Abstract
Weight gain and central obesity are associated with insulin resistance, hypertension, and dyslipidemia in type 1 diabetes. These metabolic abnormalities are risk factors for kidney disease in the general population, but data addressing the relationship of central obesity with kidney disease in type 1 diabetes are limited. Whether waist circumference is associated with incident microalbuminuria and change in creatinine clearance was examined among 1279 participants who had type 1 diabetes and were enrolled in the Epidemiology of Diabetes Interventions and Complications Study, the observational extension of the Diabetes Control and Complications Trial (DCCT). Ninety-three of 1105 participants with normal albumin excretion rate (AER) at DCCT closeout developed incident microalbuminuria over 5.8 yr of follow-up. The hazard ratio for incident microalbuminuria that was associated with each 10-cm greater waist circumference at DCCT closeout was 1.34 (95% confidence interval 1.07 to 1.68), after adjustment for DCCT closeout age, gender, duration of diabetes, treatment group, smoking status, glycosylated hemoglobin, and AER. This increased risk was modestly attenuated when additional adjustment was made for levels of BP and serum lipids. Creatinine clearance declined by an average of 0.34 ml/min per 1.73 m2 each yr over 8 yr of follow-up. Greater rate of decline in creatinine clearance was associated with greater age, conventional insulin therapy during the DCCT, smoking, and greater glycosylated hemoglobin and AER at DCCT closeout but not with waist circumference. In conclusion, waist circumference predicts the subsequent development of microalbuminuria in type 1 diabetes. In contrast, no association of waist circumference with decline in creatinine clearance was observed.
Diabetic nephropathy is the leading cause of ESRD in the United States and an important cause of morbidity and mortality for individuals with type 1 diabetes (1,2). Intensive glycemic control markedly improves renal outcomes in this population (3,4). However, weight gain complicates intensive insulin therapy, and metabolic abnormalities such as central obesity, insulin resistance, hypertension, and an atherogenic dyslipidemia have emerged as a potential new threat (5). These characteristics are associated with kidney disease in the general population (6–9), but causes of kidney disease may differ for individuals with diabetes.
This study, which establishes a relationship between central obesity measured as waist circumference and development of microalbuminuria in adult type I diabetics, is linked to a study by Mitsnefes et al. in this month’s issue of CJASN (pp. 46 –50), which found that overweight children with chronic kidney disease had relatively lower adiponectin levels and therefore possibly increased risk of cardiovascular disease as well.
Waist circumference is a measure of central obesity that reflects metabolically active visceral fat (10,11). Previous studies in patients with type 1 diabetes described correlations among central obesity, insulin resistance, and microalbuminuria, an early sign of kidney disease and an important risk factor for overt nephropathy (12–16). Such associations suggest a potential causal role for central obesity and insulin resistance in the pathogenesis of renal complications in type 1 diabetes, a disease that is characterized fundamentally by insulin deficiency. However, previous studies were limited by cross-sectional design or limited follow-up, and none has examined the relationship of central obesity with other measures of kidney function, leaving the role of central obesity in diabetic kidney disease ambiguous. To delineate further the role of central obesity in the renal complications of type 1 diabetes, we examined prospectively whether waist circumference is associated with incident microalbuminuria and change in creatinine clearance over time in a large, well-characterized cohort of individuals with type 1 diabetes.
Materials and Methods
Study Population
The Diabetes Control and Complications Trial (DCCT) was a multicenter clinical trial that examined the effects of intensive insulin therapy in individuals with type 1 diabetes (17). A total of 1441 participants between the ages of 13 and 39 yr were randomly assigned to intensive or conventional insulin therapy. The trial included two cohorts: A primary prevention cohort (1 to 5 yr duration of diabetes, albumin excretion rate [AER] <40 mg/24 h, and no retinopathy) and a secondary prevention cohort (1 to 15 yr duration, AER <200 mg/24 h, and no more than moderate nonproliferative retinopathy). Patients were followed for a mean of 6.5 yr until DCCT closeout in 1993 to 1994, the first time at which waist circumference was recorded. All DCCT participants then were invited to join the Epidemiology of Diabetes Interventions and Complications (EDIC) study, an observational extension of the DCCT, and 1375 (96% of the surviving cohort) agreed to participate. At the end of the DCCT, all former conventional treatment participants were offered instruction in intensive therapy, and all participants returned to their own health care providers for diabetes care. During the EDIC study, mean glycosylated hemoglobin (HbA1c) levels converged between the former treatment groups (4).
Our cohort study begins at DCCT closeout and follows participants through 8 yr of the EDIC study. The analytic cohort consists of all EDIC study participants excluding (1) those who were pregnant (n = 23) or did not have waist circumference (n = 40) or AER (n = 12) recorded at DCCT closeout and (2) those who had fewer than two follow-up urine collections for AER and creatinine clearance during the EDIC study (n = 13). For the analyses of incident microalbuminuria only, an additional 174 participants with prevalent microalbuminuria (AER ≥30 mg/24 h) at DCCT closeout were excluded, leaving an analytic subset of 1105 participants with normal albumin excretion at study baseline. Informed consent for DCCT/EDIC study participation was granted by all patients, and the University of Washington Institutional Review Board approved the study described here.
Study Measurements
Demographic, anthropometric, physical examination, and laboratory data were collected for all participants at DCCT closeout. Waist circumference, body mass index (BMI), and waist-to-hip ratio (WHR) each were examined as exposures. A priori, waist circumference was chosen over BMI as the primary exposure because of its superior correlations with both visceral adipose tissue and AER in cross-sectional studies (7,10,12). Waist circumference was chosen over WHR as the primary exposure because of its use in common clinical guidelines (18) and because it is a direct anthropometric measurement rather than a calculated ratio. Waist circumference was measured with the patient standing erect at the narrowest part of the torso, as seen from the rear, with the abdomen relaxed at end expiration.
