and bloom dynamics of A. tamarense in coastal waters (1). In the previous study, the existence not only of Alex-CFPB but also of Alexandrium cyst formation-inhibiting bacteria (Alex-CFIB) in Hiroshima Bay was reported (2). Many strains of Alex-CFIB were isolated from seawater samples from Hiroshima Bay. The 16S rRNA gene (rDNA)-based grouping and phylogenetic analysis showed that five ribotypes, Ia to Id and II, were determined among the strains of Alex-CFIB. Most strains belonged to ribotype I, which fell into the Alteromonas group of the γ subclass of the class Proteobacteria (2). These two reports suggest that Alex-CFPB and Alex-CFIB may play significant roles in the processes of encystment and the bloom dynamics of A. tamarense in the natural environment.
In the present study, we have undertaken the isolation of Alex-CFPB from field seawater samples of Hiroshima Bay (Japan) and have tried to examine the population structure of the Alex-CFPB assemblages by a restriction fragment length polymorphism (RFLP) analysis of the 16S rDNA regions and the phylogenetic positions of the Alex-CFPB by sequence comparison of the partial 16S rDNA regions. These analyses help us to determine the genetic diversity and structure of the Alex-CFPB population, which forms the foundation for an elucidation of the ecological roles of each strain, especially the dominant strains.
MATERIALS AND METHODS
Sample collection.
Bacterial sampling was conducted on 17 February 1997 and once a week from 8 April to 21 May 1998 at Station 11 (depth ca. 22 m) (1), a shallow coastal site in Hiroshima Bay, western Seto Inland Sea, Japan. One liter of water samples collected from the 5-m-depth layer at Station 11 with a Niskin sampling bottle (General Oceanics Inc.) was poured into acid-washed, autoclaved glass bottles and kept at 4°C in the dark during transit to the laboratory for isolation of Alex-CFPB.
Algal cultures.
Experiments were conducted with clonal and axenic isolates 6ax (mating type plus) and TNY7 (mating type minus) of A. catenella. Clonal and axenic isolate OFX191 of A. tamarense, which was originally isolated by Y. Sako in 1988 from Ofunato Bay, Iwate, Japan (46), and isolate HIM11 of A. tamarense, isolated by us in 1995 from Hiroshima Bay, Japan, were also used. These cultures were grown in f/2 with no added silicate (f/2-Si) and with 10−5 M Fe-EDTA as an iron source and chelator (6, 24). All glassware was thoroughly washed according to the method previously reported (1). Cultures of A. catenella and A. tamarense were grown at 20 and 15°C with a 14:10-h light-dark cycle at 80 μmol of photons/m2/s, respectively. The sterility of each culture was periodically determined by culture tests using a liquid peptone medium, as well as by direct bacterial observations with DAPI (4′,6′-diamidino-2-phenylindole) staining and epifluorescence microscopy as described by Adachi et al. (1).
Isolation of Alex-CFPB.
Seawater samples from Hiroshima Bay were diluted serially from 10−1 to 10−3 with sterilized seawater. Supernatants from the MPN-positive wells in the small-scale bioassay reported by Adachi et al. (1) were also diluted serially from 10−1 to 10−3 with sterilized seawater. For an MPN assay, each seawater sample was first filtered through a glass-fiber filter and then through a 0.8-μm-pore-size Millipore or 0.2-μm-pore-size Nuclepore filter. The 0.8-μm-pore-size filtrate was considered the fraction containing the bulk of the planktonic bacteria (bacterial fraction [BF]), and the 0.2-μm-pore-size filtrate was considered the bacterium-free fraction (BFF). The BF filtrate was serially diluted. BFF, BF, serially diluted BF, and f/2-Si medium (0.5 ml) were each inoculated into 16 wells of 48-well disposable sterilized tissue culture microplates (Iwaki Co., Ltd., Tokyo, Japan). In parallel, cultures of 6ax and TNY7 in the mid-exponential growth phase were mixed together as described by Adachi (1). One milliliter of the mixed cell suspension was inoculated into each of the 48 microwells, and the mixtures were mixed gently and incubated under the conditions described before (1). One month after the start of the incubation, the numbers of whole cysts formed in each well were counted under an IX-FLA inverted microscope (Olympus, Osaka, Japan). The wells in which the cyst number was more than three times the mean cyst number formed in the bacterium-free wells (n = 18) were regarded tentatively as Alex-CFPB positive (1). One hundred microliters of these diluted MPN-positive samples was spread onto FeTY agar plates (18) and incubated at 20°C for 1 month in the dark by the method described by Adachi et al. (2). Two hundred seventy-one colonies directly from the seawater samples and 777 colonies from MPN-positive wells were used for the screening. Colonies that caused the A. catenella cells to form three times more cysts than those formed in the bacterium-free wells (n = 16) were selected, streaked onto a FeTY agar plate, and incubated at 20°C for 3 weeks under dark conditions. Three colonies (diameter about 1 mm) from each plate were screened again (second screening). Colonies that caused A. catenella cells to form three times more cysts than those formed in bacterium-free wells were regarded tentatively as Alex-CFPB. Tentative cyst formation-promoting activity (CFPA) was calculated using the formula CFPA = cyst number in wells inoculated with bacteria (BW)/cyst number in bacterium-free wells (BFW).
PCR amplification and characterization of the 16S rDNA of Alex-CFPB.
The bacterial genome DNA was extracted by the method reported by Yoshinaga et al. (59). The almost-complete 16S rDNA was PCR amplified with the 27F and 1492R primers according to the method of Adachi et al. (2). Completed amplification products were purified and quantified according to the method of Adachi et al. (3). Two micrograms of the DNA was then digested with one of four restriction endonucleases, MboI, HhaI (TaKaRa Biomedicals, Osaka, Japan), RsaI (Toyobo, Osaka, Japan), and BstUI (New England Biolabs, Inc., Beverly, Mass.) according to the protocols of each manufacturer. Amplified products were electrophoresed using a 1% Agarose S gel (Nippon Gene Co., Ltd., Tokyo, Japan). Restriction fragments were resolved by electrophoresis with 4% NuSieve 3:1 agarose (FMC BioProducts, Rockland, Maine) (4). Agarose gels were stained with ethidium bromide and photographed according to standard methods (47).
PCR direct sequencing and phylogenetic analysis of the 16S rDNA.
PCR products were purified by using a GeneClean III kit according to the recommendations of the manufacturer (Bio 101, Vista, Calif.). The purified products were directly sequenced by using a BigDye Terminator Cycle Sequencing FS Ready Reaction kit (PE Biosystems, Osaka, Japan), with bacterial universal 27F, EUB338F, EUB338R, BAC514R, and BAC785R primers (2). The number in each primer name shows the corresponding position in the Escherichia coli 16S rDNA. DNA sequences were read directly to a computer with ABI PRISM 310 Genetic Analyzer (PE Biosystems). The partial 16S rDNA regions, positions 50 to 410 (E. coli numbering) reported by González et al. (22), were aligned with various bacterial 16S rDNA sequences obtained from the EMBL, GenBank, and DDBJ databases with the software ClustalW (version 1.6; EMBL, Heidelberg, Germany). Distances were inferred from sequences by using the one-parameter model of Jukes and Cantor (29). Branch lengths of phylogenetic trees were evaluated by the neighbor-joining (NJ) method (45) with ClustalW and by maximum-likelihood methods with PHYLIP DnaML (version 3.6; J. Felsenstein, Department of Genetics, University of Washington, Seattle, 1995). Bootstrap analyses of 1,000 replicates were carried out with ClustalW.
