Abstract
The ability of Mycobacterium xenopi to colonize an experimental drinking water distribution system (a Propella reactor) was investigated. M. xenopi was present in the biofilm within an hour following its introduction. After 9 weeks, it was always present in the outlet water (1 to 10 CFU 100 ml−1) and inside the biofilm (102 to 103 CFU cm−2). Biofilms may be considered reservoirs for the survival of M. xenopi.
Mycobacteria have no usual adherence factors such as pili, fimbriae, and slime, but the hydrophobic properties of the cell wall appear to contribute to the adherence process. The glycopeptidolipids present in the outermost layer of the cell walls of mycobacteria play an important role in surface colonization and biofilm formation (3, 7, 10). The biofilms represent an important risk of contamination for water distribution systems (8, 9, 12, 13). Lately, a nosocomial outbreak of vertebral osteomyelitis due to Mycobacterium xenopi (5) has been reported by a French surgical center (2). These infections were associated with invasive medical procedures and related to the presence of M. xenopi in the tap water distribution network. However, the role of M. xenopi in the biofilm formation process has not yet been investigated. The aim of this study was to evaluate the ability of M. xenopi to colonize an experimental water supply system.
A model biofilm reactor, the Propella reactor (with a distribution pipe 90 mm in diameter and 500 mm long), was used to simulate a drinking water distribution system (1). The inner surface of the pipe was covered with high-density polyethylene (HDPE), which is commonly used in drinking water pipes. The reactor had a volume of 2,080 ml and an HDPE pipe surface of 1,413 cm2. It was continuously supplied with tap water (from a local drinking water network) at a flow rate of 83.5 ml h−1. The water velocity (0.2 m s−1) was controlled with a marine propeller, which pulsed the water through an inner pipe, giving a flux parallel to the pipe wall. The formation of a biofilm on the pipe wall and the concentration of embedded bacterial cells could be studied by using 20 coupons (1.7 cm2) inserted into the inner surface of the pipe. A special device allowed the removal of the coupons without any flow interruption.
M. xenopi (CIP 104035) was subcultured on Lowenstein-Jensen medium at 40°C (optimal growth temperature). A suspension was prepared in sterile distilled water and centrifuged (4,300 × g for 30 min at 20°C). The pellet was washed twice in sterile distilled water to limit carbon contamination from culture media and suspended in sterile distilled water. The suspension was calibrated to a density equivalent to a McFarland standard of 1 and serially diluted (10−1 to 10−9) in sterile distilled water; for the enumeration of mycobacteria, 0.2 ml of each dilution was plated on Lowenstein-Jensen medium (catalog no. 55246; Bio-Rad). Media were incubated at 40°C. Five weeks later, the colonies were counted.
The investigation was performed in duplicate with two similar Propella reactors (A and B) at room temperature (20 ± 2°C). Systems were not modified during the experiments. The natural microbial flora that was present in the drinking water colonized the surface and contributed to the formation of a biofilm. Weekly, water was obtained from the inlets and outlets of the reactors and biofilm coupons were sampled to determine the biofilm formation in the reactors and to control the biofilm development. After 5 weeks, the two experimental systems were colonized by bacteria at concentrations of 3.2 × 105 bacterial cells ml−1 and 62 × 105 bacterial cells cm−2 in the outlet water and on the biofilm coupon, respectively. Then, 100 ml of the 10−2 dilution of the M. xenopi suspension of McFarland standard 1 was rapidly added to each reactor. The two systems were analyzed after 1, 2, 24, and 48 h and again once a week over a period of 9 weeks. At each time point, inlet and outlet water samples (100 ml each) and two coupons were collected.
For the biofilm analysis, HDPE coupons were aseptically removed from the sampling devices and put in 25 ml of sterile distilled water. Adherent cells were removed by sonication for 2 min (Vibra Cell 72401 cone probe; power, 2 W). For the enumeration of culturable bacteria, 1 ml of undiluted and diluted (in 10-fold steps) suspensions were incorporated into plate count agar (Merck catalog no. 1.05463). Colony counts were recorded after an incubation period of 3 days at 20 ± 2°C. Plates with a CFU count of ≤300 were selected for enumeration. The limit of detection was 1 CFU ml−1. Total cells (culturable and nonculturable bacteria) were determined by using epifluorescence microscopy and 4′,6-diamidino-2-phenylindole staining (11).