Creatinine clearance and AER were measured by timed 4-h urine collection, with each expressed per 24 h (3). Collection began after breakfast and the morning dose of insulin, resting in a sitting position, with 250 ml of water orally every 30 min. Urine albumin concentration was measured by fluoroimmunoassay, and serum and urine creatinine were measured by a variation of the Jaffe method. Coefficients of variation were 9.4% for urine albumin concentration and 2.3% for both urine and serum creatinine concentrations (4). Longitudinal measurements were obtained on alternate years throughout EDIC study follow-up. Creatinine clearance was evaluated both adjusted and unadjusted for body surface area (BSA) (19).
BP was measured by trained observers using mercury manometers with participants comfortably seated. HbA1c was measured using high-performance ion-exchange liquid chromatography (20). GFR, measured at DCCT closeout as the renal clearance of [125I]iothalamate after a subcutaneous injection without epinephrine, was used to determine whether change in creatinine clearance over follow-up differed by baseline level of kidney function (21).
Definition of Incident Microalbuminuria
Incident microalbuminuria was defined for these analyses as an AER ≥30 mg/24 h on two consecutive measurements to reduce misclassification as a result of variability in AER, consistent with recent American Diabetes Association guidelines (22). Because previous DCCT/EDIC analyses used an AER threshold of 40 mg/24 h to define microalbuminuria, this threshold was examined as a secondary outcome (3,4). Incident microalbuminuria was defined to occur at the time of the first elevated AER measurement, with the subsequent elevated AER measurement serving as a validating result. Patients were considered at risk from DCCT closeout until they developed incident microalbuminuria or until their penultimate AER measurement. When a scheduled AER measurement was missed, participants were censored at the time of the preceding AER measurement.
Statistical Analyses
Because waist circumference varies strongly by gender, waist circumference was divided into quartiles within each gender for comparison of characteristics at DCCT closeout. Because of strong rightward skew, AER also was evaluated after log transformation and as a dichotomous variable.
The unadjusted cumulative incidence of microalbuminuria was calculated as the number of individuals who met the definition of incident microalbuminuria during follow-up divided by the number of individuals at risk (AER <30 mg/24 h) at DCCT closeout. The discrete proportional hazards model, stratified by odd- versus even-year schedule of visits, was used to quantify the association of waist circumference at DCCT closeout with incident microalbuminuria, adjusting for age, gender, race, duration of diabetes, treatment group, smoking status, HbA1c, and AER at DCCT closeout (23). Because BP and serum lipids may either confound or mediate the relationship between waist circumference and incident microalbuminuria, separate models were fit with and without inclusion of these measurements at DCCT closeout. A parallel model was created with dummy variables for each gender-specific waist quartile. Adjusted risk, relative to women in the smallest waist category, then was plotted at the mean waist circumference for each group. Model assumptions were verified using both graphical and statistical methods.
Generalized estimating equation models were used to assess the relationships of waist circumference and covariates with change in creatinine clearance over follow-up. Creatinine clearance was the dependent variable in all models. Mean rate of change in creatinine clearance for the cohort as a whole was estimated with a model using time as the only independent variable. To assess the association of each covariate with change in creatinine clearance over time, we included categorical covariate variables and their corresponding covariate*time products as independent variables. Observations were clustered by patient and were assumed to have exchangeable correlation structure. Sandwich estimators ensured that SE were robust. Models were both unadjusted and adjusted for DCCT closeout age, gender, race, duration of diabetes, treatment group, smoking status, HbA11c, AER, and iothalamate GFR. A creatinine clearance of 10 ml/min per 1.73 m2 was assumed for incident dialysis or kidney transplantation (n = 9), at which time participants were censored. To determine whether waist circumference was associated with more advanced impairment of kidney function, we also tabulated by gender-specific waist quartile the cumulative incidences of a creatinine clearance <60 ml/min per 1.73 m2 and an estimated GFR <60 ml/min, derived from the abbreviated Modification of Diet in Renal Disease formula (24).
Results
Baseline Characteristics
At DCCT closeout, greater waist circumference was associated with greater age (men only), greater duration of diabetes (women only), intensive insulin therapy during the DCCT, greater BMI, higher BP, and an unfavorable lipid profile but not with HbA1c or AER (Table 1). Greater waist circumference was associated with greater creatinine clearance when unadjusted for BSA but with lesser creatinine clearance (men only) after correction for BSA. Relationships between waist circumference and covariates at DCCT closeout were unaltered when examined in the subset of participants whose AER at DCCT closeout was <30 mg/24 h.
Table 1.
