Summary
The discovery of vascular endothelial growth factor (VEGF) changed the field of angiogenesis. We have learned that VEGF has broader actions than merely a driver of tumor angiogenesis, particularly that VEGF controlled several fundamental functions and properties of endothelial cells and non-endothelial cells. The lung is one of the main organs where VEGF controls several critical physiological functions. These actions rely on tightly regulated temporal and concentration gradients of VEGF and VEGF receptor expression in the lung. Excessive or diminished VEGF have been linked to abnormal lung phenotypes and, in humans, linked to several diseases. The beneficial and detrimental actions of VEGF underscore that therapeutic targeting of VEGF in disease has to carefully consider the lung biology of VEGF.
INTRODUCTION
VEGF-A, also known as vascular permeability factor (VPF) [1], plays a fundamental role in physiological and pathophysiological forms of angiogenesis and regulation of endothelial cell differentiation. In contrast to fibroblast growth factor, which requires cell damage or basement membrane proteolysis for its release and binding to multiple cell targets, VEGF is actively secreted and has high specificity for endothelial cells. The lung contains the highest level of transcripts [2] amongst a wide range of organs that express VEGF. VEGF is necessary for the formation of vascular beds of several organs during embryo development, as demonstrated by the lethality of VEGF knockout mice and abnormal vasculogenesis of the heart and large vessels with loss of only a single copy of the VEGF gene [3]. VEGF contributes to endothelial cell nitric oxide (NO) production in coronary arteries and cultured umbilical vein endothelial cells [4]. The increase in endothelial cell nitric oxide synthase activity relies on activation of Src and MAP kinase [5] and the PI3K/Akt pathway [6]. VEGF has an anti-inflammatory action, decreasing leukocyte adhesion in an NO-dependent manner [7].
VEGF prevents death of endothelial cells, both in vitro and in animal models of oxygen-mediated retinopathy [8]. VEGF-dependent survival of endothelial cells relies on activation of PI3 kinase, Akt and Src [5]. The discovery of the parent VEGF-A molecule led to the subsequent identification of the subforms B through E. In this review, we will focus on VEGF-A (designated hereafter as VEGF).
VEGF binds to 2 tyrosine-kinase receptors present on endothelial cells, Flt or VEGF receptor 1 (VEGFR-1) and KDR or VEGFR-2. Dominant negative mutant forms of VEGFR-2 can abrogate VEGF-induced signal transduction in vitro and reduce blood vessel proliferation in vivo models of brain tumors [9]. VEGFR-1 binds VEGF with approximately 10-fold higher affinity than KDR [10], undergoes receptor autophophorylation, and stimulates Ca++ influx. VEGF binding to VEGFR-2 results in cell ruffling, mitosis, chemotaxis, and actin rearrangement [11]. VEGFR-2 undergoes autophosphorylation more efficiently than receptor 1 upon ligand binding. Inhibition of VEGFR-2 blocks proliferation of cultured umbilical vein endothelial cells, in vivo angiogenesis, and vascular permeability [12]. VEGFR-2 is also stimulated in an autocrine manner by endothelial VEGF, which was recently found to be essential for endothelial survival [13]. VEGFR-1 plays a role in the organization of development of embryonic blood vessels [14] and in enhanced monocyte adhesion to endothelial cells [15]. There is the potential for cross interaction of both VEGF receptors as they are approximately 70% homologous and possibly heterodimerize in vivo. VEGFR-1 might act as a silent receptor for VEGF since it has a poor kinase activity. However, its downstream cell signaling remains poorly delineated [16]. It may also serve as a decoy for VEGF [17], as documented by the excess numbers of endothelial cells in amniotic membrane vessels of embryonic bodies lacking VEGFR-1 in the presence of intact VEGFR-2 [18]. On the other hand, VEGFR-1 enhances VEGF-induced VEGFR-2 signaling during abnormal angiogenesis, since it prevents endothelial cell apoptosis. As there are no conditional knockouts of VEGFR1 and 2, loss-of-function experiments have relied on neutralizing antibodies (such as DC101 against VEGFR-2 and MF1 against VEGFR-1), soluble chimeric molecules with the ligand binding domain of VEGFR-1 (VEGF traps), or chemical inhibition with small molecule inhibitors such as SU5416, which prevents VEGF-induced phosphorylation of VEGF-R2 [12] and, subsequently shown, to also block VEGF-R1 [19].
