Abstract
The degree of cell clumping increased with time of storage (1% cell clumps immediately after homogenization and 3 and 6.5% after 48 and 96 h of storage, respectively), and the number of living single cells decreased. Quantitative carrier tests were carried out with these cells using ortho-phthaldialdehyde (OPA) and coco fatty aminoxethylate as biocides. In contrast to OPA, with coco fatty aminoxethylate the reductions obtained with freshly homogenized mycobacteria were significantly higher (P = 0.02) than those obtained with mycobacteria kept in the refrigerator for 4 days. Therefore, it is advisable to prepare the test suspension freshly for each test.
Recently, quantitative carrier tests for testing bactericidal, fungicidal, and mycobactericidal activities of instrument disinfectants were published as prestandards (2, 3, 4), which are based on tests carried out by Gebel et al. (6). An important aspect of all test specifications is the storage and preparation of the test organisms prior to testing. In the test specification from the German Society for Hygiene and Microbiology for proving the effectiveness of instrument disinfectants against Mycobacterium terrae in the quantitative suspension test, the test organisms could be homogenized in distilled water and stored for up to 5 days in the refrigerator at 4°C (10). In the European standards, however, it is recommended that the mycobacteria be prepared and used on the test day (1, 4). As a consequence of this recommendation, considerable extra work in the laboratory is required if tests with mycobacteria are to be carried out on several consecutive days. For this reason, the effect of refrigerated storage of the mycobacterial suspension on the mycobactericidal activity of biocides was investigated. The mycobactericidal substances tested were ortho-phthaldialdehyde (OPA) (5) and the new substance coco fatty aminoxethylate.
For the tests of effectiveness of disinfectants, M. terrae ATCC 15755 was rehydrated and cultured on Middlebrook-Cohn agar containing 10% oleic acid-albumin-catalase-dextrose enrichment (Becton-Dickinson, Heidelberg, Germany) (3 weeks, 37°C). After verification of purity and identity, the culture was harvested and the test suspension was produced as described in European standard prEN 14563 (4). To calculate the percentage of cell clumps, single cells and cell clumps of two different suspensions were counted in a Thoma counting chamber. The cell number was adjusted to 1.5 × 109 to 5 × 109 CFU/ml for the carrier tests according to the European standard.
Cells were stained with a LIVE/DEAD BacLight bacterial viability kit directly after the collection and homogenization of the mycobacteria (0 h) and after 48 and 96 h at 5°C in accordance with the manufacturer's instructions (Molecular Probes, Eugene, Oreg.). For examination of the stained preparations, an inverted microscope (L-6665 DM IRBE; Leica) was used. The samples were examined at 500 and 635 nm.
Both biocides were used in a concentration of 0.125% (wt/vol) in standard hard water (0.48 mM MgCl2, 0.25 mM CaCl2, 0.4 mM NaHCO3), with the pH of OPA adjusted with maleic acid to 4 and the pH of coco fatty aminoxethylate adjusted to 8 (1). Preliminary tests had shown that the two substances displayed their optimum effects at these pHs. In order to guarantee complete dissolution of the coco fatty aminoxethylate, 15% (wt/vol) 2-propanol was added to the solution. A 15% propanol solution exhibited no mycobactericidal effect (data not shown). Cyclodextrin (1.135%, wt/vol), sodium thiosulfate (0.5%, wt/vol), and histidine (0.1%, wt/vol) in casein-soy meal-peptone broth (Oxoid, Wesel, Germany) were used as neutralization substances. Neutralization was validated in accordance with European standard prEN 14348 (1).
