Abstract
In humans, nonstarch polysaccharides (NSP), such as arabinoxylans (AX), are not digested in the upper gut and provide fermentable carbon sources for bacteria growing in the large bowel. Despite the ubiquity of AX in nature, the microbiologic and physiologic consequences of AX digestion in the gut are poorly understood. In this study, we investigated the breakdown of ferulic acid-cross-linked AX (AXF) and non-cross-linked AX in children's intestinal microbiotas, using starch as a readily fermentable polysaccharide for comparative purposes. The experiments were performed using pH-controlled fermentation vessels under anaerobic conditions. The results demonstrated that there was variation in the metabolism of these polysaccharides by colonic microbiotas. AX was always degraded more slowly than starch, while ferulic acid cross-linking reduced the rate of AX fermentation, as shown by fermentation product measurements. Starch digestion was associated with significant acetate and butyrate production, whereas AX breakdown resulted in increased propionate formation. In general, the presence of fermentable carbohydrate significantly increased the total anaerobe counts and eubacterial rRNA concentrations (P < 0.01), while non-cross-linked AX digestion was principally associated with increased viable counts of Bacteroides fragilis group organisms, which was supported by increases in Bacteroides-Porphyromonas-Prevotella group rRNA (P < 0.01). Starch was considerably more bifidogenic than AX in these fermentations. In conclusion, in this study we found that the effects of AX and AXF on the microbial ecology and metabolism of intestinal microbiotas are similar in children and adults.
Substantial amounts (8 to 18 g) of nonstarch polysaccharides (NSP) enter the human colon daily (3). In European countries, approximately 50% of this material is derived from cereals, 31% is derived from vegetables, and 16% is derived from fruit (7). Although formed from only 10 common monosaccharides, the carbohydrate polymers found in plant cell walls are structurally diverse (31, 32). The physical properties of these polysaccharides are also affected by the presence of lignin, which provides hydrophobic surfaces and charged groups that modify their ionic properties (35).
NSP can be differentiated into celluloses, pectins, and hemicelluloses. Cellulose is an unbranched β(1-4)-linked homopolymer of up to 15,000 d-glucopyranose residues (6). Extensive hydrogen bonds link these straight chains together to form a water-insoluble fibrous substance, which is difficult to break down, although the presence of substituent groups disorders the bonds and increases the solubility (6). The majority of dietary cellulose reaches the colon intact, where it can be partially degraded by cellulolytic bacteria, although the extent of this process is dependent on physical characteristics of the polymers, such as the degree of lignification (6).
Pectins are rich in galacturonic acid, which forms the main component of the polymer through α(1-4) glycosidic linkages. Regions of the molecule are heavily substituted with arabinan, galactan, and arabinogalactan side chains, and there is evidence of extensive cross-linking through glycosidic linkages or through ferulic acid esterified to pectic components (33). Nevertheless, pectins are mainly water soluble and are rapidly fermented by human gut bacteria (12).
Hemicelluloses have β(1-4)-linked backbones of xylose, mannose, or glucose. Xyloglucan is the major hemicellulose in vegetables and, when present in cereals, is less substituted with xylose (31, 43). Arabinoxylans (AX) are some of the predominant hemicelluloses in cereals, accounting for 30% of the dry weight, compared with 5% of the dry weight in vegetables (4). AX are primary components of the walls that surround plant cells in the starchy endosperm of most cereals, although the major dietary source is likely to be wheat, since approximately 64% of some nonendosperm tissues are composed of AX (15, 31).
Cereal AX consist of backbone chains of β(1-4)-linked d-xylopyranosyl residues to which α-l-arabinofuranose units are linked as side chains. Although most of these side chains are monomeric, small proportions of oligomeric side chains consisting of two or more arabinosyl residues, linked via α(1-2), α(1-3), and α(1-5) bonds, have been reported for some AX (18). In general, AX from rice consist of more highly branched xylan backbones than AX from wheat, rye, and barley, and they may also contain galactose and glucuronic acid moieties in addition to the pentose sugars. The degree and distribution of side chains are important factors in the physiochemical properties of AX. Continuous segments of unsubstituted xylose residues permit intermolecular realignments and interchain associations, while arabinosyl moieties appear to stiffen the molecules. As a result, AX exhibit high viscosities in aqueous solutions (18).
The degree of viscosity is strongly affected by the extent of polymer cross-linking. In the presence of free radical-generating agents, such as hydrogen peroxide and peroxidase, AX form three-dimensional networks through coupling of two adjacent ferulic acid residues. Polymers with high ferulic acid contents and relatively unsubstituted xylan backbones are capable of extensive cross-linking, yielding well-developed gel networks, which greatly increases the hydration capacity of the polymers (18). Ferulic acid-cross-linked arabinoxylans (AXF) may hold up to 100 g of water per g of polymer (19). When added to wheat flour, AX compete with other constituents of dough for water and have been shown to enhance the loaf volumes of breads. Treatment of wheat flour with poor baking properties with hydrogen peroxide and peroxidase has a similarly positive effect (18, 45). AX have also been shown to affect starch retrogradation and staleness in baked products, since the increased moisture content lowers the rigidity of these substances (2). As a result, AX are becoming increasingly important additives in the food industry.
Despite the ubiquity of these polymers in the human diet, there is little information available concerning the microbiologic and physiologic consequences of AX breakdown in the large bowel. Recently, the Advisory Committee on Novel Foods and Processes sought to increase the base of knowledge regarding the fate of AX in the digestive tract (5). Children were considered to be a potentially vulnerable group with respect to possible adverse intestinal symptoms, which could result if immature gut microbiotas were unable to properly degrade these substrates or if the degradation led to excess fermentation acid production.
The aims of the present investigation, therefore, were to study the way in which AX are broken down by the intestinal bacteria in children, to investigate the fermentation products formed, as well as changes in specific groups of gut microorganisms, and to determine the effects of ferulic acid cross-linking on these processes.