The GenBank accession numbers for the sequences of the organisms used to construct the phylogenetic trees are as follows: clone GAC-1, AF007249; GAC-3, AF007251; GAC-5, AF007252; NAC11-6, AF245634; OCS84, U78943; isolate 253-13, AJ294351; 253-16, AJ294352; ATAM407-56, AF359535; 667-2, AJ294353; ATAM407-62, AF359530; DSS-8, AF098493; GAI-5, AF007256; GAI-15, AF010258; GAI-33, AF010286; GAI-36, AF007259; ISM, AF098495; PRLISY01, Y15346; SRF3, AJ002565; dimethyl sulfoniopropionate (DMSP)-degrading strain LFR, L15345; Prionitis lanceolata gall symbiont, U37762; Antarctobacter heliothermus, Y11552 Ketogulonigenium vulgare, AF136849; Marinosulfonomonas methylotropha, U62894; Octadecabacter antarcticus, U14583; Octadecabacter arcticus, U73725; Paracoccus denitrificans, X69159; Reugeria atlantica, D88526; Reugeria algicola, X78313; Reugeria gelatinovorans, D88523; Rhodobacter azotoformans, D70847; Rhodobacter veldkampii, D16421; Rhodovulum adriaticum, D16418; Roseobacter gallaeciensis, Y13244; Roseobacter litoralis, X78312; Roseovarius tolerans, Y11551; Sagittula stellata, U58356; Silicibacter lacuscaerulensis, U77644; Staleya guttiformis, Y16427; Sulfitobacter brevis, Y16425; Sulfitobacter mediterraneus, Y17387; Sulfitobacter pontiacus, Y13155; clone BD2-7, AB015537; env.S091, AJ416671; ML516-J10, AF453551; OM60, U70696; OM241, U70702; Alcanivorax sp. strain STET1, AJ416686; Marinobacterium georgiense, U58339; Neptunomonas naphthovorans, AF053734; Pseudomonas aeruginosa, AB037545; Pseudomonas alcaliphila, AB030583; Pseudomonas elongata, AB021368; Pseudomonas monteilii, AF064458; Pseudomonas oryzihabitans, D84004; Pseudomonas plecoglossicida, AB009457; Pseudomonas pseudoalcaligenes, Z76666; Pseudomonas putida, AF094746; Pseudomonas syringae, AB001441.
Determination of activities of representative Alex-CFPB.
A multi-test-tube approach was used to determine whether Alex-CFPB have encystment-promoting activities. Ten milliliters of autoclaved seawater collected from Uranouchi Inlet, Kochi, Japan, was poured into 23 sterile test tubes (diameter, 25 mm) for determination of encystment promotion activities of two representative isolates. The isolates CFPB-A9 and CFPB-C1 in late log phase (>108 cells/ml), preincubated in FeTY liquid medium for 1 week at 20°C, were diluted with the FeTY liquid medium to 5.0 × 107 cells/ml. One microliter of each of the diluted bacterial suspensions was inoculated into the respective sets of 13 test tubes containing the autoclaved seawater (BF). The same volume of FeTY liquid medium was added to the remaining 10 test tubes as a control (BFF). Twenty milliliters of culture mixtures of A. catenella 6ax and TNY7 in the mid-exponential growth phase, which had been adjusted to a concentration of 5.0 × 103 cells/ml with sterile f/2 medium, was added to each of the 13 test tubes with the BF and to the 10 tubes without bacteria (BFF), respectively, mixed gently, and incubated at 20°C for 15 days under the light conditions described above. During the incubation period, the growth of A. catenella cells in five BF and BFF test tubes was monitored once every 5 days by counting under an IX-FLA inverted microscope (Olympus). After incubation for 0, 3, 10, and 15 days, the total bacteria in triplicate tubes were monitored by the method described previously (2). After incubation for 15 days, the reactive phosphate, nitrate, and nitrite concentrations in the incubation medium of three separate tubes for each fraction were determined by means of a TRAACS-800 autoanalyzer (Bran Luebbe, Tokyo, Japan) according to the recommendations of the manufacturer. The bottoms of five test tubes of both fractions were loosened with a rubber scraper after the incubation. The cysts in the five test tubes were collected and counted by the method of Adachi et al. (1). Encystment efficiency was obtained as the cyst/peak cell concentration ratio (1).
CFPA of an isolate of CFPB-A9 on A. tamarense.
Autoclaved seawater (50 μl) was poured into 60 wells of 96-well disposable sterilized tissue culture microplates (Iwaki Co.). A culture of CFPB-A9 in late log phase (>108 cells/ml) preincubated in the FeTY liquid medium was diluted with the FeTY liquid medium to 5 × 105 cells/ml. Three microliters of the diluted bacterial suspension was inoculated at an initial density of 104 cells/ml into the 30 wells containing the autoclaved seawater (BF). The same volume of the FeTY liquid medium was added to the other 30 wells as a control (BFF). One hundred microliters of culture mixtures of A. tamarense OFX191 and HIM11, both in the mid-exponential growth phase, which had been adjusted to a concentration of 5 × 103 cells/ml with sterile f/2 medium, was added to all 96 microwells (60 wells), mixed gently, and incubated at 15°C under the light conditions described above. Thirty days after the start of the incubation, the whole cysts formed in each well were counted by the method described previously (1). CFPA of each culture was calculated by the method described previously (1).
CFPA of CFPB-A9 culture supernatant.
An 0.5-ml amount of CFPB-A9 culture (>108 cells/ml, late log phase) preincubated in the FeTY liquid medium for 1 week and of FeTY medium (negative-control fraction) was filtered through a 0.2-μm-pore-size filter (DISMIC-13 cellulose acetate; Advantec, Inc., Tokyo, Japan). One microliter of the bacterial culture filtrate and the FeTY filtrate was added into five wells each in 48-well disposable sterilized tissue culture microplates (Iwaki Co.) containing 0.4 ml of autoclaved seawater and 0.8 ml of culture mixtures of A. catenella prepared by the method described above. CFPA was calculated as follows: CFPA = cyst number in wells with bacterial culture filtrates/cyst number in wells with FeTY filtrates.
Nucleotide sequence accession numbers.
The 16S rDNA sequences of the new isolates described in this study have been assigned DDBJ numbers as follows: CFPB-A9, AB106132; CFPB-C1, AB106133; CFPB-D1, AB107215; CFPB-E1, AB106134; CFPB-F1, AB106135; CFPB-G1, AB106136; CFPB-H1, AB106137; CFPB-I1, AB106138; CFPB-L1, AB106139; CFPB-N1, AB106140.