The carrier tests were done with frosted glass strips and the same mycobacterial suspensions, whose homogeneity and viability had been checked by fluorescence microscopy after staining with the LIVE/DEAD BacLight bacterial viability kit (6). In order to simulate contamination with organic material, as occurs with medical instruments, the effectiveness of the biocides was tested under artificially “dirty” conditions, as described in European standard prEN 14563. One carrier was used per test time and concentration and for the water control. For each carrier, a polypropylene tube (volume, 15 ml; 76 by 20 mm; Sarstedt, Nümbrecht, Germany) was filled with 10 ml of active solution or standard hard water. The evaluation was carried out as described by Gebel et al. (6). The microbial reduction factor (RF), expressed as a logarithm, was calculated according to the following formula: log RF = log N0 − log Na, where N0 is the number of CFU per milliliter after the test time without the active substances (water control) and Na is the number of CFU per milliliter after the test time with the active substances. The statistical significance of the differences was evaluated by using Tukey's test (9).
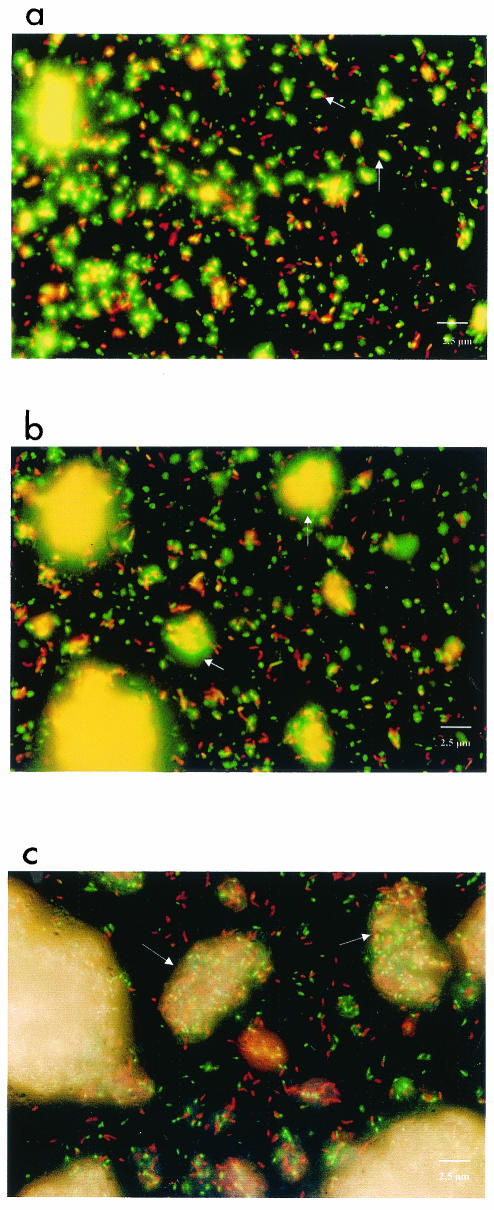
The clumping of the cells increased during refrigerated storage (Fig. 1). In the case of the freshly homogenized suspension, the mycobacteria were present largely as individual cells, but 1% clumping (mean for two batches) was calculated (Fig. 1a). The proportion of green fluorescing cells of M. terrae with an intact membrane exceeded the proportion of red fluorescing cells with a damaged membrane. After storage of the suspension for 48 h in the refrigerator, an increased amount of small cell aggregates (3%; mean for two batches) could be observed, consisting of both live and dead cells (Fig. 1b). After storage for 96 h, live and dead cells were very strongly clumped together (6.5%; mean for two batches) and the number of living single cells was smaller than that in the freshly homogenized suspension (Fig. 1a and c). The results of the carrier tests suggest that storage of mycobacteria can have an influence on the effectiveness of biocides (Table 1). This becomes particularly clear in the case of coco fatty aminoxethylate, with which, at the tested concentration and a 60-min exposure, a significantly higher reduction in bacterial count could be achieved in the case of freshly harvested and homogenized mycobacteria than in the case of bacteria stored for 96 h (RF, 3.6 and 2.1; respectively, P = 0.02). An influence of the storage of the cells on mycobactericidal effectiveness was not as strong in the case of use of OPA as in the case of use of coco fatty aminoxethylate and could be seen only after a contact time of 15 min. The difference was only a 0.6 log step and not statistically significant.