MATERIALS AND METHODS
Cross-linked AX.
AXF was produced by adding 250 ml of horseradish peroxidase (550 U/mg; Sigma, Poole, Dorset, United Kingdom) to obtain a concentration of 0.1% (wt/vol) and 500 μl of hydrogen peroxide (3%, wt/vol) to 250 μl of an AX solution (2%, wt/vol), which was then stirred for 30 s and then left overnight at 4°C. Before use, the preparation was homogenized to produce a uniform gel.
Fermentation studies.
Fecal samples from 10 children who were 16 months to seven years old (mean age, 3 years, 7 months; three males and seven females) were transferred to the laboratory for processing within 1 h of collection. Fecal slurries (20%, wt/vol) were prepared in prereduced (boiled for 5 min and cooled under a headspace of oxygen-free nitrogen) sodium phosphate buffer (0.1 M, pH 6.5) and mixed for 10 min in a stomaching homogenizer under a normal air headspace by using established methods (25, 29). Large particles were removed by passage through a 500-μm-pore-size sieve. Four fermentation vessels were set up for each sample. All of the vessels contained 25 ml of basal medium containing (per liter) 20.0 g of NaHCO3, 0.4 g of CaCl2 · 2H2O, 20.0 g of NH4Cl, 1.6 g of MgSO4 · 7H2O, 14.0 g of NaCl, 28.0 g of KH2PO4, 2.0 g of cysteine, 0.2 g of hemin, and 0.004 g of menadione. The medium also contained thiamine, p-aminobenzoic acid, and vitamin B12 at concentrations of 400, 200 and 200 μg/liter, respectively. The culture medium was made anaerobic by cooling and maintaining it under a headspace of oxygen-free gas (N2-CO2-H2, 80:10:10) at 80°C immediately following autoclaving. Aliquots (25 ml) of fecal slurry were added to each fermentor. The control vessels contained either no substrate or Lintner's starch (BDH Ltd., Poole, Dorset, United Kingdom) at a concentration of 10 g/liter. Test vessels contained 25 ml of cross-linked or non-cross-linked AX (final concentration, 10 g/liter). Experiments were done by using fecal samples from each of the children, and aliquots of culture fluid were taken at the start of incubation and after 6, 12, 24, and 48 h of incubation. Samples were immediately frozen and stored at −80°C for subsequent analysis of fermentation products, residual carbohydrate, and 16S rRNA abundance. Populations of numerically predominant bacteria in the fermentors were identified to the genus level in 5 of the 10 experiments.
Characterization of bacteria.
Fecal bacteria were cultured as follows. Samples (1 ml) were removed from the fermentors and serially diluted in an anaerobic chamber containing an H2-CO2-N2 (10:10:80) atmosphere by using prereduced half-strength peptone water as the diluent (30). Aliquots (0.1 ml) of appropriate dilutions were plated in triplicate onto a variety of selective and nonselective culture media, as follows: Wilkins-Chalgren agar (for total anaerobes), nutrient agar (for total aerobes), MRS agar (for bifidobacteria and lactobacilli), Beerens agar (1) (for total bifidobacteria), Rogosa agar (for total lactobacilli), azide blood agar base (for enterococci), MacConkey agar no. 2 (for enterobacteria and enterococci), perfringens agar with antibiotic supplements (for Clostridium perfringens), and bacteroides mineral salts medium (24) (for the Bacteroides fragilis group). Total facultative anaerobe counts were determined by using nutrient agar. Plates were incubated for up to 5 days (aerobically or in the anaerobic chamber). The predominant culturable bacteria were identified on the basis of morphology, fermentation product formation (25), the Gram reaction, and the results of biochemical tests by using API 20A and API 20E bacterial identification kits (BioMérieux UK Ltd., Basingstoke, Hampshire, United Kingdom).
Fermentation product analysis.
Short-chain fatty acids (SCFA) and other carboxylic acids were extracted and measured by gas chromatography by using a Pye model 204 gas chromatograph fitted with a flame ionization detector. SCFA were extracted by procedures described by Holdeman et al. (16), except that a tert-butylacetic acid internal standard (30 mmol/liter) was added. SCFA were separated by using Unicam 10% FFAP, 100/120 mesh Chromosorb WAW-DCMS in a glass column (1.8 m by 2 mm [inside diameter]). The injector, detector, and column temperatures were 200, 300, and 155°C, respectively. The flow rates of the nitrogen carrier gas and the hydrogen and air detector gases were 50, 30, and 390 ml/min. Lactate and succinate were measured by using the same analytical system after methylation of the samples (16). The injector, detector, and column temperatures were 175, 175, and 140°C, respectively.
Carbohydrate analysis.
The constituent monosaccharides of the test carbohydrates were measured by methods described by Englyst et al. (11). Briefly, samples (0.5 ml) were hydrolyzed by addition of 2.5 ml of sulfuric acid (2.4 M), followed by heating in a boiling water bath for 1 h. An internal standard (0.5 ml of allose [0.5 mg/ml] in 50% saturated benzoic acid) was added to 1 ml of each sample and standard hydrolysate. Samples were then derivitized, and this was followed by quantitation of the constituent sugars as alditol acetates by gas chromatography by using a Supelco SP-2330 wide-bore capillary column (30 m by 0.75 mm) with a flame ionization detector. The column temperature was 220°C, and the injector and detector were maintained at 275°C. The carrier gas (helium) flow rate was 8 ml/min.