RESULTS
Isolation of Alex-CFPB and their CFPAs.
A total of 31 isolates of Alex-CFPB were isolated from seawater samples taken directly from the field seawater samples (Field SW, Table 1) and from the MPN-positive wells (Culture sup., Table 1). Among them, 17 isolates were derived from the seawater samples collected on 28 April 1998, when the bloom of A. tamarense reached its peak. CFPA, which was determined tentatively as the ratio of cyst abundance in BW to that in BFW, ranged from 4.06 (isolate CFPB-H1) to 21.2 (isolate CFPB-C2) (Table 1). When the 62 isolates derived directly from the field seawater sample collected on 28 April 1998 (bloom peak period) were used for the screening of Alex-CFPB, five isolates showed CFPAs. CFU of the seawater sample (2.93 × 104 cells/ml) multiplied by the isolation efficiency of Alex-CFPB (8.06%) gave the abundance of Alex-CFPB (2.36 × 103 cells/ml) at that time by the colony-counting method.
TABLE 1.
List of Alex-CFPB
| Isolate | Sampling date (mo/day/yr) | Isolate origina | Dilution | No. of cysts in:
|
CFPAb | Ribotype | |
|---|---|---|---|---|---|---|---|
| BFW | BW | ||||||
| CFPB-A1 | 4/28/1998 | Field SW | 10−1 | 8.86 ± 3.99 | 53.0 | 5.98 | A |
| CFPB-A2 | 4/28/1998 | Field SW | 10−1 | 8.86 ± 3.99 | 59.0 | 6.66 | A |
| CFPB-A3 | 4/28/1998 | Field SW | 10−1 | 8.86 ± 3.99 | 80.0 | 8.86 | A |
| CFPB-A4 | 5/7/1998 | Field SW | 10−1 | 8.86 ± 3.99 | 58.0 | 6.55 | A |
| CFPB-A5 | 5/21/1998 | Field SW | 100 | 8.86 ± 3.99 | 71.0 | 8.01 | A |
| CFPB-A6 | 4/28/1998 | Culture sup. | 100 | 8.86 ± 3.99 | 90.0 | 10.2 | A |
| CFPB-A7 | 4/28/1998 | Culture sup. | 100 | 8.86 ± 3.99 | 86.0 | 9.71 | A |
| CFPB-A8 | 4/28/1998 | Culture sup. | 10−1 | 8.86 ± 3.99 | 64.0 | 7.22 | A |
| CFPB-A9 | 4/28/1998 | Culture sup. | 10−1 | 8.86 ± 3.99 | 103 | 11.6 | A |
| CFPB-B1 | 4/28/1998 | Field SW | 10−1 | 8.86 ± 3.99 | 103 | 11.6 | B |
| CFPB-B2 | 4/28/1998 | Field SW | 10−1 | 8.86 ± 3.99 | 39.0 | 4.40 | B |
| CFPB-B3 | 5/7/1998 | Field SW | 10−2 | 8.86 ± 3.99 | 58.0 | 6.55 | B |
| CFPB-B4 | 5/7/1998 | Field SW | 10−2 | 8.86 ± 3.99 | 75.0 | 8.47 | B |
| CFPB-C1 | 4/28/1998 | Culture sup. | 10−1 | 8.86 ± 3.99 | 146 | 16.5 | C |
| CFPB-C2 | 4/28/1998 | Culture sup. | 10−1 | 8.86 ± 3.99 | 188 | 21.2 | C |
| CFPB-C3 | 4/28/1998 | Culture sup. | 10−1 | 8.86 ± 3.99 | 156 | 17.6 | C |
| CFPB-D1 | 2/17/1997 | Culture sup. | 100 | 8.86 ± 3.99 | 78.0 | 8.80 | D |
| CFPB-D2 | 2/17/1997 | Culture sup. | 10−1 | 8.86 ± 3.99 | 43.0 | 4.85 | D |
| CFPB-D3 | 2/17/1997 | Culture sup. | 10−1 | 8.86 ± 3.99 | 69.0 | 7.79 | D |
| CFPB-D4 | 2/17/1997 | Culture sup. | 10−1 | 8.86 ± 3.99 | 81.0 | 9.14 | D |
| CFPB-E1 | 4/28/1998 | Culture sup. | 100 | 8.86 ± 3.99 | 49.0 | 5.53 | E |
| CFPB-E2 | 4/28/1998 | Culture sup. | 100 | 8.86 ± 3.99 | 42.0 | 4.74 | E |
| CFPB-F1 | 5/7/1998 | Field SW | 10−2 | 8.86 ± 3.99 | 53.0 | 5.98 | F |
| CFPB-G1 | 4/8/1998 | Culture sup. | 100 | 8.86 ± 3.99 | 39.0 | 4.40 | G |
| CFPB-H1 | 4/8/1998 | Culture sup. | 100 | 8.86 ± 3.99 | 36.0 | 4.06 | H |
| CFPB-I1 | 4/8/1998 | Culture sup. | 100 | 8.86 ± 3.99 | 105 | 11.9 | I |
| CFPB-J1 | 4/8/1998 | Culture sup. | 100 | 8.86 ± 3.99 | 44.0 | 4.97 | J |
| CFPB-K1 | 4/8/1998 | Culture sup. | 10−2 | 8.86 ± 3.99 | 37.0 | 4.18 | K |
| CFPB-L1 | 4/28/1998 | Culture sup. | 100 | 8.86 ± 3.99 | 37.0 | 4.18 | L |
| CFPB-M1 | 4/28/1998 | Culture sup. | 10−1 | 8.86 ± 3.99 | 48.0 | 5.42 | M |
| CFPB-N1 | 4/28/1998 | Culture sup. | 10−1 | 8.86 ± 3.99 | 37.0 | 4.18 | N |
SW, seawater; sup., supernatant in the MPN-positive wells described by Adachi et al. (1).
CFPA = cyst number in BW/cyst number in BFW.
16S rDNA-based RFLP analysis of Alex-CFPB.