FIG. 1.
Fluorescence microscopic photos of freshly homogenized M. terrae cells (a) and of M. terrae cells that had been stored at 4°C for 48 h (b) and 96 h (c) and stained with the LIVE/DEAD BacLight bacterial viability kit. The arrows indicate living (green fluorescent) cells (a) and cell aggregates (b and c). Magnification, ×1,000.
TABLE 1.
Logarithmic reduction factors of carrier tests at different contact times with freshly homogenized cells of M. terrae and cells that had been stored at 4°C
| Carrier test | Duration of storage (h) | RF at indicated contact time (min) witha:
|
|||||
|---|---|---|---|---|---|---|---|
| OPA
|
Coco fatty aminoxethylate
|
||||||
| 15 | 30 | 60 | 15 | 30 | 60 | ||
| 1 | 0 | 3.0 | 4.2 | 4.8 | 0.9 | 1.4 | 3.8 |
| 48 | 2.6 | 4.0 | 4.8 | 0.7 | 1.6 | 2.7 | |
| 96 | 2.6 | 4.6 | 4.8 | 0.8 | 1.6 | 1.9 | |
| 2 | 0 | 3.6 | 4.6 | 4.8 | 0.7 | 1.8 | 3.4 |
| 48 | 3.3 | 4.4 | 4.8 | 0.7 | 1.8 | 2.9 | |
| 96 | 2.8 | 4.8 | 4.8 | 0.8 | 1.6 | 2.3 | |
| Mean | 0 | 3.3 | 4.4 | 4.8 | 0.8 | 1.6 | 3.6* |
| 48 | 2.9 | 4.2 | 4.8 | 0.7 | 1.7 | 2.8 | |
| 96 | 2.8 | 4.7 | 4.8 | 0.8 | 1.6 | 2.1* | |
*, P = 0.02 (Tukey's test).
A particularly important factor when standardized test methods are being drawn up is the culturing and preparation of the test organisms prior to carrying out the tests. When mycobacteria are prepared for the tests, the homogenization of the harvested cells and their further use is certainly a critical step. Attention was drawn to this in a review by Sattar et al. (7). In European standard prEN 14563, the degree of homogeneity that should be required is not defined (4). Our results show that a monodispersion of the cells with 1% small cell aggregates in the suspension could be achieved directly after homogenization. When the procedure described in the European standard was followed, a lower number of cell aggregates was not obtainable, so that it has to be accepted. However, the possibility of reducing the number of cell clumps by a different homogenization method should be clarified. After 48 and 96 h of storage of the suspension in the refrigerator, the cells increasingly clumped together as a result of interactions between the hydrophobic cell walls of the mycobacteria (7). The results of the quantitative carrier test generated with coco fatty aminoxethylate correlated with the degree of cell aggregation, but only at a contact time of 60 min. With increasing storage time of the cell suspension, the reduction factor decreased (Table 1). In the case of OPA, this effect was detectable only with the short contact time of 15 min but was not statistically significant. Reasons for these differences could be different penetration properties and reaction rates of the two substances. Simons et al. concluded from studies with OPA that the aromatic component of the substance makes penetration through the outer layers of mycobacteria and gram-negative bacteria possible and thus explains the high activity against these organisms (8). In contrast, the penetration properties of the substance coco fatty aminoxethylate are unknown, so that it is difficult to interpret the results. There is a strong indication that clumping of cells and lack of penetration are responsible for the reduced efficacy of coco fatty aminoxethylate, but further investigations are necessary to confirm that.