16S rRNA analysis.
rRNA was extracted, blotted, and hybridized by using procedures described previously (41). The nucleic acid probes Bact338 (13), Entero1432 (40), Bacto1080 (40), and Bif1278 (21) were used to measure the abundance of total eubacteria, enterobacterial species, the Bacteroides-Porphyromonas-Prevotella (BPP) group, and total bifidobacteria, respectively. Samples removed from fermentors (500 mg) were subjected to direct phenol extraction by mechanical disruption in the presence of sterile zirconium beads (300 mg) in 2.2-ml screw-cap tubes with a Mini-Bead Beater (Biospec Products, Bartlesville, Okla.) (41). Glassware was baked at 300°C overnight, and solutions were prepared by using diethyl pyrocarbonate-treated double-distilled water. The mechanical disruption was followed by another extraction with phenol saturated with Tris-HCl buffer (100 mM, pH 5.1) and sequential phenol-chloroform-isoamyl alcohol (24:24:1, pH 5.1) and chloroform-isoamyl alcohol extractions. Total rRNA was precipitated at 20°C for 3 h with ammonium acetate (final concentration, 1 M). After two washes in 80% ethanol, the pellets were resuspended in 50 μl of double-distilled water. Nucleic acid concentrations were estimated spectrophotometrically (an optical density at 260 nm of 1.0 corresponded to an RNA concentration of 40 μg/ml). The quality of extracted RNA was evaluated by polyacrylamide gel electrophoresis (Mighty Small II slab gel electrophoresis unit; Hoefer Instruments, San Francisco, Calif.). Nucleic acids were then denatured and diluted to obtain a concentration of 1.5 ng/μl. Samples were applied in triplicate (50 μl per slot) to Magna Charge membranes (Micron Separation, Inc., Westboro, Mass.) by using a slot blot device (Minifold II; Schleicher & Schuell, Inc., Keene, N.H.) and a vacuum sufficient to pull the sample through the membrane in 1 to 2 min. The membranes were then air dried and baked (2 h at 80°C). The baked membranes were then prewetted in hybridization buffer (0.9 M NaCl, 50 mM sodium phosphate [pH 7.0], 5 mM EDTA, 10× Denhardt solution [41], 0.5% sodium dodecyl sulfate) and placed in hybridization tubes (Robbins Scientific, Sunnyvale, Calif.). The membranes were incubated in a rotating incubator with approximately 10 ml of hybridization buffer for 2 h at 40°C. The first hybridization buffer was discarded, and labeled probe was added by including it in 10 ml of hybridization buffer. Incubation was then continued at 40°C for 16 to 20 h. After incubation with the probe, the membranes were washed in the hybridization tubes with 100 ml of 1% sodium dodecyl sulfate-1× SSC (0.15 M NaCl plus 0.015 M sodium citrate, pH 7.0) for 2 h at 40°C. The membranes were then removed from the hybridization tubes and washed twice for 15 min in 300 ml of 1% sodium dodecyl sulfate-1× SSC at the experimentally determined dissociation temperatures for individual probes (42), as follows: Bact338, 54°C; Entero1432, 43°C; Bacto1080 50°C; and Bif1278, 56.6°C. The hybridization signals on air-dried membranes were quantitated by using an Instant Imager (Canberra Packard, Pangbourne, Berkshire, United Kingdom). The time of exposure varied depending on the intensity of the 32P signal. The signal was analyzed by using ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.). Standard curves were calculated by using reference RNA by linear regression. The levels of specific groups of organisms were expressed as percentages of the total bacterial small-subunit rRNA in a sample, and the quantities of RNA for each probe group were also expressed as the total quantities of rRNA per milliliter of fermentor fluid.
Statistics.
A one-way analysis of variance was used to compare the four in vitro fermentation vessels, and values were calculated with Data Desk software, version 6.0 (Data Description Inc., Ithaca, N.Y.).
Ethical permission.
Full parental consent was obtained for these investigations, and the study was approved by the local research ethics committee.
Chemicals.
Unless stated otherwise, all chemicals were obtained from Sigma Chemical Co. Bacterial growth media and associated antibiotic supplements were purchased from Oxoid (Basingstoke, Hampshire, United Kingdom).
RESULTS
Polysaccharide breakdown.
Table 1 summarizes the results obtained for breakdown of AX and starch carbohydrate components by fecal material obtained from 10 children. The data show that starch was rapidly degraded while AX was utilized considerably more slowly. Both the arabinose side chains and xylose backbones of AX and AXF were utilized by fecal bacteria, although AXF was broken down more slowly, as shown by the residual arabinose (P < 0.01), xylose (P < 0.01), and glucose (P < 0.05) in these cultures at 24 h. The percentages of galactose and arabinose decreased steadily, while little xylose was assimilated within the first 6 h, particularly when AXF was the substrate. Measurements of specific breakdown rates of individual substrate components showed that starch was degraded considerably more rapidly than the AX were degraded and that xylose was digested faster than arabinose (Table 1). The ratio of arabinose to xylose decreased with time in vessels supplemented with cross-linked AX, while in vessels supplemented with the non-cross-linked polymer the ratio increased after 12 h (Table 2). As a result, ferulic acid cross-linking significantly lowered the arabinose/xylose ratio at 24 h (P < 0.05). Considerable interindividual variation was observed with respect to substrate utilization, and a notable example of this was the observation that fecal bacteria from one of the children did not degrade either AX or AXF but did ferment starch (data not shown).
TABLE 1.
Maximum rates of utilization of polysaccharide carbohydrate constituents in pH-controlled incubation mixturesa
| Substrate | Utilization (μg/h/g [wet wt]) ofb:
|
||||
|---|---|---|---|---|---|
| Arabinose | Xylose | Galactose | Glucose | Total | |
| AXc | 112 (14.9) | 260 (30.0) | 31.7 (0.2) | 20.0 (4.6) | 410 (42.1) |
| AXF | 115 (10.9) | 230 (24.6) | 25.0 (2.5) | 20.0 (2.8) | 370 (4.0) |
| Starch | NAd | NA | NA | 1,150 (71.0) | 1,150 (75.9) |
Data for major sugars are shown; data for minor components are not shown.
The values are means (n = 10); the values in parentheses are standard errors of the means.
Differences in the utilization rates of AX and AXF are not statistically significant.
NA, not applicable.
TABLE 2.
