Abstract
Context
Intensive treatment of type 1 diabetes results in greater weight gain than conventional treatment.
Objective
To determine the effect of this weight gain on lipid levels and blood pressure.
Design
Randomized controlled trial; ancillary study of the Diabetes Control and Complications Trial (DCCT).
Setting
Twenty-one clinical centers.
Participants
The 1168 subjects enrolled in DCCT with type 1 diabetes who were aged 18 years or older at baseline.
Intervention
Randomized to receive either intensive (n = 586) or conventional (n = 582) diabetes treatment with a mean follow-up of 6.1 years.
Main Outcome Measures
Plasma lipid levels and blood pressure in each treatment group categorized by quartile of weight gain.
Results
With intensive treatment, subjects in the fourth quartile of weight gain had the highest body mass index (BMI) (a measure of weight adjusted for height), blood pressure, and levels of triglyceride, total cholesterol, low-density lipoprotein cholesterol (LDL-C), and apolipoprotein B compared with the other weight gain quartiles with the greatest difference seen when compared with the first quartile (mean values for the highest and lowest quartiles: BMI, 31 vs 24 kg/m2; blood pressure, 120/77 mm Hg vs 113/73 mm Hg; triglyceride, 0.99 mmol/L vs 0.79 mmol/L [88 mg/dL vs 70 mg/dL]; LDL-C, 3.15 mmol/L vs 2.74 mmol/L [122 mg/dL vs 106 mg/dL]; and apolipoprotein B, 0.89 g/L vs 0.78 g/L; all P<.001). In addition, the fourth quartile group had a higher waist-to-hip ratio; more cholesterol in the very low density lipoprotein, intermediate dense lipoprotein, and dense LDL fractions; and lower high-density lipoprotein cholesterol and apolipoprotein A-I levels compared with the first quartile. Baseline characteristics were not different between the first and fourth quartiles of weight gain with intensive therapy except for a higher hemoglobin A1c in the fourth quartile. Weight gain with conventional therapy resulted in smaller increases in BMI, lipids, and systolic blood pressure.
Conclusions
The changes in lipid levels and blood pressure that occur with excessive weight gain with intensive therapy are similar to those seen in the insulin resistance syndrome and may increase the risk of coronary artery disease in this subset of subjects with time.
PATIENTS WITH type 1 diabetes characteristically gain weight with the institution of insulin therapy but may remain leaner than nondiabetic control subjects.1,2 With intensification of diabetes therapy,continuedweightgaincorrelates inversely with improvement in hemoglobin A1c levels.3,4 After a mean follow-up of 6.5 years in the Diabetes Control and Complications Trial (DCCT), a study designed to determine the effect of intensive diabetes therapy on the microvascular complications of type 1 diabetes, the prevalence of obesity—which is determined when the body mass index (BMI), which was greater than 27.8kg/m2 for men and greater than 27.3 kg/m2 for women—reached 33.1% in the intensively treated subjects compared with 19.1% in the conventionally treated subjects.5
Despite this weight gain, lipid levels for the intensively treated group as a whole improved, including lower levels of dense low-density lipoprotein (LDL) and lipoprotein(a) (Lp[a]) at follow-up, compared with the conventionally treated group.6,7 Recent studies of adults with type 1 diabetes have highlighted, however, that a higher BMI or waist-to-hip ratio (WHR) is associated with adverse lipid levels similar to those of centrally obese subjects who do not have diabetes and those with type 2 diabetes, eg, higher triglyceride levels, normal or slightly higher low-density lipoprotein cholesterol (LDL-C) levels, and lower high-density lipoprotein cholesterol (HDL-C) levels.2,8,9
Coronary artery disease (CAD) is a leading cause of mortality in adult patients with type 1 diabetes.10,11 Because CAD prevalence in type 1 diabetes has recently been shown to be associated with a higher triglyceride level, lower HDL-C levels, a greater WHR in men, and a higher BMI in women,12 it is important to understand the effect of weight gain from intensive diabetes therapy on these risk factors.
METHODS
The methods and trial design of the DCCT have been described elsewhere.13 Briefly, it was a multicenter trial of 1441 patients with type 1 diabetes between the ages of 13 and 39 years at baseline and randomly assigned to receive conventional or intensive diabetes therapy for an average follow-up of 6.5 years. The study population included 2 separate cohorts, a primary prevention cohort (1 to 5 years’ duration of type 1 diabetes, no retinopathy, and <40 mg of albuminuria per 24 hours at baseline) and a secondary intervention cohort (1 to 15 years’ duration of type 1 diabetes,minimal to moderate non-proliferative retinopathy, and <200 mg of albuminuria per 24 hours at baseline). Exclusion criteria included a total cholesterol level of more than 3 SDs above the mean for sex and age as defined by the Lipid Research Clinics Population Studies Data Book14 or calculated LDL-C levels higher than 4.9 mmol/L (190 mg/dL); a body weight of more than 30% above ideal body weight(IBW),as defined by the 1983 Metropolitan Life Insurance norms15; and major electrocardiographic abnormalities, a clinical history of coronary heart disease, or symptoms of peripheral vascular disease. Dietary protocols were similar between the conventionally and intensively treated groups (emphasis on restricting total fat to roughly 30% of total energy intake while maintaining 50% to 55% of intake from carbohydrates and 15% to 20% from protein) with the exception of those receiving intensive therapy, following an integrated diet, and making insulin adjustments to achieve glycemic goals.16 As part of an ancillary study, WHR measurements and serum samples from 1378 subjects (96% of the original cohort) for Lp(a), apolipoprotein B (apo B), and lipoprotein density distribution were taken at the final follow-up visit and represent the subjects reported in this study.7 In order to exclude subjects who might have experienced weight gain as a result of adolescent growth, only those subjects age 18 years and older at entry (n = 1168, 586 in the conventional treatment group, mean follow-up of 6.0 years, and 582 in intensive treatment group, mean follow-up of 6.2 years) were included in this study.
Body Weight, WHR, and Blood Pressure
Height (in centimeters) was measured using a stadiometer and weight (in kilograms) was measured on the same balance-beam scale for the duration of the trial (subjects were in lightweight clothing and in stocking feet).16 The BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2). The WHR was calculated from measurements obtained at the final follow-up visit. Natural hip and waist measurements were performed twice by study-certified dietitians using inelastic tapes. If those measurements differed by more than 0.5 cm, they were repeated. The waist measurement was taken at the level of the natural waist. The hip measurement was taken at the maximum extension of the buttocks with the subject in the relaxed standing posture. Blood pressures were measured using standard techniques in the right arm.