VEGF IS A FRIEND IN THE LUNG ROLE AS A CRITICAL LUNG ENDOTHELIAL CELL MORPHOGENETIC AND MAINTENANCE FACTOR
Lung morphogenesis requires the continuous physical and molecular interaction between the mesenchymal stroma and epithelial elements [20]. During airway growth, the lung progressively acquires a rich blood supply through the growth of endothelial cells and vascular cells in the pulmonary mesenchyme. This growth is paralleled by the expression of VEGF and its receptors [21], which play central morphogenetic functions throughout fetal lung maturation. Lung VEGF is synthesized by alveolar epithelial cells, epithelial bronchial cells, smooth muscle cells, and alveolar macrophages [22;23]. This topographical compartmentalization allows for the interaction of VEGF with components of the extracellular matrix, therefore generating concentration gradients that regulate physiological functions of VEGF in the lung [24]. Overexpression of lung VEGF during development results in a markedly dysmorphic lung structure [25]. Conversely, neutralization of VEGF throughout fetal development with Fc-VEGFR1 extracellular domain promotes an overtly simplified lung in newborn mice [26].
The critical role of VEGF signaling in lung structure maintenance is supported by the findings that SU5416 impairs lung development in neonatal rats [27], decreased levels of VEGF may contribute to bronchopulmonary dysplasia [28], and mice with deleted hypoxia inducible factor-2 or treated with anti-VEGFR-2 show respiratory distress and lung prematurity. Administration of VEGF to these mice partly rescues lung immaturity and respiratory distress syndrome [29].
VEGF also plays an important role in postnatal lung growth, since blockade of VEGFR-1 and VEGFR-2 with DC101 and MF1, respectively, arrests lung growth and leads to an emphysematous mouse phenotype [30]. Inhibition of VEGF results in regression of tracheal capillaries and endothelial cell death in adult mouse lungs, while most vessels become resistant to VEGF withdrawal after embryonic development [31].
ROLE IN EMPHYSEMA
VEGFR blockade with SU5416 results in endothelial cell apoptosis and, consistent with the importance of septal endothelial cells in alveolar integrity, SU5416 causes apoptosis-dependent emphysema in rats [32]. In agreement with our findings with VEGFR- blockade by SU5416, transgenic mice in which lung VEGF was deleted by means of Cre-recombination of two Lox-P recognition sites flanking the VEGF gene show emphysema after 4 weeks of intratracheal instillation of adenoassociated virus-CRE [33]. The association between advanced human emphysema and decreased lung or plasma levels of VEGF provided evidence in support of an alveolar structural maintenance role for VEGF in humans [34]. An imbalance of VEGFR1 vs. R2 activation may also allow for apoptotic alveolar enlargement as shown in mice overexpression of placenta growth factor (Plgf) [35].
In addition to its well-known functions as a trophic and growth factor, VEGF may play novel biological roles in the maintenance of lung homeostasis. Lung cellular homeostasis requires the prompt removal of apoptotic corpses to keep the overall number of cells constant. The efficient removal of damaged cells decreases the risk of necrosis, inflammation, or neoplasia and, via binding of apoptotic cells to the phosphatidylserine receptor, a host of immunosuppressive cytokines (TGF-β, PGE2, PGI2, IL10) are released to suppress inflammation and reduce the risk of autoimmune diseases [36]. VEGF may contribute to efferocytosis [37], which in turn leads to further VEGF production, therefore favoring cell repair in face of tissue destruction [38].