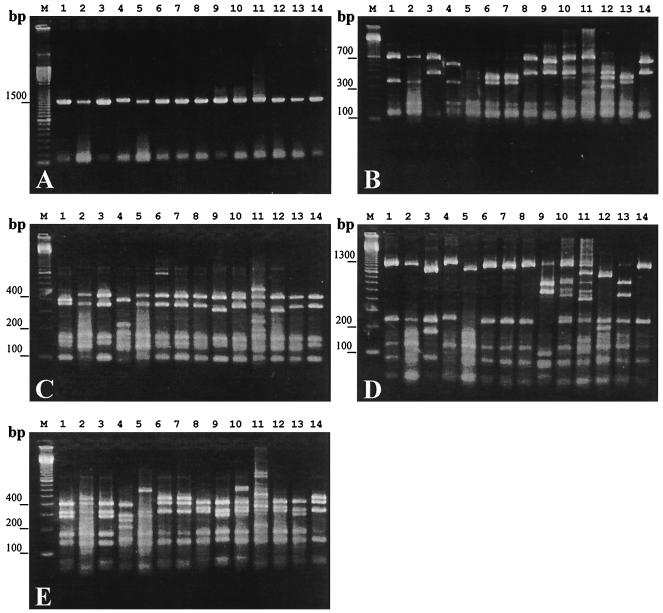
PCR amplification of the 16S rDNA from the 31 isolates of Alex-CFPB resulted in a single product of approximately 1,500 bp (Fig. 1A). Nine, seven, eight, and eight different RFLP types were detected with the restriction enzymes RsaI, BstUI, MboI, and HhaI, respectively (Fig. 1B, C, D, and E). Taken together, our results reveal 14 ribotypes among the 31 isolates of Alex-CFPB tested. Hereafter, these ribotypes are designated as A to N (Table 1). Nine of the 31 isolates belonged to ribotype A (Table 1). These ribotype A isolates were derived from the seawater samples taken at the bloom peak period or decay period (Table 1). Four isolates of ribotype B were also isolated from the seawater sampled at the bloom peak and disintegration periods (Table 1). Three isolates of ribotype C and two of ribotype E were isolated from the seawater sampled at the bloom peak period (Table 1). Four isolates of ribotype D were obtained from the seawater samples taken on 7 February 1997 at the bloom initiation period (Table 1). In the case of the other ribotypes, one isolate was obtained for each ribotype (Table 1). Among five isolates of Alex-CFPB that were derived directly from the field seawater sample collected on 28 April 1998 (bloom peak period), three belonged to ribotype A (Table 1). The abundance of Alex-CFPB (2.36 × 103 cells/ml) at that time multiplied by the isolation efficiency (60%) of Alex-CFPB ribotype A gave the abundance of Alex-CFPB ribotype A (1.41 × 103 cells/ml) at that time by the colony-counting method.
FIG. 1.
Amplified products of the 16S rDNA of various Alex-CFPB (A) and digestion of the PCR products with RsaI (B), BstUI (C), MboI (D), and HhaI (E). Lane M, 100-bp ladder markers; lane 1, CFPB-A9; lane 2, CFPB-B1; lane 3, CFPB-C1; lane 4, CFPB-D1; lane 5, CFPB-E1; lane 6, CFPB-F1; lane 7, CFPB-G1; lane 8, CFPB-H1; lane 9, CFPB-I1; lane 10, CFPB-J1; lane 11, CFPB-K1; lane 12, CFPB-L1; lane 13, CFPB-M1; lane 14, CFPB-N1.
16S rDNA-based phylogenetic analysis of Alex-CFPB.
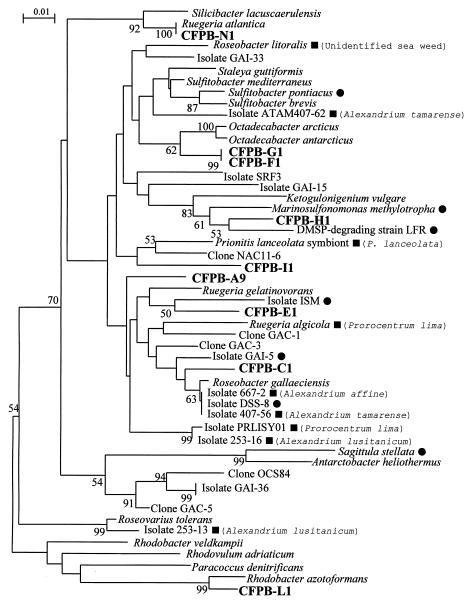
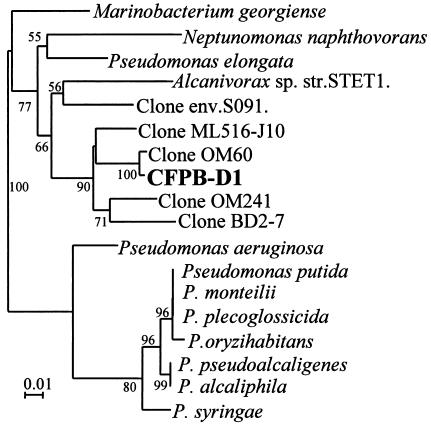
The partial 16S rDNA-based phylogenetic tree of the 10 ribotypes analyzed by the NJ method showed that nine of them fell into the Rhodobacter group of the α subclass of Proteobacteria (Fig. 2). Eight of the nine ribotypes fell into the Roseobacter subgroup (“Roseobacter group”) (22), and one type (ribotype L) fell into the Rhodobacter subgroup (Fig. 2). The other ribotype (D) fell into the Marinobacterium-Neptunomonas-Pseudomonas group of the γ subclass of Proteobacteria (Fig. 3). The topology of the trees was also confirmed by the maximum-likelihood method (data not shown).
FIG. 2.
Molecular phylogenetic tree inferred from the partial 16S rDNA for Alex-CFPB in the Rhodobacter group of the α subclass of the Proteobacteria by the NJ method. Numbers are percentages of 1,000 bootstrap repetitions. Bootstrap values greater than 50% are indicated. The tree is based on positions 50 to 410 (E. coli numbering) of the 16S rDNA described by González et al. (20, 21). Black squares indicate isolates that were found in association with phycospheres. The names of algal species associated with bacteria are shown in parentheses. Black circles indicate isolates that have abilities to transform organic and/or inorganic sulfur compounds.
FIG. 3.
Molecular phylogenetic tree inferred from the partial 16S rDNA for CFPB-D1 in the Pseudomonas group of the γ subclass of the Proteobacteria by the NJ method. Numbers are percentages of 1,000 bootstrap repetitions. Bootstrap values greater than 50% are indicated. The tree is based on positions 50 to 410 (E. coli numbering) of the 16S rDNA described by González et al. (20, 21).
Isolate CFPB-A9 was clustered with Ruegeria gelatinovorans isolated from bottom sediments in Kiel Fjord, Baltic Sea (43), Ruegeria algicola isolated from the phycosphere of the toxin-producing dinoflagellate Prorocentrum lima (32), and Roseobacter gallaeciensis isolated from the scallop Pecten maximus (44). This cluster also included the isolates CFPB-C1 and CFPB-E1. The isolate CFPB-C1 was clustered with Roseobacter gallaeciensis, isolate 407-56 from a culture of Alexandrium tamarense, isolate 667-2 from a culture of Alexandrium affine (27), and isolate DSS-8, which was isolated from coastal seawater and has the ability to degrade DMSP and dimethyl sulfide (DMS) (20). The isolate CFPB-E1 was clustered with isolate ISM, which is also able to degrade DMSP and DMS (20). The isolates CFPB-F1 and CFPB-G1 were combined into a single branch and were clustered with isolates of the genus Octadecabacter, which were isolated from polar sea ice and water samples (23). The isolate CFPB-H1 was clustered with DMSP-degrading strain LFR of Marinosulfonomonas methylotropha, which grows on methanesulfonic acid (28), and Ketogulonicigenium vulgare, which oxidizes l-sorbose to 2-keto-l-gulonic acid (54). The isolate CFPB-I1 was clustered with a bacterium associated with gall formation in the marine red alga Prionitis lanceolata (10). The isolate CFPB-N1 was clustered with Ruegeria atlantica, which was isolated from the Atlantic Ocean (43), and Silicibacter lacuscaerulensis, which is a halophilic bacterium isolated from the Blue Lagoon geothermal lake in Iceland (37). The isolate CFPB-L1 was clustered with Rhodobacter azotoformans, Rhodobacter subgroup, α Proteobacteria (26).