Severe Hypoglycemia
Episodes of severe hypoglycemia were defined as symptoms requiring the assistance of others with blood glucose levels below 2.8 mmol/L (50 mg/dL) and/ or reversal of symptoms by oral or intravenous glucose or subcutaneous glucagon. Results are expressed as the number of episodes per person over the duration of the study.
Chemistries
Following an overnight fast of at least 8 hours, blood was drawn from the subjects. After the plasma was separated and stored briefly at −20°C (−4°F), it was placed on dry ice and sent immediately to the DCCT Central Biochemistry Laboratory at the University of Minnesota, Minneapolis, where it was stored at −70°C (−94°F). Total cholesterol, triglyceride, and HDL-C levels from the final DCCT follow-up were determined by enzymatic methods.6 The LDL-C was calculated using the Friedewald equation: LDL-C = total cholesterol — (HDLC + triglycerides/5). Hemoglobin A1c and urinary albumin excretion were also measured as previously described.17
Additional samples from the final follow-up visit were routed on dry ice to the Northwest Lipid Research Laboratories in Seattle, Wash, where they were stored at −70°C (−94°F) prior to undergoing the following measurements. The Lp(a) mass was determined using a double monoclonal, antibody-based, enzyme-linked immunosorbent assay (ELISA).18 The apo B and apolipoprotein A-I (apo A-I) levels were measured by a nephelometric system calibrated with the World Health Organization (WHO) International Reference Materials.19 Lipoprotein density distribution was determined by nonequilibrium density gradient ultracentrifugation (DGUC) using a modification of a previously described technique and a vertical rotor (Beckman VTI-65, Beckman Instruments, Fullerton, Calif).7 High-density lipoprotein is located in fractions 0 to 6, LDL in fractions 7 to 18, intermediated density lipoprotein (IDL) in fractions 19 to 30, and very low-density lipoprotein (VLDL) in fractions 30 to 38. The LDL relative flotation (LDL Rf), a measure of buoyancy, was determined by dividing the density fraction number containing the peak LDL cholesterol level among fractions 7 through 19 by the total number of fractions collected (equal to 38).
Statistical Analysis
Both treatment groups were stratified into quartiles of weight gain as defined by the change in BMI from baseline to follow-up. Quartiles for weight gain were chosen because of epidemio-logical data suggesting that 25% to 30% of the general population carry traits for insulin resistance20 and small, dense LDL particles.21 Kilogram equivalents are calculated assuming that a woman is 1.65 m (5 ft 5 in) tall. Results would be higher for taller women and men.
Results among weight gain quartiles were compared within each treatment group using the Kruskal-Wallis test 1-way analysis of variance (ANOVA) on ranks to identify variables that were different across weight gain categories. If significant by ANOVA, pairwise testing between groups was performed using a Student t test of means if the results were normally distributed and a rank sum test if the results were not naturally distributed to identify specific weight gain quartiles that were different. Multiple linear regression was performed to test the independent effects of weight gain (by quartile) and glycemic control (hemoglobin A1c at final follow-up) on final lipid levels. Proportional differences between groups were tested using χ2 analysis. Differences between baseline and follow-up measurements of BMI, lipid levels, and blood pressure were tested using paired ttesting and expressed as percent change from baseline.
The cholesterol content in each fraction measured from the DGUC is expressed as percent cholesterol to adjust for differences in total cholesterol levels between subjects. This is calculated by summing the cholesterol in all 38 fractions and expressing the result for each fraction as the cholesterol in that fraction divided by the summed cholesterol and multiplied by 100. To test the significance of differences in density distributions between groups of subjects, a difference plot is generated by subtracting the mean cholesterol value of each fraction in one group from the mean cholesterol value in the same fraction of the second group and determining the 95% confidence interval (CI) for this difference. A difference in fractional cholesterol content between groups becomes significant (P<.05) when the 95% CI does not cross the zero line.
RESULTS
Baseline Parameters and Change in Body Weight
Baseline characteristics revealed no significant differences between the subjects in the conventional and intensive treatment groups chosen for this study.13 After stratification into quartiles of weight gain (Table 1), no differences in baseline values between the quartiles of the conventionally treated subjects were found except that the initial BMI was slightly higher in the first quartile compared with the other quartiles. In the intensively treated group, the only significant baseline difference between the first and fourth weight gain quartiles was a higher hemoglobin A1c level in the fourth quartile (P<.01). For each quartile of weight gain, the average increase in BMI from baseline to follow-up was roughly twice as great in the intensively treated group compared with the conventionally treated group (Figure 1). Weight gain occurred within the first year of the trial and was progressive throughout the duration of the trial in both the conventionally treated and intensively treated groups (DCCT Research Group, unpublished data, 1995).
Table 1.