ROLE IN PULMONARY HYPERTENSION
Like its participation in acute lung injury (ALI), VEGF may have a dual role in pulmonary hypertension. A recent review addressed the pathology and pathobiology of pulmonary hypertension [39], which provides a useful framework for the present discussion. Endothelial cell injury underlies the development of a severe form of experimental pulmonary hypertension, such as that caused by the alkaloid monocrotaline (MCT). MCT-pulmonary hypertension is associated with decreased VEGF expression [40] and VEFG overexpression protects against MCT-induced pulmonary hypertension [41]. Highlighting the critical prosurvival role of VEGF in lung endothelial cells, a second model of severe pulmonary hypertension was developed based on early endothelial cell apoptosis due to the combination of VEGF receptor blockade with SU5416 and chronic hypoxia [42]. These results are concordant with the observation that VEGF inhibition with haptamers causes neonatal pulmonary hypertension in an ovine model [43]. Chronic hypoxia is a common inducer of experimental pulmonary hypertension; however, hypoxic pulmonary hypertension is relatively mild and reversible upon re-exposure to normal oxygen levels. Despite the evidence of increased expression of VEGF in hypoxic lungs [23], overexpression of VEGF protects rats against hypoxic pulmonary hypertension [44]. However, once intravascular endothelial cells accumulated in the pulmonary circulation of rats with pulmonary hypertension caused by SU5416+chronic hypoxia, administration of VEGF does not afford protection against severe pulmonary hypertension. In fact, it causes a small increase in pulmonary artery pressures [45]. These findings suggest that VEGF may indeed have a dual role in pulmonary hypertension, with early protection followed by a potentially pathogenic induction of pulmonary vascular remodeling.
VEGF IS A LUNG FOE ROLE IN ACUTE LUNG INJURY (ALI)
ALI consists of an acute clinical syndrome caused by alveolar leakage of plasma proteins, alveolar epithelial cell necrosis, scattered infiltration by neutrophils, and characteristic hyaline membranes, which impair oxygen diffusion, leading to hypoxemia. This syndrome can be caused by a variety of fulminant events, including sepsis, extensive trauma, oxygen or drug toxicity, viral infections, blood transfusions, and pancreatitis, among others. Its idiopathic form is known as acute interstitial pneumonia. The pathogenetic hallmark event in ALI is a marked increase in lung capillary endothelial cell permeability and alveolar cell injury. VEGF was originally discovered as a vascular permeability factor that accounted for the increased permeability of cancers seeded in the peritoneal cavity [1]. This permeability enhancing property of VEGF occurs when the growth factor is produced in high levels and targets a suitable vasculature as demonstrated by VEGF overexpression in the rabbit ear [46]. An excellent review on the role of VEGF in ALI correctly placed the VEGF-enhanced permeability in the context of angiopoetins 1 and 2, proteins that either decrease or enhance vascular permeability, respectively [22]. VEGF, when bound to extracellular matrix, can be released by activated extracellular matrix proteases, including plasmin and metalloproteases [24], which are also involved in the pathogenesis of ALI. Moreover, inflammatory cells (neutrophils and macrophages) and parenchymal cells activated by cytokines such as IL-1, IL-6, and TGF-β cause enhanced expression of VEGF. Heightened VEGF expression allied to an increased expression of angiopoietin-2 may account for the link between VEGF and increased capillary permeability in ALI. Recent evidence supports a central role of enhanced angiopoietin-2 in alveolar oxygen injury in the neonatal lung and in ALI [47]. However, it is recognized that VEGF may play an important role in septal cell recovery from ALI since established alveolar injury is associated with decreased expression of VEGF [48]. In summary, VEGF may play a pathogenetic role in ALI when increased, leading to enhanced alveolar and capillary permeability in active sites of injury and, when decreased, precluding proper and immediate cell repair.
ROLE IN ASTHMA
We have recently summarized the aggregate of data that support a potential pathogenetic role of VEGF in airway remodeling and inflammation in asthma [34]. Increased VEGF, VEGFR, and angiopoietin-1 level are observed in lung biopsies of asthmatic patients [49] and VEGF levels correlate with airway vascularity [50]. The proinflammatory actions of VEGF in eosinophils might account for part of their migration in the asthmatic lung. Furthermore, VEGF may promote hypervascularity and edema in the asthmatic bronchial mucosa [51] and enhanced dendritic cell recruitment and maturation, therefore driving TH2 inflammation in experimental models. In murine asthma models [52], part of these VEGF actions seem to be mediated by the VEGF-NO pathway [53]. Glucocorticoids, which are used to treat asthma, suppress VEGF transcription in vitro [54] and reduce VEGF levels in proportion to decreased vascularity [55], further suggesting a role of VEGF in pathophysiology of the disease.