Arabinose/xylose ratios in pH-controlled fecal cultures supplemented with AXF and non-cross-linked AXa
| Incubation period (h) | Arabinose/xylose ratio with:a
|
|
|---|---|---|
| AX | AXF | |
| 0 | 0.51 (0.00) | 0.51 (0.00) |
| 6 | 0.42 (0.02) | 0.45 (0.01) |
| 12 | 0.42 (0.02) | 0.42 (0.02) |
| 24 | 0.48 (0.03)b | 0.41 (0.01) |
| 48 | 0.60 (0.05)b | 0.40 (0.03) |
The values are means (n = 10). The values in parentheses are standard errors of the means.
The means are significantly different (P < 0.05).
Fermentation product formation.
Table 3 shows the SCFA formed from the test polymers. The concentrations of SCFA increased rapidly in cultures incubated with starch, and the concentrations were always higher in these fermentors than in the AX fermentation vessels. This became significant when the AX form was compared to the cross-linked form at 24 h (P < 0.05). Formation of acetate and butyrate was markedly increased in starch cultures (Table 3), while fermentation of AX resulted in more propionate production. The AX and AXF substrates were metabolized similarly, although more SCFA were produced from the non-cross-linked form, particularly after 12 h. Acetate and propionate were invariably the major AX metabolites, while lactate was never detected. In contrast, lactate was a major, although transient, product of starch metabolism.
TABLE 3.
Production of fermentation acids in pH-controlled fecal incubation mixtures containing AX, AXF, and starcha
| Substrate | Incubation period (h) | Production (mM) of:
|
|||||
|---|---|---|---|---|---|---|---|
| Acetate | Propionate | Butyrate | Total SCFA | Lactate | Succinate | ||
| AX | 0 | 4.4 (0.7) | 0.7 (0.1) | 0.4 (0.1) | 5.4 (0.9) | NDb | ND |
| 6 | 18.8 (4.5) | 2.3 (0.6) | 0.5 (0.3) | 21.6 (5.2) | ND | 4.1 (1.7) | |
| 12 | 45.5 (11.8) | 6.8 (1.3) | 2.3 (1.1) | 54.3 (13.4) | ND | 8.0 (2.2) | |
| 24 | 62.1 (11.5) | 15.9 (2.6) | 4.1 (2.0) | 82.0 (14.7)c | ND | 8.7 (2.4) | |
| 48 | 72.0 (11.8) | 22.5 (2.7) | 9.7 (4.6) | 104 (15.9) | ND | 6.8 (2.1) | |
| AXF | 0 | 4.5 (0.9) | 0.8 (0.2) | 0.5 (0.1) | 5.8 (1.1) | ND | ND |
| 6 | 16.8 (3.4) | 2.5 (0.6) | 0.9 (0.3) | 20.3 (4.2) | ND | 2.0 (1.0) | |
| 12 | 43.4 (11.6) | 7.2 (1.2) | 1.7 (0.6) | 50.9 (13.2) | ND | 6.5 (1.8) | |
| 24 | 55.4 (11.4) | 14.9 (3.0) | 2.6 (0.5) | 72.9 (13.6)c | ND | 6.1 (1.4) | |
| 48 | 55.8 (8.7) | 17.7 (3.3) | 3.4 (0.6) | 73.4 (10.9) | ND | 5.1 (1.6) | |
| Starch | 0 | 3.4 (0.7) | 0.9 (0.2) | 0.5 (0.2) | 4.9 (0.9) | ND | 0.1 (0.1) |
| 6 | 54.9 (11.0) | 3.2 (0.6) | 2.7 (0.9) | 60.8 (11.7) | 9.8 (2.3) | 6.2 (1.8) | |
| 12 | 64.0 (13.8) | 7.3 (1.2) | 10.0 (2.4) | 81.4 (15.2)c | 0.7 (0.7) | 9.0 (1.7) | |
| 24 | 75.0 (12.0) | 11.6 (1.9) | 11.6 (1.9) | 98.2 (12.2) | 0.6 (0.6) | 7.3 (1.8) | |
| 48 | 81.4 (13.3) | 13.4 (2.2) | 13.1 (2.3) | 108 (13.8) | 0.3 (0.3) | 5.8 (1.5) | |
The values are means (n = 10). The values in parentheses are standard errors of the means. The concentrations of minor SCFA were <0.1 mM.
ND, not detected.
The means are significantly different for each substrate (P < 0.05).
Bacteriology.
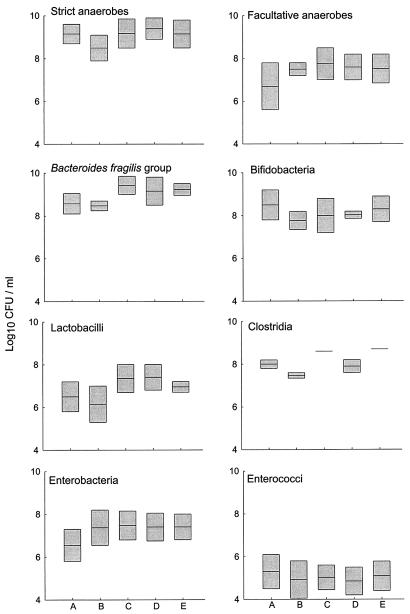
Figure 1. shows the effects of starch and AX on bacterial populations from five randomly selected children as determined in the fermentation studies (all selected samples were able to degrade AX and AXF). The presence of an added carbohydrate source increased the total anaerobe count compared with the count in the control vessel, while facultative anaerobes, particularly enterobacteria, proliferated even in the absence of an exogenous carbon source. Marked interindividual variation was apparent with respect to cell counts for the bacterial species enumerated in these investigations. Notably, clostridia were detected in only two of the five subjects.
FIG. 1.