The isolate CFPB-D1 of ribotype D fell into the Marinobacterium-Neptunomonas-Pseudomonas group of the γ Proteobacteria (Fig. 3). This isolate was clustered with clones OM60 and OM241, which were isolated from the continental shelf off Cape Hatteras, North Carolina (41); clone ML516-J10, which was obtained from the alkaline hypersaline Mono Lake, California (S. B. Humayoun, N. Bano, and J. T. Hollibaugh, unpublished data); and clone BD2-7, which was obtained from deep-sea sediment (34).
Determination of activities of representative Alex-CFPB.
Encystment promotion activities of the ribotype A isolate CFPB-A9, which was isolated at the bloom peak period, and of the ribotype C isolate CFPB-C1, which has the highest CFPA in Table 1, were analyzed by the multi-test-tube approach. The peak algal cell densities in test tubes inoculated with CFPB-A9 or CFPB-C1 and tubes not inoculated with bacteria (BFF) were (1.38 ± 0.246) × 104, (1.56 ± 0.371) × 104, and (1.02 ± 0.185) × 104 cells/ml, respectively, which were not significantly different (Student's t test; P > 0.05). In test tubes to which CFPB-A9 and CFPB-C1 had been added, the total bacteria increased from 1.00 × 104 to (2.01 ± 0.0145) × 107 cells/ml and to (1.90 ± 0.0102) × 107 cells/ml during the incubation period (15 days), respectively. Fifteen days after the start of the incubation, the phosphate concentrations in test tubes inoculated with CFPB-A9 and CFPB-C1 and those inoculated with BFF were not significantly different from one another and were 11.2, 9.81, and 14.4 μM, respectively. The nitrogen (nitrate plus nitrite) concentrations in these test tubes inoculated with bacteria and without bacteria were also not significantly different and were 411, 391, and 382 mM, respectively, after incubation for 15 days. The cyst yields in test tubes inoculated with CFPB-A9 or CFPB-C1 and without bacteria were 1.09 ± 0.170, 1.45 ± 0.530, and 0.0600 ± 0.0300 cysts/ml, respectively. The cyst yields of test tubes inoculated with CFPB-A9 and CFPB-C1 were significantly different from those of test tubes without bacteria (t test; P < 0.01). Their encystment efficiencies (the cyst yield/maximum cell ratio) were 7.98 × 10−5, 9.29 × 10−5, and 5.88 × 10−6 cysts/well, respectively. These results show that the encystment efficiencies under inoculation with ribotype A and C strains were almost 13 and 16 times those under bacterium-free conditions, respectively, although the concentrations of the macronutrients phosphate, nitrate, and nitrite were not depleted in these three fractions during the incubation periods.
CFPA of the isolate CFPB-A9 on A. tamarense.
Cyst yields of A. tamarense OFX191 and HIM11 inoculated with the isolate CFPB-A9 and without bacteria were 3.60 ± 0.121 (n = 30) and 0.309 ± 0.112 (n = 30) cysts/ml, respectively, which were significantly different (t test; P < 0.001). The CFPA of this isolate on A. tamarense, which was determined as the ratio of cyst yield under the bacterial conditions to that under the bacterium-free conditions, was 11.7.
CFPA of CFPB-A9 culture supernatant.
The cyst yields of CFPB-A9 culture supernatant and FeTY medium (negative control) on A. catenella were 11.8 ± 0.231 (n = 5) and 1.20 ± 0.163 (n = 5) cysts/ml, respectively, which showed significant differences (P < 0.001). The CFPA of this experiment was 9.8.
DISCUSSION
Ribotyping of Alex-CFPB.
Fourteen ribotypes, A to N, were determined among strains of the 31 isolates of Alex-CFPB by RFLP analysis, which suggests that the Alex-CFPB assemblage consisted of various bacterial strains. Among these isolates, nine belonged to ribotype A and were obtained from the seawater samples collected at the bloom peak and decay periods when cyst formation presumably occurred. Furthermore, 60% of total Alex-CFPB isolated at the bloom peak period (28 April 1998) belonged to ribotype A. These results suggest that the ribotype A strains may be dominant among the Alex-CFPB assemblages during this period. Four isolates of ribotype B and three of ribotype C were also isolated from the waters sampled at the bloom peak and disintegration periods. Among the isolates of these three ribotypes, those of ribotypes A and C had high CFPAs. These results suggest that the ribotype A and C strains may play an important ecological role in the process of encystment of A. tamarense in Hiroshima Bay.
Phylogenetic analysis of Alex-CFPB.
Among 10 ribotypes studied, nine types belonged to the Roseobacter subgroup of the Rhodobacter group of the α subclass of Proteobacteria (Roseobacter group reported by González et al. [22]), which has been also called the Roseobacter-Sulfitobacter-Silicibacter group (55). Our phylogenetic tree corresponded approximately to that of González et al. (20). The nine ribotypes dispersed within this cluster, which suggests that they are slightly divergent from one another. Organisms clustered in this phylogenetic lineage have been shown to form a significant part of marine bacterioplankton communities (11, 17, 19, 21, 35, 51, 56, 60). González and Moran (21) reported that this Roseobacter group was abundant in coastal seawaters of the southeastern United States, where it accounted for up to 30% of the 16S rDNA. The Roseobacter subgroup is often found in association with various phycospheres: Enteromorpha sp. (Chlorophyceae) (48), Prionitis lanceolata (Rhodophyceae) (10), the toxic dinoflagellate Prorocentrum lima (32, 39), and Alexandrium spp. (Dinophyceae) (11, 27). The strains associated with these phycospheres are marked with black squares in our phylogenetic tree (Fig. 2). Kerkhof and Kemp (30) also identified bacterial 16S rDNA sequences unique to a coastal bloom, including members of the Roseobacter subgroup of the α subclass of the Proteobacteria. Riemann et al. (42) reported that heterotrophic bacteria associated with the diatom Thalassiosira sp. blooms induced in a mesocosm were dominated by the Roseobacter 16S rDNA sequences. González et al. (22) reported that the Roseobacter lineage was numerically important in the heterotrophic bacterial community associated with the algal bloom of Emiliania huxleyi (Haptophyceae) and clarified that the abundance of Roseobacter was positively correlated with the chlorophyll a concentration during the bloom. Töbe et al. (52) reported that there was a weak but significant positive relationship between Roseobacter abundance and that of Alexandrium spp. in coastal waters of the United Kingdom. These reports and our results suggest that members in this cluster from various marine environments may have potential abilities to associate with various phycospheres and may play important ecological roles in their ecosystems.