From each water sample, total organic carbon (TOC) concentrations (Bioritech model 700 TOC analyzer), pH, and free and total chlorine levels (diethyl-para-phenylenediamine colorimetric method; IFEC-92200, Neuilly, France) were determined. Total cells and concentrations of culturable bacteria were determined as described above.
For both Propella reactors, the results showed that the chemical and bacteriological characteristics of water (carbon concentrations, pH, free and total chlorine levels, and bacterial counts) were relatively stable during the entire investigation. Thus, for each parameter, the average and the standard deviation were calculated (Table 1). For the inlet water, the averages were calculated by using results obtained from weeks 1 to 14. For the outlet water, the averages were determined by using results obtained from weeks 1 to 5 (before the introduction of M. xenopi into the reactors) and from weeks 6 to 14 (after the introduction of M. xenopi into the reactors). After 5 weeks, as all the parameters in the experiment were kept constant, the biofilm was considered to be in a pseudoequilibrium state. Enumerations of bacteria in the biofilms were performed just before the injection of the M. xenopi suspension into the reactors, 1 and 2 h later, and then weekly. Counts of culturable and unculturable bacteria were stable during the whole experiment. The averages of results and the standard deviations were calculated (Table 1).
TABLE 1.
Chemical and biological characteristics of inlet water and Propella reactors A and B before and after introduction of M. xenopia
| Water or biofilmb | pH | TOC (mg/liter) | Free chlorine (mg/liter) | Total chlorine (mg/liter) | Culturable bacteria after 72 h (CFU)c | Total cell countc |
|---|---|---|---|---|---|---|
| Inlet water | 7.5 ± 0.19 | 1.2 ± 0.2 | 0.03 ± 0.01 | 0.04 ± 0.01 | 56 ± 27 | (1.76 ± 0.16) × 105 |
| Outlet water (Propella reactors A and B) | ||||||
| Before M. xenopi (n = 5) | 7.6 ± 0.1 | 1.2 ± 0.2 | 0d | 0 | (4.5 ± 4.7) × 103 | (3.2 ± 1.4) × 105 |
| After M. xenopi (n = 9) | 7.6 ± 0.2 | 1.3 ± 0.2 | 0 | 0 | (4.5 ± 8.5) × 103 | (3.6 ± 1.3) × 105 |
| Biofilm (Propella reactors A and B) | ||||||
| Before M. xenopi (n = 2) | (1.1 ± 0.5) × 105 | (62 ± 2.9) × 105 | ||||
| After M. xenopi (n = 9) | (1.3 ± 2) × 105 | (84 ± 31) × 105 |
All values are averages ± standard deviations.
n, number of analyses used for the calculation of averages.
Values are per milliliter for water and per square centimeter for biofilm.
A value of zero indicates a measurement below the detection limit of 0.02 mg/liter.
The ability of M. xenopi to remain in this system and the distribution of the organism between the water phase and the biofilm phase were determined over a period of 9 weeks. Mycobacteria were isolated from water samples (100 ml) and biofilm suspensions (16 ml) after decontamination by a method using 3% sodium lauryl sulfate in combination with 1% NaOH (6). After neutralization and centrifugation, pellets were resuspended in 0.9 ml of sterile distilled water, 10-fold serial dilutions were carried out, and these dilutions were cultured on Lowenstein-Jensen medium. Media were incubated at 40 and 35°C. The numbers of small scotochromogenic colonies compatible with the M. xenopi type strain that were observed at 40°C were used to calculate the M. xenopi concentrations. Slopes showing more than 50 colonies were disregarded. Media incubated at 35°C were used for the detection of mycobacteria other than M. xenopi; such mycobacteria were rarely isolated (<4 CFU 100 ml−1 in outlet water and <2 CFU cm−2 on biofilm coupons).