Effects of AX and starch on culturable bacterial populations following 12 h of incubation. A, fermentor prior to incubation; B, fermentor with no additional carbohydrate; C, fermentor with AX; D, fermentor with AXF; E, fermentor with starch. The boxes indicate the standard errors of the means for data from five randomly chosen volunteers; the horizontal lines within the boxes indicate the means.
The numbers of bacteroides and bifidobacteria were generally high in these studies, and the summarized data show that bacteria belonging to these groups were able to grow on the test carbohydrates. Bacteroides exhibited some preference for AX, while lactobacilli were stimulated by both AX and AXF. However, the population differences were not statistically significant. Indeed, in the polysaccharide fermentation vessels, few consistent changes were observed in bacterial cell counts, and no statistically significant variation in viable cell numbers was observed when the different test carbohydrates were compared.
rRNA abundance.
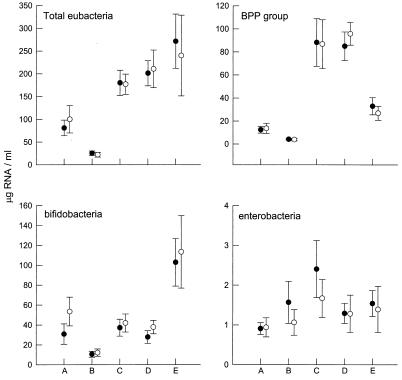
Carbohydrate fermentation resulted in highly significant increases in the levels of total bacterial rRNA, and the highest concentrations were detected in vessels to which starch had been added (Fig. 2). The bifidobacterial and BPP group signals declined in control vessels, and although all of the test carbohydrates caused increases in rRNA, substrate-specific differences were apparent. Compared to the results obtained with starch, AXF and AX caused twofold increases in the BPP group rRNA levels (P < 0.01 and P < 0.01, respectively), while the effect was reversed for bifidobacterial rRNA, the levels of which were significantly higher with starch. Ferulic acid cross-linking had little effect on 16S rRNA signals; however, AX fermentation resulted in a level of enterobacterial rRNA that was significantly greater than the level observed after AXF fermentation (P < 0.05).
FIG. 2.
Effects of AX and starch on 16S rRNA concentrations. Symbols: •, means for all 10 volunteers; ○, means for the five volunteers represented in Fig. 1. The error bars indicate standard errors of the means.
When expressed as a percentage of total bacterial rRNA, the ability of AX to increase the level of BPP group rRNA was still highly significant compared to the ability of starch (Fig. 2). The profile of bifidobacterial rRNA, however, was altered in that the AX were unable to support bifidobacterial growth. The results for enterobacteria were also affected; carbohydrate reduced the proportion of specific rRNA compared to the proportion in control vessels (P < 0.0001), although the ability of AX to induce higher levels of enterobacterial rRNA than the levels obtained with AXF was still significant (P < 0.05).
DISCUSSION
Dietary carbohydrates are important growth substrates for bacterial communities colonizing the human gut (6, 12). We found that children's gut microfloras degraded AX, AXF, and starch extensively, in a manner similar to adult gut microbiotas (12). BPP group bacteria were particularly active in AX breakdown, as shown by the increased levels of specific rRNA in fermentors amended with these substrates compared the levels in fermentors amended with starch (Fig. 2). Bacteroides is a nutritionally versatile genus, and members of this genus utilize a diverse range of NSP as fermentable carbon sources through the elaboration of a variety of hydrolytic enzymes (36). Production of xylanolytic enzymes by the colonic microflora has been well documented (27, 38), while expression of xylanase, β-xylosidase, and α-arabinofuranosidase by Bacteroides species is inducible (23).
The apparent absence of lactate formation and the production of acetate and propionate by fecal bacteria in our studies are in agreement with results obtained with adults (12), showing that the major bacterial populations responsible for NSP breakdown in adults are present in children's fecal microbiotas. The relatively high proportions of acetate, propionate, and, to a lesser degree, succinate in the AX fermentors support the contention that members of the genus Bacteroides are particularly active in AX metabolism (24).
The results of fermentation acid production and residual carbohydrate analysis reflected the structural complexity of these polysaccharides, since AX were utilized more rapidly than the viscous, cross-linked derivative of AX, AXF. The ratios of arabinose to xylose in the fermentors suggested that polymer breakdown with AX and polymer breakdown with AXF were qualitatively different (Table 2). The initial decrease in this ratio in AX vessels resulted from α-arabinofuranosidase activity removing arabinosyl moieties from the polymer, which then became available for fermentation. However, when AX polymers were linked by diferulic acid bonds, arabinose side chains were continuously utilized at a greater rate than xylose was utilized. Therefore, even after substituent groups had been removed, the xylan backbone was more resistant to degradation, probably because the gel-like structure restricted access of xylanolytic enzymes to their target sites. As a result, xylanase activity became rate limiting, and arabinose moieties were preferentially utilized.
Studies of xylan degradation in ruminal bacteria, such as Ruminococcus flavefaciens and Butyrivibrio fibrisolvens, indicated that xylanase activities could reach high levels in culture supernatants (14, 34). Many cellulolytic ruminal bacteria can extensively degrade hemicelluloses, although only a few strains are capable of significant growth on these substrates (10). When Bacteroides cells were grown in pure culture with xylan as the sole source of carbohydrate, a variety of low-molecular-weight carbohydrates were released into the growth medium, even when carbohydrate was limiting (37). Endoxylanase activity appeared to be the rate-limiting step in xylan degradation by these bacteria, and the release of xylooligosaccharides and xylose might provide a carbohydrate source for nonendoxylanolytic species that have a higher affinity for them. Yamada et al. (47) demonstrated that wheat bran oligosaccharides stimulated bifidobacterial growth, although this was not observed in our investigations. The carbon sources used in the two investigations differed not only in chain length but also in the degree of arabinose side chain substitution, since our initial arabinose/xylose ratio (0.30) was lower than that in the Japanese study. Thus, it appears that the structural complexity of AX inhibits enzymatic degradation of these polymers by bifidobacteria.