Characteristics at DCCT closeout by gender-specific quartile of waist circumferencea
| Quartile of Waist Circumference |
||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Men |
Women |
||||||
| 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | |
| Waist circumference (cm) | 77±3 | 83±1 | 89±2 | 100±7 | 68±3 | 74±2 | 79±2 | 90±7 |
| n | 170 | 172 | 170 | 172 | 147 | 150 | 148 | 150 |
| Age (yr) | 31±7 | 35±6 | 35±6 | 37±6 | 34±7 | 34±7 | 33±7 | 34±8 |
| Duration of diabetes (yr) | 11±4 | 12±5 | 12±5 | 12±5 | 11±5 | 12±5 | 13±5 | 12±5 |
| White race | 163 (96) | 165 (96) | 163 (96) | 171 (99) | 143 (97) | 147 (98) | 142 (96) | 141 (94) |
| Intensive therapy during DCCT | 76 (45) | 82 (48) | 77 (45) | 101 (59) | 68 (46) | 69 (46) | 74 (50) | 100 (67) |
| Active smoking | 37 (22) | 40 (23) | 37 (22) | 42 (24) | 37 (26) | 36 (24) | 38 (26) | 27 (17) |
| BMI (kg/m2) | 23±2 | 25±2 | 27±2 | 29±3 | 22±2 | 24±2 | 26±2 | 30±4 |
| Systolic BP (mmHg) | 116±11 | 118±10 | 121±11 | 121±11 | 111±12 | 113±11 | 113±12 | 118±11 |
| Diastolic BP (mmHg) | 73±8 | 75±9 | 78±8 | 79±8 | 70±9 | 73±8 | 72±9 | 76±8 |
| AER (mg/24 h) | 37±144 | 58±370 | 51±169 | 31±110 | 41±300 | 49±266 | 26±60 | 18±35 |
| LogAER | 2.5±1.1 | 2.4±1.2 | 2.6±1.3 | 2.5±1.0 | 2.2±1.1 | 2.3±1.2 | 2.5±1.0 | 2.3±1.0 |
| AER≥30 mg/24 h | 29 (17) | 17 (10) | 29 (17) | 20 (12) | 14 (9) | 21 (14) | 24 (16) | 20 (13) |
| HbA1c (%) | 8.4±1.6 | 8.1±1.6 | 8.4±1.5 | 8.2±1.4 | 8.3±1.7 | 8.4±1.7 | 8.3±1.8 | 8.1±1.4 |
| Total cholesterol (mg/dl) | 170±30 | 175±34 | 180±29 | 193±37 | 177±27 | 181±31 | 184±36 | 191±34 |
| Triglycerides (mg/dl) | 79±50 | 79±41 | 90±45 | 110±70 | 68±38 | 77±37 | 82±41 | 90±46 |
| HDL (mg/dl) | 50±12 | 47±11 | 47±12 | 44±10 | 60±14 | 57±12 | 56±12 | 51±11 |
| LDL (mg/dl) | 104±26 | 112±30 | 115±27 | 127±30 | 103±25 | 109±26 | 112±30 | 121±30 |
| Creatinine clearance (ml/min) | 139±26 | 144±35 | 149±30 | 154±33 | 110±25 | 116±24 | 121±27 | 129±28 |
| Creatinine clearance (ml/min per 1.73 m2) | 128±24 | 126±29 | 127±24 | 124±25 | 117±27 | 116±23 | 117±25 | 117±24 |
Continuous variables are means ± SD; categorical variables are n (%). AER, albumin excretion rate; BMI, body mass index; DCCT, Diabetes Control and Complications Trial; HbA1c, glycosylated hemoglobin.
Medication use at DCCT closeout was not available for analysis. However, angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers were discouraged during the DCCT, and only 74 (5.8%) participants were using these agents at the first EDIC study visit.
Incident Microalbuminuria
For analyses of incident microalbuminuria, participants with an AER ≥30 mg/24 h at DCCT closeout were excluded. Compared with participants who were included in the analyses of incident microalbuminuria, excluded individuals had a similar gender distribution (55 versus 53% male) and waist circumference (83 versus 83 cm); a greater likelihood of conventional therapy during the DCCT (61 versus 48%); younger age (33 versus 34 yr); longer duration of diabetes (14 versus 11 yr); higher systolic and diastolic BP (121/78 versus 116/74), HbA1c (8.9 versus 8.2%), total cholesterol (191 versus 180 mg/dl), triglycerides (107 versus 81 mg/dl), and LDL (122 versus 112 mg/dl); and lower HDL (48 versus 52 mg/dl).
During a median follow-up duration of 5.8 yr, 93 (8.4%) of 1105 at-risk individuals developed microalbuminuria. The unadjusted cumulative incidence of microalbuminuria was greater in men than in women (10.7 versus 5.8%; P = 0.004) and in those who had been assigned to conventional versus intensive therapy during the DCCT (12.8 versus 4.5%; P < 0.001). Unadjusted cumulative incidence of microalbuminuria also increased by quartile of waist circumference (Figure 1), with the exception of the quartile of greatest waist circumference for men who were treated with conventional insulin therapy during the DCCT, in which the number of participants was relatively small (n = 60).
Figure 1.