Baseline Values for Weight Gain Quartiles in Both Treatment Groups*
| Variable | Quartile of Weight Gain | |||||||
|---|---|---|---|---|---|---|---|---|
| Conventional Group | Intensive Group | |||||||
| First (n=147) |
Second (n=146) |
Third (n=146) |
Fourth (n=147) |
First (n=146) |
Second (n=145) |
Third (n=145) |
Fourth (n=146) |
|
| Age, y | 28 (6) | 29 (6) | 29 (5) | 28 (6) | 28 (6) | 29 (6) | 29 (5) | 29 (6) |
| Men/women, % | 57/43 | 58/42 | 55/45 | 58/42 | 47/53 | 63/37 | 59/41 | 40/60 |
| Body mass index, kg/m2 | 25 (3)†‡§ | 23 (3) | 24 (3) | 24 (3) | 24 (2) | 23 (2) | 23 (3) | 24 (3)†‡ |
| Hemoglobin A1c, % | 8.6 (1.4) | 8.6 (1.6) | 8.9 (1.6) | 8.9 (1.6) | 8.7 (1.6) | 8.5 (1.4) | 8.9 (1.5) | 9.2 (1.5)∥† |
| Triglyceride, mmol/L [mg/dL] | 0.95 (0.72) [84 (64)] |
0.88 (0.47) [78 (42)] |
0.96 (0.61) [85 (54)] |
0.93 (0.56) [82 (50)] |
0.93 (0.49) [82 (43)] |
0.87 (0.49) [77 (43)] |
0.86 (0.3) [76 (27)] |
0.93 (0.43) [82 (38)] |
| Total cholesterol, mmol/L [mg/dL] | 4.58 (0.85) [177 (33)] |
4.52 (0.85) [175 (33)] |
4.60 (0.96) [178 (37)] |
4.58 (0.83) [177 (32)] |
4.78 (0.91) [185 (35)] |
4.45 (0.80)¶ [172 (31)] |
4.58 (0.85) [177 (33)] |
4.68 (0.83) [181 (32)] |
| LDL, mmol/L [mg/dL] | 2.87 (0.75) [111 (29)] |
2.82 (0.75) [109 (29)] |
2.87 (0.83) [111 (32.1)] |
2.84 (0.72) [110 (28)] |
3.00 (0.78) [116 (30.2)] |
2.74 (0.67)∥ [106 (26)] |
2.87 (0.78) [111 (30)] |
2.95 (0.72) [114 (28)] |
| HDL, mmol/L [mg/dL] | 127 (0.31) [49 (12)] |
1.29 (0.34) [50 (13)] |
1.32 (0.34) [51 (13)] |
1.29 (0.31) [50 (12)] |
1.37 (0.31) [53 (12)] |
1.32 (0.34) [51 (13)] |
1.32 (0.34) [51 (13)] |
1.32 (0.31) [51 (12)] |
| Systolic BP, mm Hg | 116 (11) | 115 (11) | 116 (12) | 116 (11) | 114 (11) | 114 (12) | 114 (12) | 114 (12) |
| Diastolic BP, mm Hg | 74 (8.4) | 73 (8.9) | 73 (8.3) | 73 (8.4) | 72 (8.5) | 72 (8.7) | 73 (9.0) | 73 (9) |
Results are expressed as mean (SD) unless otherwise indicated. Body mass index is a measure of weight in kilograms divided by the square of height in meters; LDL indicates low-density lipoprotein; HDL, high-density lipoprotein; and BP, blood pressure.
P<.001 vs second quartile.
P<.001 vs third quartile.
P<.001 vs fourth quartile.
P<.01 vs first quartile.
P<.001 vs first quartile.
Figure 1.
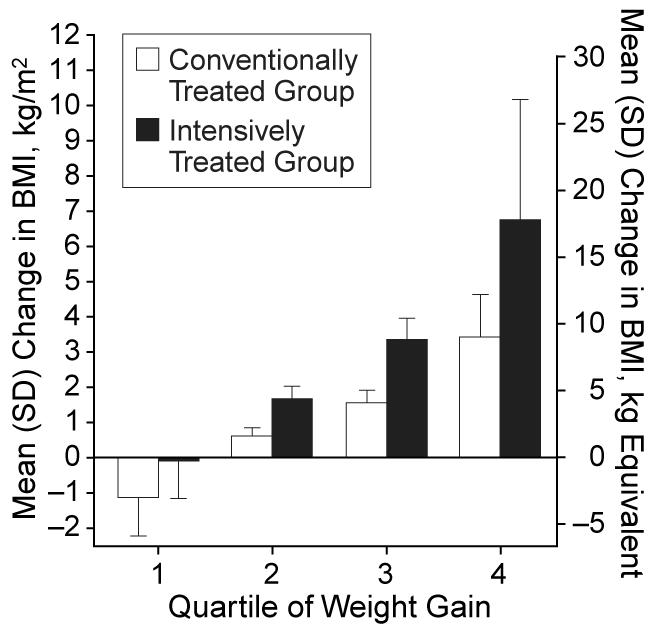
Increases in body mass index (BMI) (a measure of weight in kilograms divided by the square of height in meters) and the equivalent weight in kilograms from baseline to follow-up in the quartiles of weight gain in the conventionally and intensively treated groups.
Conventionally Treated Group at Follow-up
Hemoglobin A1c levels at follow-up were lower with increasing weight gain (significantly lower in fourth quartile of weight gain vs the first quartile, P<.01) (Table 2). The BMI was highest in the fourth quartile and significantly higher in the third quartile compared with the first 2 quartiles. Systolic blood pressure, insulin dose, and total cholesterol, LDL, and apo B levels at follow-up were significantly higher in the fourth quartile only when compared with the first or second quartile (Tables 2 and 3). Diastolic blood pressure, WHR, triglyceride levels, Rf, HDL, apo A-I, and the number of episodes of severe hypoglycemia were not different among the quartiles of weight gain. No significant differences in cholesterol content in the IDL or LDL fractions (95% CI include the zero line in these fractions) between the fourth and first weight gain quartiles were found (Figure 2).
Table 2.
Follow-up Characteristics of Quartiles of Weight Gain of Conventionally Treated Group*
| Variable | Quartile of Weight Gain | |||
|---|---|---|---|---|
| First | Second | Third | Fourth | |
| Hemoglobin A1c , % | 9.5 (1.6) [+11]† |
9.2 (1.5) [+7.0]† |
9.1 (1.5) [+2.2] |
8.9 (1.3)‡ [0] |
| Body mass index, kg/m2 | 24 (2.6) [−4.0]† |
24 (2.5) [+4.4]† |
25 (2.6)§∥ [+4.2]† |
27 (3.3)]§∥¶ [+13]† |
| Systolic BP, mm Hg | 113 (15) [−2.6]# |
115 (11) [0] |
116 (13) [0] |
119 (13)‡# [+2.6]** |
| Diastolic BP, mm Hg | 74 (11) [0] |
73 (10) [0] |
75 (8) [+2.7]** |
76 (9) [+4.1]** |
| Insulin dose, u/kg per day | 0.60 (0.19) | 0.61 (0.15) | 0.62 (0.15) | 0.66 (0.17)§ |
| Waist-to-hip ratio | 0.82 (0.07) | 0.81 (0.08) | 0.82 (0.08) | 0.84 (0.1) |
| Severe hypoglycemia, episodes per subject |
1 (2) | 2 (5) | 1 (2) | 1 (2) |
Results are expressed as mean (SD). Results in brackets are percent change from baseline. Body mass index is a measure of weight in kilograms divided by the square of height in meters. BP indicates blood pressure.