The Roseobacter subgroup members are proving to be important in various biogeochemical processes such as lignin transformation (21), degradation of aromatic compounds (12), degradation of DSMP produced by algae and coastal vascular plants (20, 33), and transformations of organic and inorganic sulfur compounds (28, 31, 40). González et al. (22) reported a positive correlation between Roseobacter abundance and DMSP or total organic sulfur (DMS plus DMSP plus dimethyl sulfoxide) concentrations during the bloom of Emiliania huxleyi and hypothesized that this lineage plays a role in cycling organic sulfur compounds produced within the bloom. The black circles in Fig. 2 indicate the strains having abilities to transform organic and/or inorganic sulfur compounds. The bacterial strains marked with the black circles dispersed in the cluster of the Roseobacter subgroup. The finding that many Alex-CFPB clustered with the Roseobacter lineage containing these strains marked with black circles in our phylogenetic tree suggests that Alex-CFPB may also have potential abilities to transform organic and/or inorganic sulfur compounds and may increase with the bloom of A. tamarense utilizing organic sulfur compounds such as DMSP, when they may coincidentally promote encystment of A. tamarense.
The phylogenetic analysis of ribotype D clarified that this type fell into the cluster containing the Marinobacterium-Neptunomonas-Pseudomonas group of the γ subclass of the Proteobacteria. This cluster also contained some DNA clones which were obtained from various marine environments, open ocean and bottom sediment. The mechanism of cyst formation promotion of this ribotype D strain may be different from those of the Roseobacter subgroup strains because of the genetic divergence between them.
Activities of Alex-CFPB.
In the multi-test-tube experiments, strains CFPB-A9 (ribotype A) and CFPB-C1 (ribotype C) did not affect the growth of A. catenella but did show strong encystment-promoting activities. It is reported that sexuality in Alexandrium is controlled by the initial availability in batch culture of macronutrients, such as nitrogen and/or phosphorus, and is induced by nitrogen (nitrate, nitrite, and ammonium) and/or phosphorus depletion (6, 7). In this report, macronutrient (phosphate-nitrite-nitrate) concentrations in cultures inoculated with bacteria (CFPB-A9 or CFPB-C1) and without bacteria were not significantly different, and the macronutrients in these cultures were concluded not to be depleted during the incubation periods. Furthermore, the very small bacterial culture supernatant (1 μl), which hardly affected the macronutrient or micronutrient concentrations of the algal cultures (1,200 μl), showed high CFPAs. These results suggest that the promotion was caused not by the indirect effect of changes in concentrations of macronutrients (nitrogen and/or phosphorus) but by a direct effect of a substance excreted by Alex-CFPB or by the phage released from Alex-CFPB under our laboratory conditions.
Specific members of Proteobacteria affect cyst formation of Alexandrium.
Recently bacteria that affect the sexuality of the harmful dinoflagellate Alexandrium have been isolated from the natural environment. Adachi et al. (2) reported that Alex-CFIB were detected in seawater sampled from Hiroshima Bay throughout the year and suggested that Alex-CFIB may be ubiquitous in the coastal waters where the toxic dinoflagellate A. tamarense occurs annually. They reported the isolation of many Alex-CFIB and clarified that most Alex-CFIB fell into the Alteromonas group of the γ subclass of the Proteobacteria (2). Adachi et al. (1) observed a clear positive correlation between the abundance of A. tamarense and that of Alex-CFPB in Hiroshima Bay. In this study, we reported that many Alex-CFPB were isolated and some of them had strong Alexandrium CFPAs. We also clarified the population structure of Alex-CFPB: most belong to a specific clade, the Roseobacter subgroup of the Rhodobacter group of the α subclass of the Proteobacteria, which is clearly differentiated from the Alex-CFIB previously studied. These results suggest that specific members of the α (Alex-CFPB) and γ (Alex-CFIB) subclasses of the Proteobacteria may play significant roles in the process of sexual differentiation and bloom dynamics of the harmful dinoflagellate Alexandrium in the natural environment.
Acknowledgments
This work was supported by Grants-in-Aid for Encouragement of Young Scientists (09760179, 11760137, and 13760143) from the Ministry of Education, Science, Sports and Culture of Japan.
We thank R. Kondo, N. Nishibori, and Y. Sako for providing cultures of Alexandrium. We also thank K. Fukami for valuable advice. We thank T. Shiomi for his technical assistance.
REFERENCES
- 1.Adachi, M., T. Kanno, T. Matsubara, T. Nishijima, S. Itakura, and M. Yamaguchi. 1999. Promotion of cyst formation in the toxic dinoflagellate Alexandrium (Dinophyceae) by natural bacterial assemblages from Hiroshima Bay, Japan. Mar. Ecol. Prog. Ser. 191:175-185. [Google Scholar]
- 2.Adachi, M., T. Matsubara, R. Okamoto, T. Nishijima, S. Itakura, and M. Yamaguchi. 2001. Inhibition of cyst formation in the toxic dinoflagellate Alexandrium (Dinophyceae) by bacteria from Hiroshima Bay, Japan. Aquat. Microb. Ecol. 26:223-233. [Google Scholar]
- 3.Adachi, M., Y. Sako, and Y. Ishida. 1996. Analysis of Alexandrium (Dinophyceae) species using sequences of the 5.8S ribosomal DNA and internal transcribed spacer regions. J. Phycol. 32:424-432. [Google Scholar]
- 4.Adachi, M., Y. Sako, and Y. Ishida. 1994. Restriction fragment length polymorphism of ribosomal DNA internal transcribed spacer and 5.8S regions in Japanese Alexandrium species (Dinophyceae). J. Phycol. 30:857-863. [Google Scholar]
- 5.Anderson, D. M., S. W. Chisholm, and C. J. Watras. 1983. Importance of life cycle events in the population dynamics of Gonyaulax tamarensis. Mar. Biol. 76:179-189. [Google Scholar]
- 6.Anderson, D. M., D. M. Kulis, and B. J. Binder. 1984. Sexuality and cyst formation in the dinoflagellate Gonyaulax tamarensis: cyst yield in batch cultures. J. Phycol. 20:418-425. [Google Scholar]
- 7.Anderson, D. M., and N. L. Lindquist. 1985. Time-course measurements of phosphorus depletion and cyst formation in the dinoflagellate Gonyaulax tamarensis Lebour. J. Exp. Mar. Biol. Ecol. 86:1-13. [Google Scholar]
- 8.Anderson, D. M., and F. M. M. Morel. 1979. The seeding of two red tide blooms by the germination of benthic Gonyaulax tamarensis hypnocyst. Estuar. Coast. Mar. Sci. 8:279-293. [Google Scholar]
- 9.Anderson, D. M., and D. Wall. 1978. The potential importance of benthic cysts of Gonyaulax tamarensis and Gonyaulax excavata in initiating toxic dinoflagellate blooms. J. Phycol. 14:224-234. [Google Scholar]
- 10.Ashen, J. B., and L. J. Goff. 1996. Molecular identification of a bacterium associated with gall formation in the marine red alga Prionitis lanceolata. J. Phycol. 32:286-297. [Google Scholar]
- 11.Brinkmeyer, R., M. S. Rappé, S. Gallacher, and L. Medlin. 2000. Development of clade- (Roseobacter and Alteromonas) and taxon-specific oligonucleotide probes to study interactions between toxic dinoflagellates and their associated bacteria. Eur. J. Phycol. 35:315-330. [Google Scholar]
- 12.Buchan, A., L. S. Collier, E. L. Neidle, and M. A. Moran. 2000. Key aromatic-ring-cleaving enzyme, protocatechuate 3,4-dioxygenase, in the ecologically important marine Roseobacter lineage. Appl. Environ. Microbiol. 66:4662-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole, J. J., G. E. Likens, and D. L. Strayer. 1982. Photosynthetically produced dissolved organic carbon: an important carbon source for planktonic bacteria. Limnol. Oceanogr. 27:1080-1090. [Google Scholar]
- 14.Dale, B. 1977. Cysts of the toxic red-tide dinoflagellate Gonyaulax excavata (Braarud) Balech from Oslofjorden, Norway. Sarsia 63:29-34. [Google Scholar]
- 15.Doucette, G. J. 1995. Interactions between bacteria and harmful alga. Nat. Toxins 3:65-74. [DOI] [PubMed] [Google Scholar]
- 16.Doucette, G. J., M. Kodama, S. Franca, and S. Gallacher. 1998. Bacterial interaction with harmful algal bloom species: bloom ecology, toxigenesis, and cytology, p. 619-648. In D. M. Anderson, A. D. Cembella, and G. M. Hallegraeff (ed.), Physiological ecology of harmful algal blooms. Springer-Verlag, Berlin, Germany.