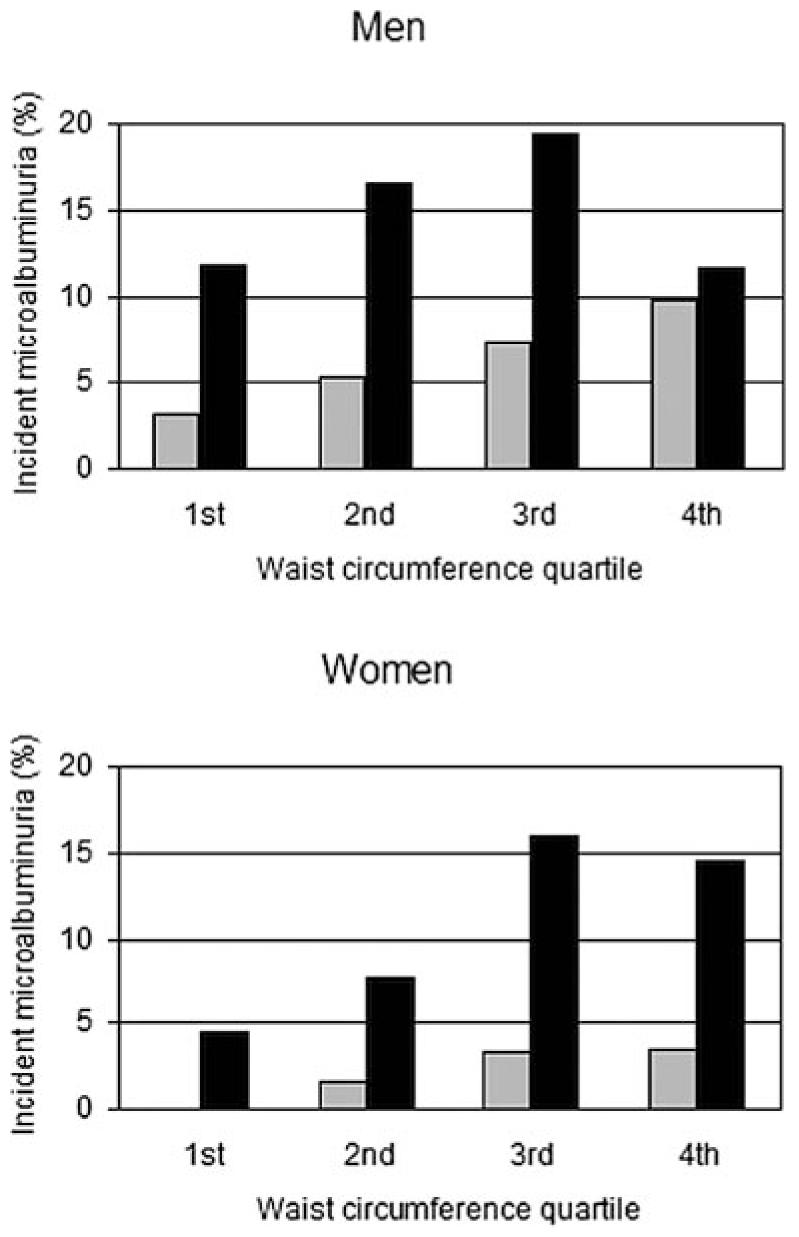
Unadjusted cumulative incidence of microalbuminuria by gender-specific quartile of waist circumference and Diabetes Control and Complications Trial (DCCT) treatment group. ■, conventional insulin therapy;  , intensive insulin therapy.
, intensive insulin therapy.
The hazard ratio (HR) for incident microalbuminuria that was associated with each 10-cm greater waist circumference was 1.34 (95% confidence interval [CI] 1.07 to 1.68), adjusting for DCCT closeout age, gender, race, duration of diabetes, treatment group, smoking status, HbA1c, and AER (Table 2). When systolic and diastolic BP were added to the model, the risk that was associated with waist circumference was attenuated slightly (HR 1.29; 95% CI 1.02 to 1.63). When serum total cholesterol, triglyceride, LDL, and HDL concentrations also were added to the model, an additional small attenuation was observed for the risk that was associated with waist circumference (HR 1.23; 95% CI 0.96 to 1.58). Adjusting for angiotensin-converting enzyme inhibitor use at the first EDIC study visit or restricting to those without use did not alter the risk that was associated with waist circumference.
Table 2.
Adjusted HR for incident microalbuminuriaa
| Variable at DCCT Closeout | Adjusted HR | 95% CI |
|---|---|---|
| Waist circumference (10 cm) | 1.34 | 1.07 to 1.68 |
| Age (yr) | 1.03 | 1.00 to 1.07 |
| Gender (male) | 1.44 | 0.85 to 2.41 |
| Race (nonwhite) | 2.05 | 0.78 to 5.40 |
| Duration of diabetes (yr) | 1.00 | 0.96 to 1.05 |
| Smoking (active) | 1.48 | 0.91 to 2.40 |
| DCCT treatment group (intensive) | 0.87 | 0.48 to 1.55 |
| HbA1c (1%) | 1.80 | 1.54 to 2.10 |
| AER (mg/24 h) | 1.11 | 1.07 to 1.14 |
Adjusted for age, gender, race, duration of diabetes, treatment group, smoking status, waist circumference, HbA1c, and AER, each measured at DCCT closeout. CI, confidence interval; HR, hazard ratio.
Greater waist circumference was associated with an increased risk for incident microalbuminuria in each gender (HR 1.65 among women, 1.23 among men; P = 0.209 for interaction), in each DCCT treatment group (HR 1.40 with intensive therapy, 1.29 with conventional therapy; P = 0.697 for interaction), and across categories of other covariates (Table 3, Figure 2). All results were similar using 40 mg/24 h as the AER threshold for incident microalbuminuria.
Table 3.