P<.001 compared with baseline.
P<.01 vs first quartile.
P<.001 vs first quartile.
P<.001 vs second quartile.
P<.001 vs third quartile.
P<.01 vs second quartile.
P <.05 compared with baseline.
Table 3.
Lipid Levels at Follow-up by Quartile of Weight Gain in the Conventionally Treated Group*
| Variable | Quartile of Weight Gain | |||
|---|---|---|---|---|
| First | Second | Third | Fourth | |
| Triglyceride, mmol/L [mg/dL] | 0.89 (0.50) [79 (44)] [−5.4] |
0.93 (0.54) [82 (48)] [4.7] |
0.99 (0.62) [88 (55)] [3.2] |
1.05 (0.60) [93 (53)] [11.1]† |
| Total cholesterol, mmol/L [mg/dL] | 4.55 (0.85) [176 (33)] [−0.7] |
4.71 (0.91) [1.82 (35)] [4.2]† |
4.84 (0.96) [187 (37)] [5.2]§ |
4.97 (0.91) [192 (35)]‡ [8.5]§ |
| LDL, mmol/L [mg/dL] | 2.82 (0.75) [109 (29)] [−1.7] |
2.90 (0.83) [112 (32)] [2.8] |
3.05 (0.83) [118 (32)] [6.3]§ |
3.18 (0.78) [123 (30)]†∥ [12]§ |
| HDL, mmol/L [mg/dL] | 1.32 (0.31) [51 (12)] [3.9] |
1.37 (0.36) [53 (14)] [6.2]§ |
1.34 (0.34) [52 (13)] [1.5] |
1.29 (0.31 [50 (12)] [0] |
| Apo B, g/L | 0.81 (0.21) | 0.83 (0.21) | 0.87 (0.23) | 0.92 (0.22)ঠ|
| LDL Rf | 0.30 (0.03) | 0.30 (0.03) | 0.30 (0.04) | 0.30 (0.03) |
| Apo A-I, g/L | 1.38 (0.23) | 1.43 (0.24) | 1.41 (0.22) | 1.38 (0.20) |
| Lp(a), g/L | 1.3 | 1.2 | 1.6 | 1.1 |
Results are expressed as mean (SD) except Lp(a), which is reported as median. Results listed last in brackets are percent change from baseline. LDL indicates low-density lipoprotein; HDL, high-density lipoprotein; aĀpo B, apolipoprotein B; Rf, relative flotation; apo A-I, apolipoprotein A-I; and Lp(a), lipoprotein(a).
P<.01 compared with baseline.
P<.001 vs first quartile.
P<.001 compared with baseline.
P<.01 vs second quartile.
P<.001 vs second quartile.
Figure 2.
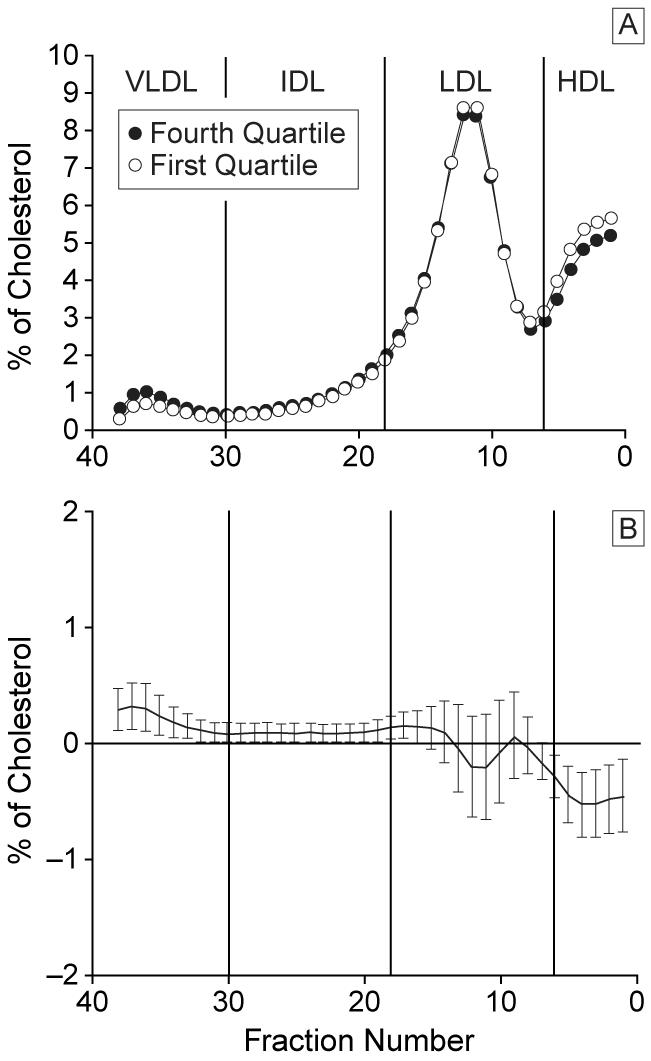
Distribution of lipoprotein cholesterol across nonequilibrium density gradient (from very low-density lipoprotein [VLDL], intermediate density lipoprotein [IDL], low-density lipoprotein [LDL], to high-density lipoprotein [HDL]) ultracentrifugation comparing the fourth quartile with the first quartile of weight gain with conventional therapy (A) and difference plot of mean cholesterol levels of each fraction with 95% confidence interval (B).
Compared with baseline, changes in BMI were significant in all quartiles with weight loss occurring in the first quartile and weight gain in the remaining groups (Table 2). Systolic blood pressure fell in the first quartile but rose in the fourth quartile and diastolic blood pressures were higher in the third and fourth quartiles but not the first 2 (Table 2). With the greatest weight gain (third and fourth quartiles), levels of triglyceride, total cholesterol and LDL-C rose but HDL-C levels remained stable (Table 3).