Interestingly, an arabinofuranohydrolase that is specific for AX has been isolated from a strain of Bifidobacterium adolescentis, and this enzyme was unique because of its ability to remove arabinosyl moieties from double-substituted xylose units in the polymer (44). However, given the comparatively low abundance of bifidobacteria in our studies (Fig. 2), this type of xylose unit was unlikely to have been a major component of the polymers, or a similar enzyme was associated with BPP group organisms. Strains of B. adolescentis and Bifidobacterium infantis have been reported to ferment xylan, although the extent and rate of degradation were not noted (39). While some strains of bifidobacteria evidently produce glycosidases capable of degrading AX (9), no endoxylanase has been accurately described for these organisms, and the low metabolic activity of bifidobacteria in our experiments indicates that the organisms may not be able to produce such enzymes.
The rate of transcription in cells lacking genes for endoxylanase is likely to be lower than the rate of transcription in cells actively expressing this enzyme, although by scavenging free xylose and small oligomers such bacteria could obtain sufficient carbon and energy for growth. This could partially explain discrepancies between viable counts, which showed little change in the fermentation studies, and the 16S rRNA measurements, which were significantly different (Fig. 2). However, since the concentration of BPP group rRNA was more than twice that of bifidobacterial rRNA, population size was probably also a factor.
Utilization of AX by species lacking endoxylanase activity is dependent on the rate and the extent to which these organisms can assimilate the oligomers and monosaccharides released by primary polymer-degrading species. Previous studies showed that xylooligosaccharides were poor substrates for bifidobacteria when they were compared to other carbohydrates (17), and this conclusion is supported by the relatively low rRNA levels specific for these bacteria in the AX cultures. Jaskari et al. (20) used low-molecular-weight oligomers made from xylan as growth substrates for pure-culture studies with several bacterial species commonly isolated from the human large intestine. Bifidobacteria were found to be more efficient at degrading the xylooligomers than the three species of Bacteroides tested were. Interestingly, the Bacteroides species were unable to utilize oligomers with a degree of polymerization of five, while bifidobacteria degraded all the larger oligomers and the concentration of xylose in the media actually increased. This suggests that the bifidobacteria produced high levels of β-xylosidase, although they were not able to cleave oligomers from AX, these organisms were outcompeted for this substrate in fecal cultures.
Feruloyl esterase activity may be an important factor determining the rate of degradation in the AXF cultures, since this enzyme cleaves ferulic acid from the arabinose side chain, thereby releasing xylan polymers from the gel matrix. Feruloyl esterases have only recently been discovered, partly because xylans are often isolated by alkaline extraction, during which the ester groups are saponified. Hence, relatively little information regarding this enzyme has been available. Although experiments with the intestinal microflora of rats have revealed high levels of feruloyl esterase activity (46), it is not yet clear which intestinal bacteria are responsible for production of this enzyme. Figure 2 shows that formation of ferulic acid cross-links between AX polymers significantly reduced the abundance of enterobacteria. Unlike the activity of bifidobacteria, a selective increase in the colonic activity of Bacteroides spp. is not normally considered to be beneficial per se (29), although these bacteria are often numerically dominant in healthy adults (23). Conversely, enterobacteria are frequently identified as the causative agent of diarrhea in humans, and thus, the formation of diferulic acid bonds between AX polymers in foodstuffs could be viewed as being beneficial since these carbohydrates are less efficient enterobacterial growth substrates. Moreover, while over 85% of the AX was utilized within 48 h, cross-linking was found to reduce the extent of bacterial degradation, indicating that this form is more likely to have greater fecal bulking properties in vivo.
It should be noted, however, that bacterial digestion of AX in the large gut is strongly affected by the presence of other fermentable carbohydrates. The metabolic pathways of bacteria involved in the depolymerization of complex carbohydrates and the uptake and fermentation of sugars and oligosaccharides are controlled by a number of catabolite regulatory processes (28). Previous studies have demonstrated that starch and xylan are coutilized by mixed populations of fecal bacteria (12). In vitro experiments in which polysaccharide degradation by human intestinal bacteria was investigated under multi-substrate-limiting conditions provided clear evidence of bacterial substrate preferences and the sequential utilization of some carbohydrates (28). At high bacterial growth rates, xylan degradation was found to be catabolite repressed, and carbohydrates such as starch and pectin were preferentially utilized. This suggests that utilization of AX by colonic bacteroides is greatest in the distal bowel, where carbohydrate concentrations are low (22), although this could be affected by diet and intestinal transit time (8).
In agreement with many previous studies in which adult microbiotas were used, starch was rapidly fermented, and over 70% of the starch was degraded during the first 6 h of incubation (Table 1). rRNA analysis demonstrated that bifidobacteria were able to compete effectively for glucose polymers in fecal bacterial cultures, indicating that resistant starch could have a considerable influence on bacterial metabolism in the large intestine. Many species of bacteria are capable of digesting this polysaccharide (26), although Bacteroides spp. and enterobacteria were less active in starch breakdown than bifidobacteria (Fig. 1 and 2). Human feces are known to have very high extracellular amylase activity (26), which could provide nonamylolytic species with fermentable substrates. Pure-culture studies have shown that glucose improved the growth of bifidobacteria and lactobacilli compared to the growth of Bacteroides and Escherichia coli (17).
Acetate, propionate, and butyrate were the principal fermentation products of starch utilization (Table 2), which together with the transient formation of lactate correlated well with the results of previous work in which adult stool samples were used (26). The initially rapid production of acetate and lactate supported the hypothesis that species belonging to the genus Bifidobacterium and/or the genus Lactobacillus are stimulated by starch, since these are the major fermentation products of these organisms. However, the formation of succinate and the later accumulation of butyrate suggest involvement of other species, such as Bacteroides and Clostridium species (16).