ROLE IN PULMONARY HYPERTENSION
Although the aforementioned experimental data support a beneficial role of VEGF in pulmonary hypertension, there is evidence of increased expression of VEGF in remodeled hypertensive arteries (summarized in [34]). The coordinated expression of VEGF and VEGFR-2 in plexiform lesions in idiopathic pulmonary hypertension [56] further support the concept that these angioproliferative lesions have features in common to neoplastic processes [57] and with angiogenic cancers such as Kaposi’s sarcoma [58]. Of note, experimental studies of pulmonary vascular remodeling and pulmonary hypertension caused by cigarette smoke inhalation also demonstrate that VEGF is upregulated early in the pulmonary vascular pathology and may potentiate pulmonary vascular remodeling in combination with upregulated endothelin [59]. However, there is evidence for a friendly role of VEGF in pulmonary vascular disease as discussed previously.
CONCLUSION
The lung’s requirement on VEGF is more complex than that documented with most organs. For the past 13 years, we have learned that VEGF is both a friend and a foe. Too much or too little of VEGF is catastrophic to the lung-an observation that is shared with manipulations of other highly important molecules. However, the final verdict for VEGF still awaits clear therapeutic interventions, which will fulfill the central elements of Koch’s postulate on the role of VEGF in lung diseases. At the present time, the evidence supports a beneficial role of VEGF, as its loss may underlie lung immaturity, pulmonary hypertension, emphysema, and the repair stage of ALI. Furthermore, the broader actions of VEGF in the lung warrant that the pathobiological effects of VEGF supplementation or blockade be carefully considered in therapeutic trials.
Figure 1.
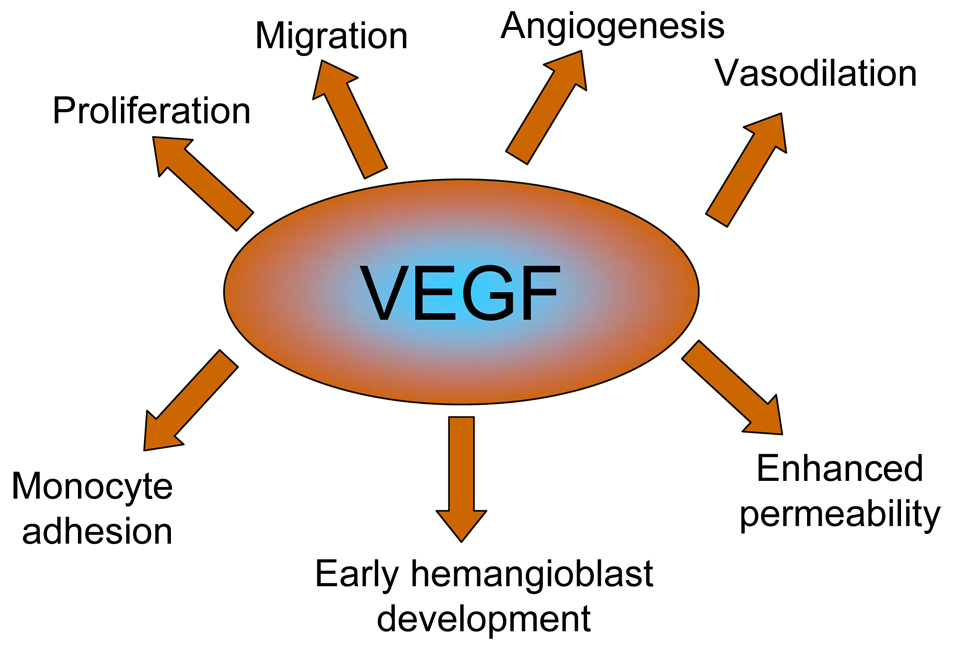
Biological actions of VEGF.
Figure 2.