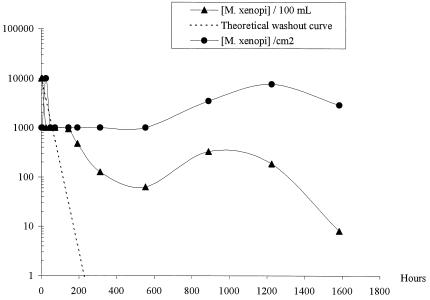
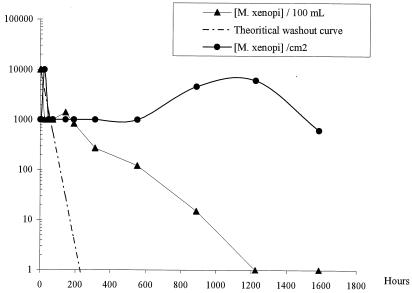
Before its introduction, M. xenopi was not detected in the local drinking water or in the biofilms in the Propella reactors. Biofilms in the local drinking water system were not analyzed. The concentration of the initial M. xenopi suspension (McFarland standard 1) was determined to be 108 CFU ml−1, so the theoretical concentration of M. xenopi in each Propella reactor was 4.8 × 106 CFU 100 ml−1. As shown in Fig. 1 and 2, for each Propella reactor, M. xenopi was always isolated from the outlet water but its concentration regularly decreased. In the last samples analyzed, the concentrations of M. xenopi were 8 CFU 100 ml−1 for Propella reactor A and 1 CFU 100 ml−1 for Propella reactor B. Curves corresponding to the theoretical washout relationship of M. xenopi concentration (log) over time are presented in Fig. 1 and 2. These curves were established assuming that no deposition of cells occurred on the pipe wall of the Propella reactor that was continously fed with drinking water (dilution rate, 0.04 h−1). In biofilms 1 h after the introduction of M. xenopi into the systems, M. xenopi concentrations of 103 CFU cm−2 were found. After this initial phase, the concentrations remained stable (102 to 103 CFU cm−2) until the end of the experiments.
FIG. 1.
M. xenopi organisms in the outlet water (in CFU per 100 ml) or the biofilm (in CFU per square centimeter) from Propella reactor A.
FIG. 2.
M. xenopi organisms in the outlet water (in CFU per 100 ml) or the biofilm (in CFU per square centimeter) from Propella reactor B.
The evolution of M. xenopi in the Propella reactors was compared with that suggested by the washout curves. Within 2 to 3 days following the injection of M. xenopi into the reactors, the mycobacterial concentration in outlet water was lower than the theoretical concentration. This suggests that a phenomenon other than washout, such as loss of culturability, bacterial death, or attachment, contributed to the absence of mycobacteria. After this period, the slopes of the experimental curves changed and clearly crossed the washout curves. Actually, 144 h (Propella reactor A) and 192 h (Propella reactor B) after the M. xenopi injection, the concentrations of M. xenopi were higher than the expected theoretical values. These observations suggest that most of the injected M. xenopi bacteria were trapped in the reactors, from which they were progressively eliminated. M. xenopi concentrations were maintained in the biofilm, whereas they decreased and tended to disappear in water. Twenty days after the injection, the total quantity of M. xenopi bacteria was more than 10 times higher in the biofilm than in the bulk water. This indicates an important contribution of the biofilm to the accumulation of M. xenopi. The first evidence of the attachment of M. xenopi on the biofilm was obtained just after the introduction of the organism into the Propella reactor. This finding suggests fast transfer of planktonic mycobacterial cells from liquid to solid surfaces and strong adherence of these cells to the existing biofilm. These results confirm those reported by Hall-Stoodley and Lappin-Scott (4), who showed that Mycobacterium fortuitum may form a dense biofilm within 48 h.