Adjusted HR for incident microalbuminuria associated with each 10-cm greater waist circumference, by covariate subgroup
| Group | No. of Events/No. at Risk | Adjusted HR (95% CI)a | Pb |
|---|---|---|---|
| All patients | 93/1105 | 1.34 (1.07 to 1.68) | |
| Age (yr) | |||
| <30 | 23/276 | 1.31 (0.84 to 2.04) | 0.890 |
| 30 to 40 | 36/514 | 1.30 (0.93 to 1.81) | |
| >40 | 34/222 | 1.44 (1.03 to 1.99) | |
| Gender | |||
| female | 30/486 | 1.65 (1.12 to 2.42) | 0.209 |
| male | 63/526 | 1.23 (0.94 to 1.62) | |
| Race | |||
| white | 88/983 | 1.35 (1.08 to 1.70) | 0.867 |
| nonwhite | 5/29 | 0.82 (0.20 to 3.45) | |
| DCCT treatment group | |||
| intensive | 26/554 | 1.40 (1.03 to 1.89) | 0.697 |
| conventional | 67/458 | 1.29 (0.95 to 1.75) | |
| HbA1c (%) | |||
| <7 | 4/240 | 1.71 (0.81 to 3.63) | 0.470 |
| 7 to 9 | 24/527 | 1.45 (1.06 to 1.99) | |
| >9 | 65/245 | 1.18 (0.88 to 1.58) | |
| AER (mg/24 h) | |||
| <10 | 31/598 | 1.23 (0.83 to 1.80) | 0.805 |
| 10 to 20 | 35/342 | 1.29 (0.93 to 1.77) | |
| 20 to 30 | 27/72 | 1.45 (0.99 to 2.10) |
Discrete proportional hazards model includes waist circumference (scaled to 10 cm), age, gender, race, duration of diabetes, DCCT treatment group, smoking status, HbA1c, and AER, each measured at DCCT closeout.
Tests hypothesis that HR differs among subgroups of covariate.
Figure 2.
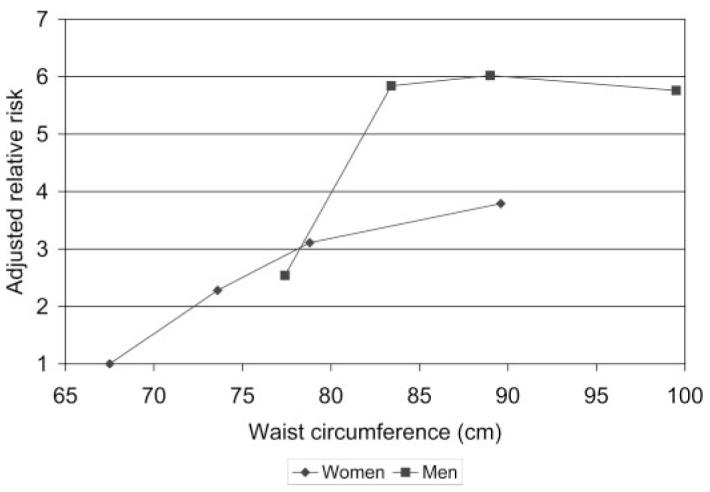
Adjusted relative risk for incident microalbuminuria by gender-specific quartile of waist circumference. Risk is presented relative to that of women in the category of smallest waist circumference (reference group), adjusted for age, race, duration of diabetes, treatment group, smoking status, glycosylated hemoglobin, and albumin excretion rate, each measured at DCCT closeout. Gender-specific waist quartiles are plotted at the mean waist circumference for each group.
Waist circumference was highly correlated with both BMI (r = 0.74) and WHR (r = 0.70). BMI also was associated with an increased risk for incident microalbuminuria when it replaced waist circumference in the model, and a similar trend was noted for WHR. When each obesity measure was scaled to its SD, the magnitude of effect that was associated with each measurement was similar, with widely overlapping 95% CI: Waist circumference HR 1.36 (1.07 to 1.72), BMI HR 1.46 (1.17 to 1.80), and WHR HR 1.33 (0.97 to 1.81).
Change in Creatinine Clearance
Eighty-eight percent of participants had five creatinine clearance measurements available for analysis, including DCCT closeout measurement; the remainder had three or four. Median follow-up duration was 8.0 yr. The mean change in creatinine clearance per year was -0.34 ml/min per 1.73 m2 (95% CI -0.55 to -0.13). Waist circumference was not associated with change in creatinine clearance over time in unadjusted or adjusted analyses (Table 4). Similarly, neither BMI nor WHR was associated with change in creatinine clearance over time (data not shown). No association between waist circumference and change in creatinine clearance was observed among subgroups that were defined by DCCT closeout GFR (<90, 90 to 150, or >150 ml/min per 1.73 m2) or AER (> or <30 mg/24 h) or when creatinine clearance measurements that were unadjusted for BSA were examined as the outcome variable (data not shown). However, several covariates (greater age, conventional insulin treatment during the DCCT, active smoking, greater HbA1c, and greater AER) were associated with a greater decline in creatinine clearance over time (Table 4).
Table 4.