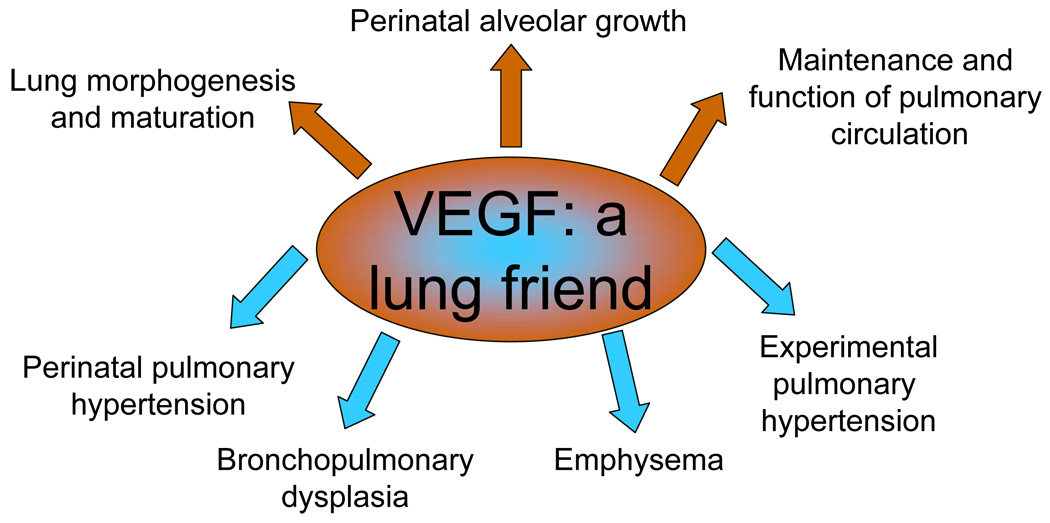
VEGF is a friend. Shown are the biological functions of VEGF form the basis of its role in the diseases, given the impact of decreased VEGF levels or protective effects of VEGF overexpression.
Figure 3.
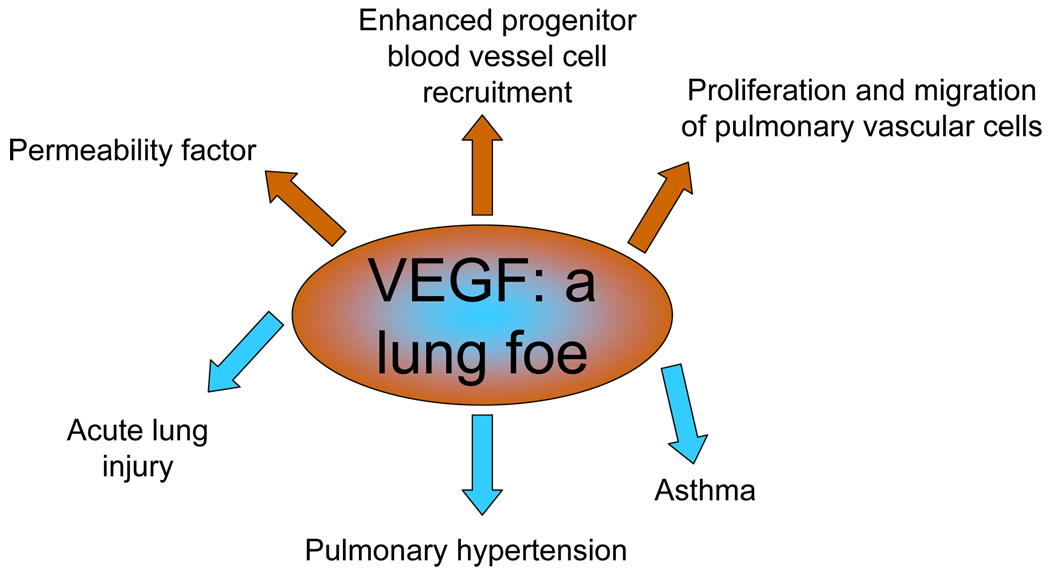
VEGF is a foe. Shown are the biological properties of VEGF that underlie its detrimental actions in the context of the listed diseases.
Acknowledgments
Grant support: This work has been supported by the CMREF Center for Tissue Processing Center of the Pulmonary Hypertension Breakthrough Initiative, P150 HL 084946-01 (Pathology Core and Project 5), and R01HL 66554 to RMT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562.This is the first description of VEGF in the literature. It was then known as vascular permeability factor. Its discovery was based on the ability of cancers to enhance permeability, a central feature of VEGF.
- 2.Monacci WT, Merrill MJ, Oldfield EH. Expression of vascular permeability factor/vascular endothelial growth factor in normal rat tissues. Amer J Physiol. 1993;264:C995–C1002. doi: 10.1152/ajpcell.1993.264.4.C995. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0.This is the first study to demonstrate that VEGF is absolutely required for fetal development and blood vessel formation.