Intensively Treated Group at Follow-up
In the intensively treated group, all quartiles of weight gain achieved the same glycemic control at follow-up as measured by hemoglobin A1c(Table4). Final BMI increased significantly in each weight gain quartile and was the highest in the fourth quartile (average BMI in fourth quartile, 31 kg/m2). Systolic and diastolic blood pressures were significantly higher only in the fourth quartile of weight gain compared with the first 3 quartiles. Insulin requirements and WHR at follow-up also trended upward with increasing weight gain with the highest levels of these values found in the fourth quartile with intensive therapy (insulin dose in fourth quartile vs first and second quartiles, P<.001; WHR in fourth quartile vs first quartile, P<.01). The average number of episodes of severe hypoglycemia that occurred per subject over the duration of the study was slightly but significantly higher in the fourth quartile compared with the first quartile (5 episodes per subject vs 4 episodes per subject, P<.05) but not the other quartiles.
Table 4.
Follow-up Characteristics of Quartiles of Weight Gain of Intensively Treated Groupa
| Variable | Quartile of Weight Gain | |||
|---|---|---|---|---|
| First | Second | Third | Fourth | |
| Hemoglobin A1c, % | 7.3 (1.3) [−16.1]b |
7.2 (1.0) [−15.3]b |
7.1 (1.0) [−20.2]b |
7.3 (0.9) [−20.7]b |
| Body mass index, kg/m2 | 24 (2.3) [0] |
25 (2.5)c [+8.7]b |
27 (2.9)cd [+17]b |
31 (4.7)cde [+29]b |
| Systolic BP, mm Hg | 113 (11) [−0.9] |
117 (11) [+2.6]f |
115 (15) [+0.9] |
120 (12)cgh [+5.3]b |
| Diastolic BP, mm Hg | 73 (8) [+1.4] |
74 (9) [+2.8] |
74 (11) [+1.4] |
77 (8)cih [+5.5]b |
| Insulin dose, u/kg per day | 0.61 (0.19) | 0.64 (0.17) | 0.69 (0.22)c | 0.74 (0.22)cd |
| Severe hypoglycemia, episodes per subject |
4 (8) | 5 (8) | 5 (7) | 5 (8)j |
| Waist-to-hip ratio | 0.81 (0.09) | 0.82 (0.09) | 0.82 (0.09) | 0.83 (0.12)k |
Results are expressed as mean (SD). Results in brackets are percent change from baseline.
P<.001 compared with baseline.
P<.001 vs first quartile.
P<.001 vs second quartile.
P<.001 vs third quartile.
P<.05 compared with baseline.
P<.05 vs fourth quartile.
P<.01 vs third quartile.
P<.01 vs second quartile.
P<.05 vs first quartile.
P<.01 vs first quartile.
Compared with baseline, BMI did not change in the first quartile at follow-up but was significantly higher in each of the other 3 quartiles (Table 4). Only in the fourth quartile did both systolic and diastolic blood pressures rise significantly.
Lipid Levels of Intensively Treated Group at Follow-up
Levels of triglyceride, total cholesterol, LDL-C, and apo B at follow-up were significantly higher in the fourth quartile of weight gain compared with each of the first 3 quartiles (Table 5). A trend for reduction in HDL-C and apo A-I levels at follow-up was seen with weight gain, though these levels were significantly lower only in the fourth compared with the first quartile. The LDL peak buoyancy at follow-up was significantly less (LDL was denser) in the fourth quartile compared with the first quartile (LDL Rf, 0.30 vs 0.31, respectively, P<.01). Comparing the distribution of cholesterol in the lipoprotein fractions between the fourth and first weight gain quartiles at follow-up demonstrated significantly greater cholesterol in VLDL, IDL, and dense LDL fractions in the fourth quartile compared with the first quartile (Figure 3). The 0.41-mmol/L (16-mg/dL) increase in calculated LDL-C between the first and fourth quartiles (Table 5) was the result of cholesterol accumulation in IDL and dense LDL fractions (Figure 3) and resulted in 53 subjects with LDL levels greater than or equal to 3.36 mmol/L (130 mg/dL) in the fourth quartile compared with 23 subjects with similar levels in the first quartile (χ2= 22.59; P = .001).
Table 5.
Lipid Levels at Follow-up by Quartile of Weight Gain in the Intensively Treated Group*
| Variable | Quartile of Weight Gain | |||
|---|---|---|---|---|
| First | Second | Third | Fourth | |
| Triglyceride, mmol/L [mg/dL] | 0.79 (0.38) [70 (34)] [−14.4]† |
0.82 (0.52) [73 (46)] [−4.8] |
0.91 (0.59) [81 (52)] [6] |
0.99 (0.45) [88 (40)]‡§∥ [7.8] |
| Total cholesterol, mmol/L [mg/dL] | 4.50 (0.80) [174 (31)] [−5.9]† |
4.53 (0.72) [175 (28)] [1.8] |
4.63 (0.78) [179 (30)] [1.1] |
4.86 (0.72) [188 (28)]द [3.8]# |
| LDL, mmol/L [mg/dL] | 2.74 (0.70) [106 (27)] [−8.7]† |
2.79 (0.65) [108 (25)] [1.8] |
2.92 (0.72) [113 (28)] [0.7] |
3.15 (0.65) [122 (25)]‡§∥ [6.8]† |
| HDL, mmol/L [mg/dL] | 1.40 (0.34) [54 (13)] [2.2] |
1.34 (0.36) [52 (14)] [1.5] |
1.29 (0.34) [50 (14)] [−2.3] |
1.27 (0.31) [49.1 (12)]‡ [−3.8]† |
| Apo B, g/L | 0.78 (0.18) | 0.80 (0.18) | 0.84 (0.27) | 0.89 (0.17)‡§∥ |
| LDL Rf | 0.31 (0.02) | 0.31 (0.03) | 0.31 (0.03) | 0.30 (0.03)** |
| Apo A-I, g/L | 1.42 (0.22) | 1.39 (0.23) | 1.36 (0.22) | 1.36 (0.23)†† |
| Lp(a), g/L | 1.3 | 8.6 | 1.0 | 1.3 |
Results are expressed as mean (SD) except Lp(a), which is reported as median. Results listed last in brackets are percent change from baseline. LDL indicates low-density lipoprotein; HDL, high-density lipoprotein; apo B, apolipoprotein B; Rf, relative flotation; apo A-I, apolipoprotein A-I; and Lp(a), lipoprotein(a).
P<.001 compared with baseline.
P<.001 vs first quartile.
P<.001 vs second quartile.
P<.001 vs third quartile.
P<.05 vs third quartile.
P<.01 compared with baseline.
P<.01 vs first quartile.
P<.05 vs first quartile.
Figure 3.