Change in creatinine clearance over follow-up by covariate subgroup
| Parameter Covariate (at DCCT Closeout) | Unadjusted Model |
Adjusted Modela |
||
|---|---|---|---|---|
| Change in Creatinine Clearance (ml/min per 1.73 m2 each yr; 95% CI) |
Pb | Change in Creatinine Clearance (ml/min per 1.73 m2 each yr; 95% CI) |
Pb | |
| All participants | -0.34 (-0.55 to -0.13) | -0.32 (-0.55 to -0.09) | ||
| Age (yr) | ||||
| <30 | -0.28 (-0.68 to 0.12) | 0.031 | -0.43 (-0.87 to 0.01) | 0.123 |
| 30 to 40 | -0.16 (-0.45 to 0.14) | -0.10 (-0.44 to 0.24) | ||
| >40 | -0.82 (-1.22 to -0.42) | -0.65 (-1.07 to -0.23) | ||
| Gender | ||||
| women | -0.48 (-0.77 to -0.19) | 0.213 | -0.46 (-0.78 to -0.14) | 0.278 |
| men | -0.22 (-0.51 to 0.08) | -0.21 (-0.53 to 0.11) | ||
| Race | ||||
| white | -0.31 (-0.52 to -0.11) | 0.287 | -0.30 (-0.53 to -0.07) | 0.496 |
| nonwhite | -1.06 (-2.42 to 0.30) | -1.75 (-2.26 to 0.63) | ||
| Treatment group | ||||
| intensive | -0.04 (-0.32 to 0.25) | 0.004 | -0.11 (-0.43 to 0.21) | 0.060 |
| conventional | -0.65 (-0.93 to -0.34) | -0.55 (-0.87 to -0.22) | ||
| Smoking | ||||
| active | -0.77 (-1.26 to -0.27) | 0.047 | -0.77 (-1.34 to -0.20) | 0.061 |
| not active | -0.22 (-0.43 to 0.01) | -0.18 (-0.42 to 0.06) | ||
| Waist quartile, women | ||||
| 1st | -0.91 (-1.45 to -0.37) | 0.305 | -1.00 (-1.60 to -0.41) | 0.254 |
| 2nd | -0.54 (-1.07 to -0.01) | -0.31 (-0.90 to 0.28) | ||
| 3rd | -0.18 (-0.86 to 0.51) | -0.23 (-0.95 to 0.49) | ||
| 4th | -0.30 (-0.82 to 0.22) | -0.30 (-0.89 to 0.29) | ||
| Waist quartile, men | ||||
| 1st | -0.06 (-0.64 to 0.52) | 0.872 | -0.17 (-0.85 to 0.51) | 0.984 |
| 2nd | -0.19 (-0.80 to 0.44) | -0.14 (-0.81 to 0.53) | ||
| 3rd | -0.42 (-1.03 to 0.20) | -0.32 (-1.00 to 0.35) | ||
| 4th | -0.21 (-0.73 to 0.34) | -0.20 (-0.76 to 0.36) | ||
| Systolic BP (mmHg) | ||||
| <110 | -0.24 (-0.65 to 0.17) | 0.983 | -0.17 (-0.61 to 0.27) | 0.935 |
| 110 to 130 | -0.21 (-0.51 to -0.10) | -0.25 (-0.58 to 0.09) | ||
| >130 | -0.19 (-0.54 to 0.17) | -0.14 (-0.50 to 0.23) | ||
| HbA1c (%) | ||||
| <7 | 0.01 (-0.45 to 0.48) | 0.004 | -0.09 (-0.60 to 0.41) | 0.054 |
| 7 to 9 | -0.14 (-0.40 to 0.13) | -0.15 (-0.45 to 0.16) | ||
| >9 | -0.90 (-1.3 to -0.47) | -0.77 (-1.2 to -0.32) | ||
| AER (mg/24 h) | ||||
| <30 | -0.20 (-0.42 to 0.02) | 0.004 | -0.20 (-0.44 to 0.03) | 0.038 |
| 30 to 300 | -0.94 (-1.58 to -0.30) | -0.79 (-1.50 to -0.08) | ||
| >300 | -3.04 (-4.9 to -0.78) | -2.95 (-5.49 to -0.40) | ||
Generalized estimating equation model adjusted for age, gender, race, duration of diabetes, treatment group, smoking status, waist circumference, systolic BP, diastolic BP (except with systolic BP), HbA1c, logAER, and iothalamate GFR, each measured at DCCT closeout.
Tests hypothesis that change in creatinine clearance differs among subgroups of covariate.
Finally, 62 participants developed a creatinine clearance <60 ml/min per 1.73 m2 during follow-up, and 83 developed an estimated GFR <60 ml/min per 1.73 m2. The incidence of neither measure of impaired kidney function differed by waist circumference, BMI, or WHR (data not shown).
Discussion
The results of this study demonstrate that central obesity, as measured by waist circumference, is an independent risk factor for incident microalbuminuria in individuals with type 1 diabetes. This temporal association suggests that metabolic abnormalities that are associated with central obesity may contribute to the pathogenesis of microalbuminuria in type 1 diabetes. In contrast, no association was observed between waist circumference and change in creatinine clearance over time, further suggesting that microalbuminuria and loss of excretory kidney function may have different risk factors and pathogenic mechanisms in this population.
Previous studies in type 1 diabetes support the observed association between central obesity and incident microalbuminuria. Two cross-sectional analyses, one using data midway through the EDIC study, observed that WHR was correlated positively with AER (12,25). In the Eurodiab study, baseline WHR was greater in those who had microalbuminuria at follow-up than in those who did not (16). Although the Eurodiab study was limited by loss to follow-up and a lack of serial AER measurements, the magnitude of risk that was associated with central obesity, relative to established risk factors such as HbA1c and baseline AER, is consistent with the results of our study.
Central obesity also has been associated with microalbuminuria among individuals without diabetes and among individuals with type 2 diabetes, suggesting that the effective mechanism is not unique to type 1 diabetes (7,26–28). Because adjusting for BP and serum lipid concentrations resulted in only modest attenuation of the risk for incident microalbuminuria that was associated with central obesity, it is likely that other pathways contribute to this effect. Insulin resistance is associated with both central obesity and microalbuminuria and may play a prominent mediating role. No direct measures of insulin sensitivity are available in this study, but in another prospective study of individuals with type 1 diabetes, lower estimated glucose disposal rate was associated with an increased risk for overt nephropathy (14). These observations suggest that individuals with type 1 diabetes and superimposed features of insulin resistance, or “double diabetes,” are at increased risk for microalbuminuria. Additional possible mediators of the association between central obesity and incident microalbuminuria include inflammatory proteins as well as circulating hormones that are released by visceral adipose tissue, such as adiponectin and components of the renin-angiotensin system.