- 4.Tsurumi Y, Murohara T, Krasinski K, Chen D, Witzenbichler B, Kearney M, Couffinhal T, Isner JM. Reciprocal relation between VEGF and NO in the regulation of endothelial integrity. Nat Med. 1997;3:879–886. doi: 10.1038/nm0897-879. [DOI] [PubMed] [Google Scholar]
- 5.He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem. 1999;274:25130–25135. doi: 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Medhora M, Falck JR, Pritchard KA, Jr., Jacobs ER. Mechanisms of activation of eNOS by 20-HETE and VEGF in bovine pulmonary artery endothelial cells. AJP - Lung Cellular and Molecular Physiology. 2006;291:L378–L385. doi: 10.1152/ajplung.00424.2005. [DOI] [PubMed] [Google Scholar]
- 7.Scalia R, Booth G, Lefer DJ. Vascular endothelial growth factor attenuates leukocyte-endothelium interaction during acute endothelial dysfunction: essential role of endothelium-derived nitric oxide. Faseb J. 1999;13:1039–1046. doi: 10.1096/fasebj.13.9.1039. [DOI] [PubMed] [Google Scholar]
- 8.Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 9.Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367:576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- 10.Vaisman N, Gospodarowicz D, Neufeld G. Characterization of the receptors for vascular endothelial growth factor. J Biol Chem. 1990;265:19461–19466. [PubMed] [Google Scholar]
- 11.Quinn TP, Peters KG, De VC, Ferrara N, Williams LT. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci U S A. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99–106. [PubMed] [Google Scholar]
- 13.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF Signaling Is Required for Vascular Homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 15.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 16.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 17.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bon F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 18.Kearney JB, Ambler CA, Monaco KA, Johnson N, Rapoport RG, Bautch VL. Vascular endothelial growth factor receptor Flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood. 2002;99:2397–2407. doi: 10.1182/blood.v99.7.2397. [DOI] [PubMed] [Google Scholar]
- 19.Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O'Reilly T, Persohn E, Rosel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch KH, Schneider MR, Drevs J, Martiny-Baron G, Totzke F. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178–2189. [PubMed] [Google Scholar]
- 20.Mason RJ, Williams MC, Moses HL, Mohla S, Berberich MA. Stem cells in lung development, disease, and therapy. Am J Resp Cell Mol Biol. 1997;16:355–363. doi: 10.1165/ajrcmb.16.4.9115744. [DOI] [PubMed] [Google Scholar]
- 21.Acarregui MJ, Penisten ST, Gross KL, Ramirez K, Snyder JM. Vascular endothelial growth factor gene expression in human fetal lung in vivo. Am J Resp Cell Mol Biol. 1999;20:14–23. doi: 10.1165/ajrcmb.20.1.3251. [DOI] [PubMed] [Google Scholar]
- 22.Mura M, dos Santos CC, Stewart D, Liu M. Vascular endothelial growth factor and related molecules in acute lung injury. J.Appl.Physiol. 2004;97:1605–1617. doi: 10.1152/japplphysiol.00202.2004. [DOI] [PubMed] [Google Scholar]
- 23.Tuder RM, Flook BE, Voelkel NF. Increased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxide. J Clin Invest. 1995;95:1798–1807. doi: 10.1172/JCI117858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keyt BA, Berleau LT, Nguyen HV, Chen H, Heinsohn H, Vandlen R, Ferrara N. The carboxyl-terminal domain (111–165) of vascular endothelial growth factor is critical for its mitogenic potency. J Biol Chem. 1996;271:7788–7795. doi: 10.1074/jbc.271.13.7788. [DOI] [PubMed] [Google Scholar]
- 25.Zeng X, Wert SE, Federici R, Peters KG, Whitsett JA. VEGF enhances pulmonary vasculogenesis and disrupts lung morphogenesis. Dev Dyn. 1998;211:215–227. doi: 10.1002/(SICI)1097-0177(199803)211:3<215::AID-AJA3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 26.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 27.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. American Journal of Physiology - Lung Cellular & Molecular Physiology. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 28.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am.J.Respir.Crit.Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 29.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat.Med. 2002;8:702–710. doi: 10.1038/nm721.This paper delineated the critical role of VEGF in lung maturation and that lack of VEGF would cause a syndrome of lung prematurity.