The results show that, in order to improve the reproducibility of results, the mycobacterial suspension should be used within a day after homogenization and that this recommendation in the European standards is correct, even though more work is necessary. A possibility would be to prolong the duration of the use of the mycobacterial suspension by means of daily rehomogenization. For that, however, tests would be needed to rule out false results caused by injured microorganisms. The question of why work is carried out in order to obtain a homogenized suspension even though such a suspension certainly does not correspond to natural conditions can be answered as follows. Monodispersed bacterial suspensions are certainly not representative of what happens in nature. However, this argument is counterproductive, because the vital aspect of reproducibility of results in a standard test would otherwise be neglected (7). Unfortunately, nothing is said in the European standard prEN 14563 about the number of repeats and the statistical evaluation. Therefore, it is very important to describe the method very clearly to reduce the causes of the fault.
Acknowledgments
We are grateful to S. A. Sattar from the Center for Research on Environmental Microbiology, University of Ottawa, for critical reading of the manuscript. We thank Mirjana Jevtic for excellent technical assistance.
REFERENCES
- 1.European Committee for Standardisation. 2002. European standard prEN 14348: chemical disinfectants and antiseptics—quantitative suspension test for the evaluation of mycobactericidal activity of chemical disinfectants in the medical area including instrument disinfectants. Test methods and requirements (phase 2/step 1). European Committee for Standardisation, Brussels, Belgium.
- 2.European Committee for Standardisation. 2002. European standard prEN 14561: chemical disinfectants—quantitative carrier test for evaluation of bactericidal activity of chemical disinfectants for instruments used in medical area. Test method and requirements (phase 2/step 2). European Committee for Standardisation, Brussels, Belgium.
- 3.European Committee for Standardisation. 2002. European standard prEN 14562: chemical disinfectants—quantitative carrier test for evaluation of fungicidal activity of chemical disinfectants for instruments used in medical area. Test method and requirements (phase 2/step 2). European Committee for Standardisation, Brussels, Belgium.
- 4.European Committee for Standardisation. 2002. European standard prEN 14563: chemical disinfectants—quantitative carrier test for evaluation of mycobactericidal activity of chemical disinfectants for instruments used in medical area. Test method and requirements (phase 2/step 2). European Committee for Standardisation, Brussels, Belgium.
- 5.Fraud, S., J.-Y. Maillard, and A. D. Russell. 2001. Comparison of the mycobactericidal activity of ortho-phthaldialdehyde, glutaraldehyde and other dialdehydes by a quantitative suspension test. J. Hosp. Infect. 48:214-221. [DOI] [PubMed] [Google Scholar]
- 6.Gebel, J., K.-P. Bansemir, M. Exner, P. Goroncy-Bermes, A. Kirsch, F. von Rheinbaben, and H.-P. Werner. 2000. Evaluating the efficacy of chemical disinfectants for medical instruments. Hyg. Med. 25:451-457. [Google Scholar]
- 7.Sattar, S. A., M. Best, V. S. Springthorpe, and G. Sanani. 1995. Mycobacterial testing of disinfectants: an update. J. Hosp. Infect. 30(Suppl.):372-382. [DOI] [PubMed] [Google Scholar]
- 8.Simons, C., S. E. Walsh, J.-Y. Maillard, and A. D. Russell. 2000. A note: ortho-phthaldialdehyde: proposed mechanism of action of a new antimicrobial agent. Lett. Appl. Microbiol. 31:299-302. [DOI] [PubMed] [Google Scholar]
- 9.Sokal, R. R., and F. J. Rohlf. 1995. Biometry, 3rd ed. W. H. Freeman and Company, New York, N.Y.
- 10.Werner, H.-P., J. Gebel, A. Kirsch, K. Bansemir, B. Christiansen, M. Exner, P. Goroncy-Bermes, U. Kirschner, P. Mecke, C. Ostermeyer, M. Pfeiffer, H.-J. Rödger, R. Schubert, H.-G. Sonntag, and H. Weiβ. 2000. Quantitative suspension test with Mycobacterium terrae ATCC 15755 for the testing of instrument disinfectants. Hyg. Med. 25:239-248. [Google Scholar]