Central obesity is a modifiable risk factor. Weight loss has been associated with a decrease in AER among obese individuals with overt diabetic nephropathy and among individuals without diabetes and with severe obesity (29,30). Whether weight gain or weight loss is associated with change in AER among individuals with diabetes and normoalbuminuria or microalbuminuria has not been reported.
Creatinine clearance estimates GFR, which reflects excretory kidney function. In cross-section at DCCT closeout, unadjusted creatinine clearance was associated positively with waist circumference, reflecting the known relationship between body size and GFR. This association was nullified (for women) or reversed (for men) when creatinine clearance was adjusted for BSA, in accordance with standard practice. More important, however, waist circumference was not associated with rate of decline in creatinine clearance over time in this study, the first to our knowledge that has examined this relationship in type 1 diabetes. These results contrast with observations from the general population, in which central obesity has been associated with lower estimated GFR and a decline in estimated GFR over time (6,8).
Although variation in creatinine clearance measurements in this study was wide, poor accuracy and/or precision alone likely is not the sole cause of the lack of an observed association with waist circumference, because associations of several covariates (age, DCCT treatment group, smoking status, HbA1c, and AER) with change in creatinine clearance were identified. The slow rate of decline in creatinine clearance, relative to that expected with age alone (31), also did not prevent observation of associations with these covariates. Alternative explanations include (1) a time lag between microalbuminuria and decreased GFR that extends beyond the follow-up period of this study and (2) a true disparity of the effect of central obesity on microalbuminuria versus GFR.
An effect of central obesity on GFR delayed beyond 8 yr of follow-up is feasible and cannot be excluded. Because elevated AER was among the strongest determinants of decline in GFR over time in this study, one reasonably could expect that microalbuminuria that is induced in part by central obesity eventually would lead to a decline in GFR. However, waist circumference was not a risk factor for decline in creatinine clearance among participants who already had elevated AER at DCCT closeout.
Not all individuals with type 1 diabetes and microalbuminuria progress to a significant loss of GFR, suggesting that these two measures of kidney disease are not inextricably linked (32,33). Such a dissociation is consistent with the observation that some EDIC study participants lost GFR with sustained normal AER (34). Moreover, our results are consistent with those that were observed among patients who had type 2 diabetes and were enrolled in the United Kingdom Prospective Diabetes Study, in which greater waist circumference was associated with incident albuminuria (both microalbuminuria and clinical grade albuminuria) but not with subsequent decreased kidney function (26). Thus, microalbuminuria may reflect diffuse vascular damage that is related more directly to central obesity than is GFR (35). The relationships between microalbuminuria and other components of kidney function, including excretory and synthetic function, merit further investigation, as does the role of central obesity in these relationships.
Weight gain and central obesity are well-characterized complications of intensive insulin therapy in type 1 diabetes (5). Previous DCCT and EDIC study analyses have shown clearly that, on balance, intensive insulin therapy reduces the incidence of microalbuminuria and clinical grade albuminuria (3,4). Thus, even with the associated weight gain, intensive therapy still has a beneficial effect on microalbuminuria and clinical grade albuminuria. Results from this study suggest that weight gain that results from intensive therapy may identify those who are at increased risk for renal complications, perhaps as a result of an inherited predisposition to insulin resistance, and may modify the pathways through which renal complications occur in type 1 diabetes (36).
Results from this study identify preservation of GFR as an additional benefit of intensive insulin therapy. Compared with those who had received conventional therapy during the DCCT, individuals who had received intensive therapy had a slower decline in creatinine clearance over EDIC study follow-up. Similarly, lower HbA1c at DCCT closeout was associated with relative preservation of creatinine clearance. These observations are consistent with previously published analyses showing that intensive therapy during the DCCT resulted in a reduced likelihood of developing a creatinine clearance <70 ml/min per 1.73 m2 and a trend toward greater mean creatinine clearance during EDIC study follow-up (4).
This study has several limitations. First, radiographic quantification of body fat distribution was not available. Multiple measurements of body size were associated with incident microalbuminuria, and because these measurements were highly correlated, we could not establish conclusively that the relationship of obesity with incident microalbuminuria was attributable specifically to visceral adipose tissue. Second, the measurement method for creatinine clearance was not optimal and the duration of follow-up for this outcome may have been insufficient to detect an association with central obesity. Finally, results from a clinical trial setting may not generalize to the broader population with type 1 diabetes, who may have differing levels of self-care, medication adherence, and physical fitness, and findings from this predominantly white population may not apply to individuals of other race/ethnicity.
Conclusion
Central obesity predicts the subsequent development of microalbuminuria in the EDIC study. No association between waist circumference and decline in creatinine clearance was observed, suggesting a possible differential effect of central obesity on these two measures of kidney disease.
Acknowledgments
This study was supported by National Institutes of Health grants DK007247, HL004136, and Roadmap 5 K12 RR023265-03 (I.H.d.B.); DK599445 (S.D.S.); DK02456 (J.D.B.); and M01-RR01066. This study was supported further by the Endocrine Fellows Foundation (I.H.d.B.).
We greatly appreciate the generous and continuing dedication of the DCCT/EDIC study participants.
Footnotes
Disclosures
None.