- 30.McGrath-Morrow SA, Cho C, Cho C, Zhen L, Hicklin DJ, Tuder RM. Vascular endothelial growth factor receptor 2 blockade disrupts postnatal lung development. Am J Respir.Cell Mol.Biol. 2005;32:420–427. doi: 10.1165/rcmb.2004-0287OC. [DOI] [PubMed] [Google Scholar]
- 31.Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, McDonald DM. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ.Physiol. 2006;290:H547–H559. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 32.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman SH, Hirth P, Waltenberger J, Voelkel NF. Inhibition of vascular endothelial growth factor receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259.This work showed for the first time that blockade of VEGF signaling caused an emphysema-like process in rats, dependent of alveolar cell apoptosis. It led to a new paradigm in the study of this highly relevant disease.
- 33.Tang K, Rossiter HB, Wagner PD, Breen EC. Lung-targeted VEGF inactivation leads to an emphysema phenotype in mice. J Appl.Physiol. 2004;97:1559–1566. doi: 10.1152/japplphysiol.00221.2004. [DOI] [PubMed] [Google Scholar]
- 34.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am.J.Physiol Lung Cell Mol Physiol. 2006;290:L209–L221. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- 35.Tsao PN, Su YN, Li H, Huang PH, Chien CT, Lai YL, Lee CN, Chen CA, Cheng WF, Wei SC, Yu CJ, Hsieh FJ, Hsu SM. Overexpression of placenta growth factor contributes to the pathogenesis of pulmonary emphysema. Am J Respir Crit Care Med. 2004;169:505–511. doi: 10.1164/rccm.200306-774OC. [DOI] [PubMed] [Google Scholar]
- 36.Henson PM, Bratton DL, Fadok VA. The phosphatidylserine receptor: a crucial molecular switch? Nat.Rev Mol Cell Biol. 2001;2:627–633. doi: 10.1038/35085094. [DOI] [PubMed] [Google Scholar]
- 37.Vandivier RW, Tuder RM, Morimoto K, Voelkel NF, Henson PM. Vascular endothelial growth factor regulates clearance of apoptotic cells: implications for emphysema. Proc Am Thorac Soc. 2006;3:552. [Google Scholar]
- 38.Golpon HA, Fadok VA, Taraseviciene-Stewart L, Scerbavicius R, Sauer C, Welte T, Henson PM, Voelkel NF. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. Faseb J. 2004;18:1716–1718. doi: 10.1096/fj.04-1853fje. [DOI] [PubMed] [Google Scholar]
- 39.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin.Chest Med. 2007;28:23–42. doi: 10.1016/j.ccm.2006.11.010. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Partovian C, Adnot S, Eddahibi S, Teiger E, Levame M, Dreyfus P, Raffestin B, Frelin C. Heart and lung VEGF mRNA expression in rats with monocrotaline- or hypoxia-induced pulmonary hypertension. Amer J Physiol -Heart Circ Phy. 1998;44:H1948–H1956. doi: 10.1152/ajpheart.1998.275.6.H1948. [DOI] [PubMed] [Google Scholar]
- 41.Campbell AIM, Zhao YD, Sandhu R, Stewart DJ. Cell-based gene transfer of vascular endothelial growth factor attenuates monocrotaline-induced pulmonary hypertension. Circulation. 2001;104:2242–2248. doi: 10.1161/hc4201.097838. [DOI] [PubMed] [Google Scholar]
- 42.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon GG, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. Faseb J. 2001;15:427–438. doi: 10.1096/fj.00-0343com.This paper delineated that proliferation of endothelial cells in pulmonary arteries caused severe pulmonary hypertension and required early death of endothelial cells due to VEGF receptor blockade.