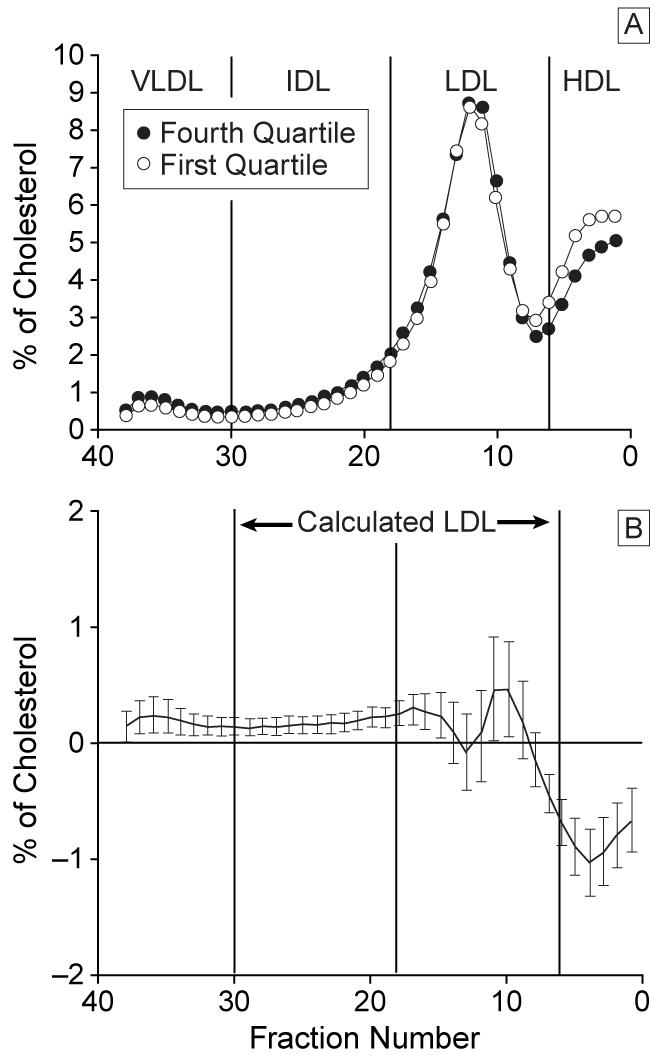
Distribution of lipoprotein cholesterol across nonequilibrium density gradient (from very low-density lipoprotein [VLDL], intermediate density lipoprotein [IDL], low-density lipoprotein [LDL], to high-density lipoprotein [HDL]) ultracentrifugation comparing the fourth quartile with the first quartile of weight gain with intensive therapy (A) and difference plot of mean cholesterol levels of each fraction with 95% confidence interval (B).
Compared with baseline, the first quartile experienced decreases in triglyceride, total cholesterol, and LDL-C levels (Table 5). The fourth quartile, on the other hand, experienced significant increases in total cholesterol and LDL-C and a decrease in HDL-C levels.
Multivariate Analysis
Since glycemic control affects lipid levels independently of body weight, multiple linear regression was performed in both treatment groups to determine if the affect of weight gain on plasma lipid levels was independent of final hemoglobin A1c. After controlling for hemoglobin A1c, weight gain remained independently associated with triglyceride, total cholesterol, LDL, and apo B levels in both treatment groups (P<.001), and HDL and apo A-I levels in the intensively treated group (P<.001).
Other Baseline and Follow-up Comparisons
No differences were found between weight gain quartiles in either treatment group for the following: albumin excretion rate and levels of Lp(a) at follow-up, the number of smokers at baseline who became nonsmokers at follow-up, duration of diabetes at entry, baseline insulin dose, and baseline stimulated C-peptide levels (data not shown). Men and women in the intensive treatment group experienced similar changes in BMI, lipid levels, apolipoprotein levels, and cholesterol distribution in lipoproteins with weight gain (data not shown).
The proportion of pregnancies in women was similar for each treatment group (102 pregnancies in 287 women with intensive therapy vs 91 pregnancies in 252 women with conventional therapy, P = .10). Within each treatment group, the highest proportion of pregnancies occurred in the women in the fourth quartiles: 42 pregnancies in 88 women in fourth quartile of weight gain with intensive therapy (χ2= 9.71; P<.05) compared with other quintiles of weight gain with intensive therapy; 31 pregnancies in 62 women in fourth quartile of weight gain with conventional therapy (χ2= 13.09; P<.005) compared with other quintiles of weight gain with conventional therapy. The proportion of pregnancies in the fourth quartiles of the intensively and conventionally treated groups, however, was not different (χ2= .08; P<.78). When women who had been pregnant at any time during the study were excluded from analysis of the follow-up parameters in Tables 2 through 5, the results did not change.
COMMENT
Prior to instituting insulin treatment for type 1 diabetes, patients who have poor metabolic control and hypovolemia are underweight and have hypertriglyceridemia due in large part to depressed lipoprotein lipase (LpL) activity in adipose and muscle tissue. After rehydration and institution of insulin therapy, metabolic control improves, LpL activity normalizes, and despite the subsequent weight gain, lipid levels usually become normal.9 The DCCT showed that despite greater weight gain compared with conventional therapy, continued improvement in metabolic control with intensive diabetes therapy resulted in improvements in lipid levels and the distribution of cholesterol in lipoprotein particles at follow-up.6,7 Concern has arisen, however, that this weight gain with intensive therapy may have adverse effects on lipid levels and blood pressure and increase the risk for macrovascular complications in some individuals.
Since the glycemic control at follow-up was not uniform among the weight gain quartiles with conventional therapy, final lipid levels in these groups represent the interaction of changes in glycemic control and weight that occurred during the study. Even though glycemic control in the first quartile deteriorated compared with baseline, lipid levels remained stable at follow-up, perhaps as a result of the small amount of weight loss that occurred in this group. On the other hand, despite stable glycemic control in the fourth quartile compared with baseline, all lipid and blood pressure parameters deteriorated significantly (except HDL-C, which remained stable) with weight gain. Looking at the cross-sectional data at follow-up, the fourth quartile had better glycemic control than the first quartile, which might have been expected to result in improved lipid levels,6,7 and yet the extra weight gain was associated with higher total cholesterol, LDL-C, and apo B levels and systolic blood pressure. No effect of weight gain on follow-up triglyceride levels, HDL-C, the distribution of cholesterol in IDL or dense LDL fractions (fourth vs first quartile), WHR, or diastolic blood pressure, however, could be demonstrated between the weight gain quartiles with conventional therapy. The implication of this data is that even the more modest weight gain with conventional therapy compared with that seen with intensive therapy (see below) has deleterious effects on lipid levels and systolic blood pressure.