- 17.Eilers, H., J. Pernthaler, F. O. Glöckner, and R. Amann. 2000. Culturability and in situ abundance of pelagic bacteria from the North Sea. Appl. Environ. Microbiol. 66:3044-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukami, K., A. Yuzawa, T. Nishijima, and Y. Hata. 1992. Isolation and properties of a bacterium inhibiting the growth of Gymnodinium nagasakiense. Nippon Suisan Gakkaishi 58:1073-1077. [Google Scholar]
- 19.Giovannoni, S. J., T. B. Britschgi, C. L. Moyer, and K. G. Field. 1990. Genetic diversity in Sargasso Sea bacterioplankton. Nature 345:60-63. [DOI] [PubMed] [Google Scholar]
- 20.González, J. M., R. P. Kiene, and M. A. Moran. 1999. Transformation of sulfur compounds by an abundant lineage of marine bacteria in the alpha-subclass of the class Proteobacteria. Appl. Environ. Microbiol. 65:3810-3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González, J. M., and M. A. Moran. 1997. Numerical dominance of a group of marine bacteria in the alpha-subclass of the class Proteobacteria in coastal seawater. Appl. Environ. Microbiol. 63:4237-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González, J. M., R. Simo, R. Massana, J. S. Covert, E. O. Casamayor, C. Pedros-Alio, and M. A. Moran. 2000. Bacterial community structure associated with a dimethyl sulfoniopropionate-producing North Atlantic algal bloom. Appl. Environ. Microbiol. 66:4237-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gosink, J. J., R. P. Herwig, and J. T. Staley. 1997. Octadecabacter arcticus gen. nov., sp. nov., and O. antarcticus, sp. nov., nonpigmented, psychrophilic gas vacuolate bacteria from polar sea ice and water. Syst. Appl. Microbiol. 20:356-365. [Google Scholar]
- 24.Guillard, R. R., and J. H. Ryther. 1962. Studies of marine planktonic diatoms. 1. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 8:229-239. [DOI] [PubMed] [Google Scholar]
- 25.Hallegraeff, G. M. 1993. A review of harmful algal blooms and their apparent global increase. Phycologia 32:79-99. [Google Scholar]
- 26.Hiraishi, A., K. Muramatsu, and Y. Ueda. 1996. Molecular genetic analyses of Rhodobacter azotoformans sp. nov. and related species of phototrophic bacteria. Syst. Appl. Microbiol. 19:168-177. [Google Scholar]
- 27.Hold, G. L., E. A. Smith, T. H. Birkbeck, and S. Gallacher. 2001. Comparison of paralytic shellfish toxin (PST) production by the dinoflagellates Alexandrium lusitanicum NEPCC 253 and Alexandrium tamarense NEPCC 407 in the presence and absence of bacteria. FEMS Microbiol. Ecol. 36:223-234. [DOI] [PubMed] [Google Scholar]
- 28.Holmes, A. J., D. P. Kelly, S. C. Baker, A. S. Thompson, P. De Marco, E. M. Kenna, and J. C. Murrell. 1997. Methylosulfonomonas methylovora gen. nov., sp. nov., and Marinosulfonomonas methylotropha gen. nov., sp. nov.: novel methylotrophs able to grow on methanesulfonic acid. Arch. Microbiol. 167:46-53. [DOI] [PubMed] [Google Scholar]
- 29.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism. Academic Press, New York, N.Y.
- 30.Kerkhof, L., and P. Kemp. 1999. Small ribosomal RNA content in marine Proteobacteria during non-steady-state growth. FEMS Microbiol. Ecol. 30:253-260. [DOI] [PubMed] [Google Scholar]
- 31.Kiene, R. P., L. J. Linn, J. González, M. A. Moran, and J. A. Bruton. 1999. Dimethylsulfoniopropionate and methanethiol are important precursors of methionine and protein-sulfur in marine bacterioplankton. Appl. Environ. Microbiol. 65:4549-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lafay, B., R. Ruimy, C. R. de Traubenberg, V. Breittmayer, M. J. Gauthier, and R. Christen. 1995. Roseobacter algicola sp. nov., a new marine bacterium isolated from the phycosphere of the toxin-producing dinoflagellate Prorocentrum lima. Int. J. Syst. Bacteriol. 45:290-296. [DOI] [PubMed] [Google Scholar]
- 33.Ledyard, K. M., E. F. DeLong, and J. W. H. Dacey. 1993. Characterization of a DMSP-degrading bacterial isolate from the Sargasso Sea. Arch. Microbiol. 160:312-318. [Google Scholar]
- 34.Li, L., C. Kato, and K. Horikoshi. 1999. Microbial diversity in sediments collected from the deepest cold-seep area, the Japan Trench. Mar. Biotechnol. 1:391-400. [DOI] [PubMed] [Google Scholar]
- 35.Mullins, T. D., T. B. Britschgi, R. L. Krest, and S. J. Giovannoni. 1995. Genetic comparisons reveal the same unknown bacterial lineages in Atlantic and Pacific bacterioplankton communities. Limnol. Oceanogr. 40:148-158. [Google Scholar]
- 36.Perez, C. C., S. Roy, M. Levasseur, and D. M. Anderson. 1998. Control of germination of Alexandrium tamarense (Dinophyceae) cysts from the Lower St. Lawrence Estuary (Canada). J. Phycol. 34:242-249. [Google Scholar]
- 37.Petursdottir, S. K., and J. K. Kristjansson. 1997. Silicibacter lacuscaerulensis gen. nov., sp. nov., a mesophilic moderately halophilic bacterium characteristic of the Blue Lagoon geothermal lake in Iceland. Extremophiles 1:94-99. [DOI] [PubMed] [Google Scholar]
- 38.Pfiester, L., and D. M. Anderson. 1987. Dinoflagellate reproduction, p. 611-648. In F. J. R. Taylor (ed.), Biology of dinoflagellates. Blackwell Scientific Publications, Oxford, United Kingdom.