- 43.Grover TR, Parker TA, Zenge JP, Markham NE, Kinsella JP, Abman SH. Intrauterine hypertension decreases lung VEGF expression and VEGF inhibition causes pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol. 2003;284:L508–L517. doi: 10.1152/ajplung.00135.2002. [DOI] [PubMed] [Google Scholar]
- 44.Partovian C, Adnot S, Raffestin B, Louzier V, Levame M, Mavier IM, Lemarchand P, Eddahibi S. Adenovirus-mediated lung vascular endothelial growth factor overexpression protects against hypoxic pulmonary hypertension in rats. Am J Respir Cell Mol Biol. 2000;23:762–771. doi: 10.1165/ajrcmb.23.6.4106. [DOI] [PubMed] [Google Scholar]
- 45.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Cool C, Wood K, Tuder RM, Burns N, Kasper M, Voelkel NF. Simvastatin Causes Endothelial Cell Apoptosis and Attenuates Severe Pulmonary Hypertension. Am.J Physiol Lung Cell Mol Physiol. 2006 doi: 10.1152/ajplung.00491.2005. In Press. [DOI] [PubMed] [Google Scholar]
- 46.Sundberg C, Nagy JA, Brown LA, Feng D, Eckelhoefer IA, Manseau EJ, Dvorak AM, Dvorak HF. Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery. Am J Pathol. 2001;168:1145–1160. doi: 10.1016/S0002-9440(10)64062-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS.Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thickett DR, Armstrong L, Christie SJ, Millar AB. Vascular endothelial growth factor may contribute to increased vascular permeability in acute respiratory distress syndrome. Am.J.Respir.Crit Care Med. 2001;164:1601–1605. doi: 10.1164/ajrccm.164.9.2011071. [DOI] [PubMed] [Google Scholar]
- 49.Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. Journal of Allergy and Clinical Immunology. 2001;107:1034–1038. doi: 10.1067/mai.2001.115626. [DOI] [PubMed] [Google Scholar]
- 50.Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. Journal of Allergy and Clinical Immunology. 2001;107:295–301. doi: 10.1067/mai.2001.111928. [DOI] [PubMed] [Google Scholar]
- 51.Kanazawa H, Nomura S, Asai K. Roles of Angiopoietin-1 and Angiopoietin-2 on Airway Microvascular Permeability in Asthmatic Patients. Chest. 2007;131:1035–1041. doi: 10.1378/chest.06-2758. [DOI] [PubMed] [Google Scholar]
- 52.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, Elias JA. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–1103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhandari V, Choo-Wing R, Chapoval SP, Lee CG, Tang C, Kim YK, Ma B, Baluk P, Lin MI, McDonald DM, Homer RJ, Sessa WC, Elias JA. Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung. Proc.Natl.Acad.Sci.U.S.A. 2006;103:11021–11026. doi: 10.1073/pnas.0601057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen FQ, Liu X, Manda W, Terasaki Y, Kobayashi T, Abe S, Fang Q, Ertl R, Manouilova L, Rennard SI. TH2 Cytokine-enhanced and TGF-beta-enhanced vascular endothelial growth factor production by cultured human airway smooth muscle cells is attenuated by IFN-gamma and corticosteroids. J Allergy Clin.Immunol. 2003;111:1307–1318. doi: 10.1067/mai.2003.1455. [DOI] [PubMed] [Google Scholar]
- 55.Feltis BN, Wignarajah D, Reid DW, Ward C, Harding R, Walters EH. Effects of inhaled fluticasone on angiogenesis and vascular endothelial growth factor in asthma. Thorax. 2007;62:314–319. doi: 10.1136/thx.2006.069229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tuder RM, Chacon M, Alger LA, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci MW, Semenza GL, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol. 2001;195:367–374. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 57.Voelkel NF, Cool CD, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest. 1999;114:225S–230S. doi: 10.1378/chest.114.3_supplement.225s. [DOI] [PubMed] [Google Scholar]
- 58.Cool CD, Rai MD, Yeager ME, Hernandez-Saavedra D, Serls AE, Bull TM, Geraci MW, Brown KK, Routes JM, Tuder RM, Voelkel NF. Expression of human herpesvirus 8 in primary pulmonary hypertension. N Engl J Med. 2003;349:1113–1122. doi: 10.1056/NEJMoa035115. [DOI] [PubMed] [Google Scholar]
- 59.Wright JL, Levy RD, Churg A. Pulmonary hypertension in chronic obstructive pulmonary disease: current theories of pathogenesis and their implications for treatment. Thorax. 2005;60:605–609. doi: 10.1136/thx.2005.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]