Intensive therapy resulted in similar glycemic control at follow-up in all the weight gain quartiles. Final lipid levels in these groups, therefore, reflect improvements from intensified diabetes therapy counter balanced by changes due to weight gain. The first quartile of weight gain with intensive therapy did not significantly change weight during the study and experienced improvement in triglyceride, total cholesterol, and LDL-C levels compared with baseline. This demonstrates the benefits of improved glycemic control on lipid levels in the absence of weight gain. On the other hand, those in the fourth quartile had a significant deterioration of lipid levels and blood pressure compared with baseline and had twice as many subjects with LDL levels above the recommended acceptable limit compared with the first quartile.25
In the cross-sectional analysis at follow-up, the mean final BMI in the fourth quartile weight gain group receiving intensive therapy was 31 kg/m2 and was the highest BMI compared with all other weight gain quartiles in either treatment group. Since this fourth quartile of weight gain was the only quartile to meet obesity criteria by BMI,22 the term excessive weight gain is used to describe this group. In addition to becoming clinically obese, the fourth quartile had significantly higher levels of triglyceride, total cholesterol, LDL-C, and apo B at follow-up compared with remaining quartiles, whereas no significant differences between the bottom 3 quartiles for these values could be demonstrated. This implies that the beneficial effects of intensive therapy on total lipid and apo B levels are present in those experiencing modest weight gain and that deterioration in these levels does not occur unless subjects experience excessive weight gain.
As already mentioned, because the first quartile of weight gain with intensive therapy remained weight-stable throughout the study, follow-up results in this group represent the effects of intensive therapy without effects from weight gain or loss. This quartile is therefore an ideal control group for comparison with the fourth quartile to isolate the effects of excessive weight gain on lipid levels, cholesterol distribution in lipoprotein particles, and blood pressure. The increase in total cholesterol and calculated LDL-C that occurred between the first and the fourth quartile resulted from greater cholesterol in the VLDL, IDL, and dense LDL particle fractions (Figure 3). In nondiabetic subjects, accumulation of cholesterol in small, dense LDL is frequently accompanied by higher levels of triglyceride, lower levels of HDL, more insulin resistance, higher blood pressure, and greater abdominal obesity.23,24,26,27 This clustering of metabolic abnormalities has been termed the central obesity–insulin resistance syndrome and is associated with an increased risk for heart disease.23,24,26 In addition to the lipid abnormalities found when the fourth quartile is compared with the first quartile, other components of insulin resistance syndrome can be demonstrated between these groups, including more insulin resistance (higher insulin dose), higher blood pressure, and greater abdominal obesity (higher WHR). Many of the metabolic abnormalities associated with the central obesity–insulin resistance syndrome demonstrated with excessive weight gain from intensive treatment, however, did not emerge with the more modest weight gain from conventional treatment. This suggests the degree of glycemic control may play an important role in allowing full expression of this disorder. These changes in lipid levels and blood pressure with excessive weight gain, especially when accompanied by a central distribution of body weight and insulin resistance, may have an undesirable effect on macrovascular risk in this subset of patients. The findings of excessive weight gain and obesity in subjects undergoing intensive diabetes management may therefore be reason to modify glycemic goals to prevent further weight gain.
The only baseline characteristic that distinguished the group that gained the most amount of weight with intensive therapy from the group that gained the least was a higher hemoglobin A1c level in the former. If the greatest weight gain with intensive therapy was due to this group being underweight at baseline as a result of poorer glycemic control, then the initial BMI of the fourth quartile subjects would have been expected to be the lowest of all the quartiles. In fact, the initial BMI of the fourth quartile was slightly higher than the 2 middle quartiles and the same as the first quartile. An alternative explanation might be that since baseline insulin doses were not different between the weight gain quartiles, the higher hemoglobin A1c level in the fourth quartile at baseline represents the presence of greater insulin resistance in this group. Such insulin resistance might be a marker for those subjects who have a predisposition for excessive weight gain through mechanisms that might include the need for greater exogenous insulin or a genetic potential for weight gain. The frequency of severe hypoglycemia was slightly more common in the fourth quartile of weight gain with intensive therapy compared with the first quartile, but it is unlikely that this small difference accounted for the weight gain seen. Unfortunately, many important determinants of weight gain, such as the frequency of mild-to-moderate hypoglycemia symptoms and alterations in energy expenditure, were not measured as part of the DCCT.
Interestingly, the highest proportions of pregnancies were found in the fourth quartiles of each treatment group. An explanation for this association of increased frequency of pregnancy and weight gain may come from the fact that women in the conventionally treated group who became pregnant under went in ten sification of therapy during pregnancy to achieve the same goals as the intensively treated group.17 Once postpartum, those in the conventionally treated group were then expected to resume their treatment assignment. It may be that with intensification of diabetes therapy some women will be less likely to return to their baseline weight postpartum and may represent those predisposed to remain obese after pregnancy. Because the proportion of pregnancies among the women was equal in the fourth quartiles of weight gain of both treatment groups, however, this finding alone does not account for the excessive weight gain found with intensive therapy.
In summary, the changes in lipid levels and cholesterol content in lipoprotein fractions that occur with excessive weight gain from intensive therapy of type 1 diabetes include higher triglyceride levels, higher total cholesterol and LDL-C levels, lower HDL levels, and greater cholesterol distribution in VLDL, IDL, and dense LDL particles. These lipid changes—along with the findings of higher blood pressures, an increased WHR, and greater insulin requirements in this group—suggest similarities to the insulin resistance syndrome that occurs with central obesity and type 2 diabetes. Over a long follow-up period, these lipid and metabolic changes may contribute to an increased risk of macrovascular disease in the subset of subjects who gain an excess amount of weight with intensive therapy despite improved glycemic control.