References
- 1.US Renal Data System . USRDS 2004 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda: 2004. [Google Scholar]
- 2.Borch-Johnsen K, Kreiner S. Proteinuria: Value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. BMJ (Clin Res Ed) 1987;294:1651–1654. doi: 10.1136/bmj.294.6588.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications (DCCT) Research Group. Kidney Int. 1995;47:1703–1720. doi: 10.1038/ki.1995.236. [DOI] [PubMed] [Google Scholar]
- 4.Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: The Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159–2167. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial. JAMA. 1998;280:140–146. doi: 10.1001/jama.280.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J. The metabolic syndrome and chronic kidney disease in US adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 7.Pinto-Sietsma SJ, Navis G, Janssen WM, de Zeeuw D, Gans RO, de Jong PE. A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis. 2003;41:733–741. doi: 10.1016/s0272-6386(03)00020-9. [DOI] [PubMed] [Google Scholar]
- 8.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 9.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 10.Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Despres JP. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am J Clin Nutr. 1996;64:685–693. doi: 10.1093/ajcn/64.5.685. [DOI] [PubMed] [Google Scholar]
- 11.Nieves DJ, Cnop M, Retzlaff B, Walden CE, Brunzell JD, Knopp RH, Kahn SE. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes. 2003;52:172–179. doi: 10.2337/diabetes.52.1.172. [DOI] [PubMed] [Google Scholar]
- 12.Sibley SD, Thomas W, de Boer I, Brunzell JD, Steffes MW. Gender and elevated albumin excretion in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort: Role of central obesity. Am J Kidney Dis. 2006;47:223–232. doi: 10.1053/j.ajkd.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Sibley SD, Hokanson JE, Steffes MW, Purnell JQ, Marcovina SM, Cleary PA, Brunzell JD. Increased small dense LDL and intermediate-density lipoprotein with albuminuria in type 1 diabetes. Diabetes Care. 1999;22:1165–1170. doi: 10.2337/diacare.22.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE. Nephropathy in type 1 diabetes: A manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study. Kidney Int. 2002;62:963–970. doi: 10.1046/j.1523-1755.2002.00507.x. [DOI] [PubMed] [Google Scholar]
- 15.Yip J, Mattock MB, Morocutti A, Sethi M, Trevisan R, Viberti G. Insulin resistance in insulin-dependent diabetic patients with microalbuminuria. Lancet. 1993;342:883–887. doi: 10.1016/0140-6736(93)91943-g. [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi N, Bandinelli S, Mangili R, Penno G, Rottiers RE, Fuller JH. Microalbuminuria in type 1 diabetes: Rates, risk factors and glycemic threshold. Kidney Int. 2001;60:219–227. doi: 10.1046/j.1523-1755.2001.00789.x. [DOI] [PubMed] [Google Scholar]
- 17.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 18.Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 19.DuBois D, DuBois E. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 20.Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial A multicenter study. The DCCT Research Group. Clin Chem. 1987;33:2267–2271. [PubMed] [Google Scholar]
- 21.Levey AS, Greene T, Schluchter MD, Cleary PA, Teschan PE, Lorenz RA, Molitch ME, Mitch WE, Siebert C, Hall PM, et al. Glomerular filtration rate measurements in clinical trials. Modification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group. J Am Soc Nephrol. 1993;4:1159–1171. doi: 10.1681/asn.v451159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW. Nephropathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S79–S83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- 23.Lachin J. Biostatistical Methods: The Assessment of Relative Risks. John Wiley & Sons; New York: 2000. [Google Scholar]
- 24.Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract] J Am Soc Nephrol. 2000;11:A0828. [Google Scholar]
- 25.Stuhldreher WL, Becker DJ, Drash AL, Ellis D, Kuller LH, Wolfson SK, Orchard TJ. The association of waist/hip ratio with diabetes complications in an adult IDDM population. J Clin Epidemiol. 1994;47:447–456. doi: 10.1016/0895-4356(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 26.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: UK Prospective Diabetes Study 74. Diabetes. 2006;55:1832–1839. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 27.Palaniappan L, Carnethon M, Fortmann SP. Association between microalbuminuria and the metabolic syndrome: NHANES III. Am J Hypertens. 2003;16:952–958. doi: 10.1016/s0895-7061(03)01009-4. [DOI] [PubMed] [Google Scholar]
- 28.Anderson PJ, Chan JC, Chan YL, Tomlinson B, Young RP, Lee ZS, Lee KK, Metreweli C, Cockram CS, Critchley JA. Visceral fat and cardiovascular risk factors in Chinese NIDDM patients. Diabetes Care. 1997;20:1854–1858. doi: 10.2337/diacare.20.12.1854. [DOI] [PubMed] [Google Scholar]
- 29.Solerte SB, Fioravanti M, Schifino N, Ferrari E. Effects of diet-therapy on urinary protein excretion albuminuria and renal haemodynamic function in obese diabetic patients with overt nephropathy. Int J Obes. 1989;13:203–211. [PubMed] [Google Scholar]
- 30.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14:1480–1486. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 31.Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG, Larson TS. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43:112–119. doi: 10.1053/j.ajkd.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 33.Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M. The early natural history of nephropathy in type 1 diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes. 2005;54:2164–2171. doi: 10.2337/diabetes.54.7.2164. [DOI] [PubMed] [Google Scholar]
- 34.Molitch ME, Rutledge B, Steffes M, Cleary PA. Renal insufficiency in the absence of albuminuria among adults with type 1 diabetes in the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Presented at the American Diabetes Association Scientific Sessions; Washington, DC. June 9 to 13, 2006. [Google Scholar]
- 35.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32:219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 36.Purnell JQ, Dev RK, Steffes MW, Cleary PA, Palmer JP, Hirsch IB, Hokanson JE, Brunzell JD. Relationship of family history of type 2 diabetes, hypoglycemia, and autoantibodies to weight gain and lipids with intensive and conventional therapy in the Diabetes Control and Complications Trial. Diabetes. 2003;52:2623–2629. doi: 10.2337/diabetes.52.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]