In conclusion, our investigations demonstrated that children's gut microbiotas degrade xylose-containing polysaccharides in a way analogous to the way in which adult microbiotas degrade them, that Bacteroides plays a major role in the degradation of AX in children's fecal microbiotas, and that, with the exception of enterobacterial metabolism, bacterial metabolism was not markedly affected by ferulic acid cross-linking between polymers. The introduction of diferulic acid bridges did, however, alter the way in which xylanolytic enzymes digested AX, and competition for xylose residues between fecal bacteria was found to be more intense than competition for arabinose. Overall, our results show that the digestive fates of AX and AXF in children are likely to be similar to the fates of other NSP.
REFERENCES
- 1.Beerens, H. 1990. An elective and selective isolation medium for Bifidobacterium spp. Lett. Appl. Bacteriol. 11:155-157. [Google Scholar]
- 2.Biliaderis, C. G., M. S. Izydorczyk, and O. Rattan. 1995. Effect of arabinoxylans on bread-making quality of wheat flours. Food Chem. 53:165-171. [Google Scholar]
- 3.Bingham, S. A. 1990. Mechanisms and experimental and epidemiological evidence relating dietary fibre (non-starch polysaccharides) and starch to protection against large bowel cancer. Proc. Nutr. Soc. 49:153-171. [DOI] [PubMed] [Google Scholar]
- 4.Cartano, A. V., and B. O. Juliano. 1970. Hemicelluloses of milled rice. J. Agric. Food Chem. 18:40-42. [DOI] [PubMed] [Google Scholar]
- 5.Charalampopoulos, D., R, Wang, S. S. Pandiella, and C. Webb. 2002. Application of cereals and cereal components in functional foods: a review. Int. J. Food Microbiol. 79:131-141. [DOI] [PubMed] [Google Scholar]
- 6.Cummings, J. H. 1984. Cellulose and the human gut. Gut 25:805-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings, J. H. 1995. Dietary fibre intakes in Europe: overview and summary of European research activities, conducted by members of the Management Committee of COST 92. Eur. J. Clin. Nutr. 49:S5-S9. [PubMed] [Google Scholar]
- 8.Cummings, J. H., and G. T. Macfarlane. 1991. The control and consequences of bacterial fermentation in the human colon: a review. J. Appl. Bacteriol 70:443-459. [DOI] [PubMed] [Google Scholar]
- 9.Degnan, B. A., and G. T. Macfarlane. 1995. Arabinogalactan utilization in continuous cultures of Bifidobacterium longum: effect of co-culture with Bacteroides thetaiotaomicron. Anaerobe 1:103-112. [DOI] [PubMed] [Google Scholar]
- 10.Dehority, B. A. 1965. Degradation and utilization of isolated hemicelluloses by pure cultures of cellulolytic rumen bacteria. J. Bacteriol. 89:1515-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Englyst, H. N., M. E. Quigley, G. J. Hudson, and J. H. Cummings. 1992. Determination of dietary fibre as non-starch polysaccharides by gas-liquid chromatography. Analyst 115:1707-1714. [DOI] [PubMed] [Google Scholar]
- 12.Englyst, H. N., S. Hay, and G. T. Macfarlane. 1987. Polysaccharide breakdown by mixed populations of human faecal bacteria. FEMS Microbiol. Ecol. 95:163-171. [Google Scholar]
- 13.Harmsen, H. J., G. C. Raangs., T. He, J. E. Degener, and G. W. Welling. 2002. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl Environ. Microbiol. 68:2982-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hespell, R. B., R, Wolf, and R. J. Bothast. 1987. Fermentation of xylans by Butyrivibrio fibrisolvens and other ruminal bacteria. Appl. Environ. Microbiol. 53:2849-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman, R. A., J. P. Kamerling, and J. F. Vliegenthart. 1992. Structural features of a water-soluble arabinoxylan from the endosperm of wheat. Carbohydr. Res. 226:303-311. [DOI] [PubMed] [Google Scholar]
- 16.Holdeman, L. V., E. P. Cato, and W. E. C. Moore. 1977. Anaerobe laboratory manual, 4th ed. Virginia Polytechnic Institute and State University, Blacksburg.
- 17.Hopkins, M. J., J. H. Cummings, and G. T. Macfarlane. 1998. Inter-species differences in maximum specific growth rates and cell yields of bifidobacteria cultured on oligosaccharides and other simple carbohydrate sources. J. Appl. Microbiol. 85:381-386. [Google Scholar]
- 18.Izydorczyk, M. S., and C. G. Biliaderis. 1995. Cereal arabinoxylans: advances in structure and physiochemical properties. Carbohydr. Polym. 28:33-48. [Google Scholar]
- 19.Izydorczyk, M. S., C. G. Biliaderis, and W. Bushuk. 1990. Oxidative gelation studies of water-soluble pentosans from wheat. J. Cereal Sci. 11:153-169.