In our study, the positive growth of M. xenopi in the biofilm was not measured, so it is difficult to ascertain whether M. xenopi survives and/or multiplies in the biofilm, but bacterial multiplication may be suspected. Indeed, 9 weeks after a single massive injection of M. xenopi, the bacteria were always present in the outlet water. The concentration was low but was higher than the theoretical values corresponding to a simple elution by the water flow. Considering that the multiplication of slowly growing mycobacteria is limited in water and that a pseudoequilibrium of the mycobacterial concentration in the biofilm was observed over a 9-week period, it is conceivable that M. xenopi injected into the drinking water system adapted, persisted, and grew preferentially in the biofilm. Future studies should be carried out to investigate the growth rates of mycobacteria in biofilms. Regardless of these rates, biofilms may be considered reservoirs for the proliferation of mycobacteria in water distribution systems and contributors to the continuous bacteriological contamination of the water via an erosion process.
REFERENCES
- 1.Appenzeller, B. M., M. Batté, L. Mathieu, J. C. Block, V. Lahoussine, J. Cavard, and D. Gatel. 2001. Effect of adding phosphate to drinking water on bacterial growth in slightly and highly corroded pipes. Water Res. 35:1100-1105. [DOI] [PubMed] [Google Scholar]
- 2.Astagneau, P., N. Desplaces, V. Vincent, V. Chicheportiche, H. Bortherel, S. Maugat, K. Lebascle, P. Leonard, J. C. Desenclos, J. Grosset, J. M. Ziza, and G. Brücker. 2001. Mycobacterium xenopi spinal infections after discovertebral surgery: investigation and screening of a large outbreak. Lancet 358:747-751. [DOI] [PubMed] [Google Scholar]
- 3.Bardouniotis, E., H. Ceri, and M. E. Olson. 2003. Biofilm formation and biocide susceptibility testing of Mycobacterium fortuitum and Mycobacterium marinum. Curr. Microbiol. 46:28-32. [DOI] [PubMed] [Google Scholar]
- 4.Hall-Stoodley, L., and H. M. Lappin-Scott. 1998. Biofilm formation by the rapidly growing mycobacterial species Mycobacterium fortuitum. FEMS Microbiol. Lett. 168:77-84. [DOI] [PubMed] [Google Scholar]
- 5.Jiva, T. M., H. M. Jacoby, L. A. Weymouth, D. A. Kaminski, and A. C. Portmore. 1997. Mycobacterium xenopi: innocent bystander or emerging pathogen? Clin. Infect. Dis. 24:226-232. [PubMed] [Google Scholar]
- 6.Kamala, T., C. N. Paramasivan, D. Herbert, P. Venkatesan, and R. Prabhakar. 1994. Evaluation of procedures for isolation of nontuberculous mycobacteria from soil and water. Appl. Environ. Microbiol. 60:1021-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez, A., S. Torello, and R. Kolter. 1999. Sliding motility in mycobacteria. J. Bacteriol. 181:7331-7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyers, H., B. A. Brown-Elliott, D. Moore, J. Curry, C. Truong, Y. Zhang, and R. J. Wallace, Jr. 2002. An outbreak of Mycobacterium chelonae infection following liposuction. Clin. Infect. Dis. 34:1500-1507. [DOI] [PubMed] [Google Scholar]
- 9.Plankhurst, C. L. 2003. Risk assessment of dental unit waterline contamination. Prim. Dent. Care 10:5-10. [DOI] [PubMed] [Google Scholar]
- 10.Recht, J., and R. Kolter. 2001. Glycopeptidolipid acetylation affects sliding motility and biofilm formation in Mycobacterium smegmatis. J. Bacteriol. 183:5718-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saby, S., I. Sibille, L. Mathieu, J. L. Paquin, and J. C. Block. 1997. Influence of water chlorination on the counting of bacteria with DAPI (4′,6-diamidino-2-phenylindole). Appl. Environ. Microbiol. 63:1564-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulze-Röbbecke, R., C. Feldman, R. Fischeder, B. Janning, M. Exner, and G. M. Wahl. 1989. Dental units: an environmental study of sources of potentially pathogenic mycobacteria. Tuber. Lung Dis. 76:318-323. [DOI] [PubMed] [Google Scholar]
- 13.Schulze-Röbbecke, R., B. Janning, and R. Fischeder. 1992. Occurrence of mycobacteria in biofilm samples. Tuber. Lung Dis. 73:141-144. [DOI] [PubMed] [Google Scholar]