Acknowledgments
This work was supported by grant DK 2456 from the National Institutes of Health, Rockville, Md, and the Juvenile Diabetes Foundation International, New York, NY. The DCCT was supported by the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes, Digestive, and Kidney Disease, National Institutes of Health. A portion of these studies was performed at the Clinical Research Center at the University of Washington, Seattle, under grant RR 37.
We would like to thank Chris Casazza for his invaluable aid in performing the DGUC measurements and the participants and investigators who took part in the DCCT.
References
- 1.Brunzell JD. Obesity and risk for cardiovascular disease. In: Greenwood MRC, editor. Contemporary Issues in Clinical Nutrition, Obesity. Churchill Livingston Inc; New York, NY: 1983. pp. 3–16. [Google Scholar]
- 2.Laakso M, Pyorala K. Adverse effects of obesity on lipid and lipoprotein levels in insulin-dependent and non–insulin-dependent diabetes. Metabolism. 1990;39:117–122. doi: 10.1016/0026-0495(90)90062-h. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group Weight gain associated with intensive therapy in the Diabetes Control and Complications Trial. Diabetes Care. 1988;11:567–573. doi: 10.2337/diacare.11.7.567. [DOI] [PubMed] [Google Scholar]
- 4.Wing RR, Klein R, Moss SE. Weight gain associated with improved glycemic control in population-based sample of subjects with type I diabetes. Diabetes Care. 1990;13:1106–1109. doi: 10.2337/diacare.13.11.1106. [DOI] [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial Research Group Adverse events and their association with treatment regimens in the Diabetes Control and Complications Trial. Diabetes Care. 1995;18:1415–1427. doi: 10.2337/diacare.18.11.1415. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial Research Group Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol. 1995;75:894–903. doi: 10.1016/s0002-9149(99)80683-3. [DOI] [PubMed] [Google Scholar]
- 7.Purnell JQ, Marcovina SM, Hokanson JE, et al. Levels of Lp(a), apolipoprotein B, and lipoprotein cholesterol distribution in IDDM: results from follow-up in the Diabetes Control and Complications Trial. Diabetes. 1995;44:1218–1226. doi: 10.2337/diab.44.10.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuhldreher WL, Orchard TJ, Ellis D. The association of waist-hip ratio and risk factors for development of IDDM complications in an IDDM adult population. Diabetes Res Clin Pract. 1992;17:99–109. doi: 10.1016/0168-8227(92)90155-k. [DOI] [PubMed] [Google Scholar]
- 9.Brunzell JD, Chait A. Diabetic dyslipidemia-pathology and treatment. In: Porte D Jr, Sherwin J, editors. Ellenberg and Rifkin’s Diabetes Mellitus. Appleton & Lange Inc; East Norwalk, Conn: 1997. pp. 1077–1098. [Google Scholar]
- 10.Dorman JS, Laport RE, Kuller LH, et al. The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study: mortality results. Diabetes. 1984;33:271–276. doi: 10.2337/diab.33.3.271. [DOI] [PubMed] [Google Scholar]
- 11.Krolewski AS, Kosinski EJ, Warram JH, et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59:750–755. doi: 10.1016/0002-9149(87)91086-1. [DOI] [PubMed] [Google Scholar]
- 12.Koivisto VA, Stevens LK, Mattock M, et al. Cardiovascular disease and its risk factors in IDDM in Europe. Diabetes Care. 1996;19:689–697. doi: 10.2337/diacare.19.7.689. [DOI] [PubMed] [Google Scholar]
- 13.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services . The Prevalence Study. Vol. 1. Public Health Service, National Institutes of Health; Bethesda, Md: 1980. [Google Scholar]
- 15.Metropolitan height and weight tables. Stat Bull Metrop Life Ins Co. 1993;43:410–418. [PubMed] [Google Scholar]
- 16.The Diabetes Control and Complications Trial Research Manual of Operations 1993US Dept of Commerce, National Technical Information Service; Springfield, Va; Publication 93-183382. [Google Scholar]
- 17.The Diabetes Control and Complications Trial Research Group The Diabetes Control and Complications Trial (DCCT): design and methodologic considerations for the feasibility phase. Diabetes. 1986;35:530–545. [PubMed] [Google Scholar]
- 18.Marcovina SM, Albers JJ, Gabel B, Koshinsky ML, Guar VP. Effect of the number of apolipoprotein (a) kringle 4 domains on immunochemical measurements of lipoprotein (a) Clin Chem. 1995;41:246–255. [PubMed] [Google Scholar]
- 19.Marcovina SM, Albers JJ, Kennedy H, Mei JV, Henderson LO, Hannon WH. International Federation of Clinical Chemistry Standardization Project for measurements of apolipoproteins A-I and B, IV: comparability of apolipoprotein B values by use of International Reference Material. Clin Chem. 1994;40:586–592. [PubMed] [Google Scholar]
- 20.Hollenbeck C, Reaven GM. Variations in insulin-stimulated glucose uptake in healthy individuals with normal glucose tolerance. J Clin Endocrinol Metab. 1987;64:1169–1173. doi: 10.1210/jcem-64-6-1169. [DOI] [PubMed] [Google Scholar]
- 21.Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype: a proposed marker for coronary artery disease risk. Circulation. 1990;82:495–506. doi: 10.1161/01.cir.82.2.495. [DOI] [PubMed] [Google Scholar]
- 22.Kuczmarski R, Flegal K, Campbell S, Johnson C. Increasing prevalence of overweight among US adults. JAMA. 1994;272:205–211. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Gardner DC, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276:875–881. [PubMed] [Google Scholar]
- 24.Stampfer MJ, Krauss RM, Ma J, et al. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882–888. [PubMed] [Google Scholar]
- 25.American Diabetes Association Detection and management of lipid disorders in diabetes. Diabetes Care. 1996;19(suppl 1):S96–S102. doi: 10.2337/diacare.16.5.828. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto WY, Abbate SL, Kahn SE, Hokan-son JE, Brunzell JD. The visceral adiposity syndrome in Japanese-American men. Obes Res. 1994;2:364–371. doi: 10.1002/j.1550-8528.1994.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 27.Tchernof A, Lamarche B, Prud’Homme D, et al. The dense LDL phenotype: association with plasma lipoprotein levels, visceral obesity, and hyperinsulinemia in men. Diabetes Care. 1996;19:629–637. doi: 10.2337/diacare.19.6.629. [DOI] [PubMed] [Google Scholar]