- 39.Prokic, I., F. Brümmer, T. Brigge, H. D. Görtz, G. Gerdts, C. Schütt, H. Elbrächter, and W. E. G. Müller. 1998. Bacteria of the genus Roseobacter associated with the toxic dinoflagellate Prorocentrum lima. Protist 149:347-357. [DOI] [PubMed] [Google Scholar]
- 40.Pukall, R., D. Buntefuß, A. Fruhling, M. Rohde, R. M. Kroppenstedt, J. Burghardt, P. Lebaron, L. Bernard, and E. Stackebrandt. 1999. Sulfitobacter mediterraneus sp. nov., a new sulfite-oxidizing member of the alpha-Proteobacteria. Int. J. Syst. Bacteriol. 49:513-519. [DOI] [PubMed] [Google Scholar]
- 41.Rappé, M. S., P. E. Kemp, and S. J. Giovannoni. 1997. Phylogenetic diversity of marine coastal picoplankton 16S rRNA genes cloned from the continental shelf off Cape Hatteras, North Carolina. Limnol. Oceanogr. 42:811-826. [Google Scholar]
- 42.Riemann, L., G. F. Steward, and F. Azam. 2000. Dynamics of bacterial community composition and activity during a mesocosm diatom bloom. Appl. Environ. Microbiol. 66:578-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruger, H. J., and M. G. Hofle. 1992. Marine star-shaped-aggregate-forming bacteria: Agrobacterium atlanticum sp. nov.; Agrobacterium meteori sp. nov.; Agrobacterium ferrugineum sp. nov., nom. rev.; Agrobacterium gelatinovorum sp. nov., nom. rev.; and Agrobacterium stellulatum sp. nov., nom. rev. Int. J. Syst. Bacteriol. 42:133-143. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz-Ponte, C., V. Cilia, C. Lambert, and J. L. Nicolas. 1998. Roseobacter gallaeciensis sp. nov., a new marine bacterium isolated from rearings and collectors of the scallop Pecten maximus. Int. J. Syst. Bacteriol. 48:537-542. [DOI] [PubMed] [Google Scholar]
- 45.Saitou, N., and M. Nei. 1987. The neighbor-joining method. A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 46.Sako, Y., C. H. Kim, H. Ninomiya, M. Adachi, and Y. Ishida. 1990. Isozyme and cross analysis of mating populations in the Alexandrium catenella/tamarense species complex, p. 320-330. In E. Granéli, B. Sundström, and D. M. Anderson (ed.), Toxic marine phytoplankton. Elsevier Science Publishing, New York, N.Y.
- 47.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 48.Shiba, T. 1991. Roseobacter litoralis gen. nov., sp. nov., and Roseobacter denitrificans sp. nov., aerobic pink-pigmented bacteria which contain bacteriochlorophyll a. Syst. Appl. Microbiol. 14:140-145. [Google Scholar]
- 49.Shumway, S. E. 1993. A review of the effects of algal blooms on shellfish and aquaculture. J. World Aquacult. Soc. 21:65-104. [Google Scholar]
- 50.Steidinger, K. A. 1975. Basic factors influencing red tides, p. 153-162. In V. R. LoCicero (ed.), Toxic dinoflagellate blooms. Elsevier Science Publishing, New York, N.Y.
- 51.Suzuki, M. T., M. S. Rappé, Z. W. Haimberger, H. Winfield, N. Adair, J. Strobel, and S. J. Giovannoni. 1997. Bacterial diversity among small-subunit rRNA gene clones and cellular isolates from the same seawater sample. Appl. Environ. Microbiol. 63:983-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Töbe, K., C. Ferguson, M. Kelly, S. Gallacher, and L. Medlin. 2001. Seasonal occurrence at a Scottish PSP monitoring site of purportedly toxic bacteria originally isolated from the toxic dinoflagellate genus Alexandrium. Eur. J. Phycol. 36:243-256. [Google Scholar]
- 53.Turpin, D. H., P. E. R. Dobell, and F. J. R. Taylor. 1978. Sexuality and cyst formation in Pacific strains of the toxic dinoflagellate Gonyaulax tamarense. J. Phycol. 14:235-238. [Google Scholar]
- 54.Urbance, J. W., B. J. Bratina, S. F. Stoddard, and T. M. Schmidt. 2001. Taxonomic characterization of Ketogulonigenium vulgare gen. nov., sp. nov. and Ketogulonigenium robustum sp. nov., which oxidize l-sorbose to 2-keto-l-gulonic acid. Int. J. Syst. Evol. Microbiol. 51:1059-1070. [DOI] [PubMed] [Google Scholar]
- 55.Wagner-Döbler, I., H. Rheims, A. Felske, R. Pukall, and B. J. Tindall. 2003. Jannaschia helgolandensis, gen. nov., sp. nov., a novel abundant member of the marine Roseobacter clade from the North Sea. Int. J. Syst. Evol. Microbiol. 53:731-738. [DOI] [PubMed] [Google Scholar]
- 56.Weidner, S., W. Arnold, E. Stackebrandt, and A. Pühler. 2000. Phylogenetic analysis of bacterial communities associated with leaves of the seagrass Halophila stipulacea by a culture-independent small-subunit rRNA gene approach. Microb. Ecol. 39:22-31. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimatsu, S. 1981. Sexual reproduction of Protogonyaulax catenella in culture. Bull. Plankton Soc. Jpn. 28:131-139. [Google Scholar]
- 58.Yoshinaga, I., T. Kawai, T. Takeuchi, and Y. Ishida. 1995. Distribution and fluctuation of bacteria inhibiting the growth of a marine red tide phytoplankton Gymnodinium mikimotoi in Tanabe Bay (Wakayama Pref., Japan). Fish. Sci. 61:780-786. [Google Scholar]
- 59.Yoshinaga, I., M.-C. Kim, N. Katanozaka, I. Imai, A. Uchida, and Y. Ishida. 1998. Population structure of algicidal marine bacteria targeting the red tide forming alga Heterosigma akashiwo (Raphidophyceae), determined by restriction fragment length polymorphism analysis of the bacterial 16S ribosomal RNA genes. Mar. Ecol. Prog. Ser. 33:33-44. [Google Scholar]
- 60.Zubkov, M. V., B. M. Fuchs, P. H. Burkill, and R. Amann. 2001. Comparison of cellular and biomass specific activities of dominant bacterioplankton groups in stratified waters of the Celtic Sea. Appl. Environ. Microbiol. 67:5210-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]