- 20.Jaskari, J., P. Kontula, A. Siitonen, H. Jousimies-Somer, T. Mattila-Sandholm, and K. Poutanen. 1998. Oat β-glucan and xylan hydrosylates as selective substrates for Bifidobacterium and Lactobacillus strains. Appl. Microbiol. Biotechnol. 49:175-181. [DOI] [PubMed] [Google Scholar]
- 21.Langendijk, P. S., F. Schut, G. J. Jansen, G. C. Raangs, G. R. Kamphuis, M. H. Wilkinson, and G. W. Welling. 1995. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 61:3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macfarlane, G. T., G. R. Gibson, and J. H. Cummings. 1992. Comparison of fermentation reactions in different regions of the human colon. J. Appl. Bacteriol. 72:57-64. [DOI] [PubMed] [Google Scholar]
- 23.Macfarlane, G. T., and G. R. Gibson. 1991. Formation of glycoprotein degrading enzymes by Bacteroides fragilis. FEMS Microbiol. Lett. 61:289-293. [DOI] [PubMed] [Google Scholar]
- 24.Macfarlane, G. T., and G. R. Gibson. 1991. Co-utilization of polymerized carbon sources by Bacteroides ovatus grown in a two-stage continuous culture system, Appl. Environ. Microbiol. 57:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macfarlane, G. T., J. H. Cummings, and C. Allison,. 1986. Protein degradation by human intestinal bacteria. J. Gen. Microbiol. 132:1647-1656. [DOI] [PubMed] [Google Scholar]
- 26.Macfarlane, G. T., and H. N. Englyst. 1986. Starch utilization by the human large intestinal microflora. J. Appl. Bacteriol. 60:195-201. [DOI] [PubMed] [Google Scholar]
- 27.Macfarlane, G. T., S. Hay, S. Macfarlane, and G. R. Gibson. 1990. Effect of different carbohydrates on growth, polysaccharidase and glycosidase production by Bacteroides ovatus, in batch and continuous culture. J. Appl. Bacteriol. 68:179-187. [DOI] [PubMed] [Google Scholar]
- 28.Macfarlane, S., M. E. Quigley, M. J. Hopkins, D. F. Newton, and G. T Macfarlane. 1998. Polysaccharide degradation by human intestinal bacteria during growth under multi-substrate limiting conditions in a three-stage continuous culture system. FEMS Microbiol. Ecol. 26: 231-243. [Google Scholar]
- 29.McBain, A. J., and G. T. Macfarlane. 1998. Ecological and physiological studies on large intestinal bacteria in relation to production of hydrolytic and reductive enzymes involved in formation of genotoxic metabolites. J. Med. Microbiol. 47:407-416. [DOI] [PubMed] [Google Scholar]
- 30.McBain, A. J., and G. T. Macfarlane. 2001. Modulation of genotoxic enzyme activities by non-digestible oligosaccharide metabolism in in-vitro human gut bacterial ecosystems. J. Med. Microbiol. 50:833-842. [DOI] [PubMed] [Google Scholar]
- 31.McDougall, G. J., I. M. Morrison, D. Stewart, and J. R. Hillman. 1996. Plant cell walls as dietary fibre: range, structure, processing and function. J. Sci. Food Agric. 70:133-150. [Google Scholar]
- 32.Obel, N., A. C. Porchia, and H. V. Scheller. 2002. Dynamic changes in cell wall polysaccharides during wheat seedling development. Phytochemistry 60:603-610. [DOI] [PubMed] [Google Scholar]
- 33.Oosterveld, A., G. Beldman, and A. G. Voragen. 2000. Oxidative cross-linking of pectic polysaccharides from sugar beet pulp. Carbohydr. Res. 328:199-207. [DOI] [PubMed] [Google Scholar]
- 34.Pettipher, G. L., and M. J. Latham. 1979. Production of enzymes degrading plant cell walls and fermentation of cellobiose by Ruminococcus flavefaciens in batch and continuous culture. J. Gen. Microbiol. 110:29-38. [Google Scholar]
- 35.Rodriguez-Arcos, R. C., A. C. Smith, and K. W. J. Waldron. 2002. Effect of storage on wall-bound phenolics in green asparagus. J. Agric. Food Chem. 50:3197-3203. [DOI] [PubMed] [Google Scholar]
- 36.Salyers, A. A. 1979. Energy sources of major intestinal fermentative anaerobes. Am. J. Clin. Nutr. 32:158-163. [DOI] [PubMed] [Google Scholar]
- 37.Salyers, A. A., F. Gherardini, and M. O'Brien. 1981. Utilization of xylan by two species of human colonic Bacteroides. Appl. Environ. Microbiol. 41:1065-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salyers, A. A., J. K Palmer, and T. D. Wilkins. 1978. Degradation of polysaccharides by intestinal bacterial enzymes. Am. J. Clin. Nutr 31:S128-S130. [DOI] [PubMed] [Google Scholar]
- 39.Salyers, A. A., S. E. H. West, J. R. Vercellotti, and T. D. Wilkins. 1977. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl. Environ. Microbiol. 34:529-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sghir, A., G, Gramet, A. Suau, V. Rochet, P. Pochart, and J. Dore. 2000. Quantification of bacterial groups within human fecal flora by oligonucleotide probe hybridization. Appl. Environ. Microbiol. 66:2263-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharp, R., and G. T. Macfarlane. 2000. Chemostat enrichments of human feces with resistant starch are selective for adherent butyrate-producing clostridia at high dilution rates. Appl. Environ. Microbiol. 66:4212-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stahl, D. A., B. Flesher, H. R. Mansfield, and L. Montgomery. 1988. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl. Environ. Microbiol. 54:1079-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tharanathan, R. N. 2002. Food-derived carbohydrates—structural complexity and functional diversity. Crit. Rev. Biotechnol. 22:65-84. [DOI] [PubMed] [Google Scholar]
- 44.Van Laere, K. M. J., G. Beldman, and, A. G. J. Voragen. 1997. A new arabinofuranohydrolase from Bifidobacterium adolescentis able to remove arabinosyl residues from double-substituted xylose units in arabinoxylan. Appl. Microbiol. Biotechnol. 47:231-235. [DOI] [PubMed] [Google Scholar]
- 45.Vinkx, C. J. A., and J. A. Delcour. 1996. Rye (Secale cereale L.) arabinoxylans: a critical review. J. Cereal Sci. 24:1-14. [Google Scholar]
- 46.Wende, G., C. J. Buchanan, and S. C Fry. 1997. Hydrolysis and fermentation by rat gut microoganisms of 2-O-β-d-xylopyranosyl-(5-O-feruloyl)-l-arabinose derived from grass cell wall arabinoxylan. J. Sci. Food Agric. 73:296-300. [Google Scholar]
- 47.Yamada, H., K. Itoh, Y. Morishita, and H. Taniguchi. 1993. Advances in cereal chemistry and technology in Japan. Structure and properties of oligosaccharides from wheat bran. Cereal Foods World 38:490-492. [Google Scholar]