Abstract
An apparent consensus governs the management of carrier status information generated incidentally through newborn screening: results cannot be withheld from parents. This normative stance encodes the focus on autonomy and distaste for paternalism that characterize the principles of clinical bioethics.
However, newborn screening is a classic public health intervention in which paternalism may trump autonomy and through which parents are—in effect—required to receive carrier information. In truth, the disposition of carrier results generates competing moral infringements: to withhold information or require its possession.
Resolving this dilemma demands consideration of a distinctive body of public health ethics to highlight the moral imperatives associated with the exercise of collective authority in the pursuit of public health benefits.
NEWBORN SCREENING programs identify serious conditions for which early detection reduces mortality or morbidity.1 Yet, in the pursuit of information about targeted disorders, screening may incidentally generate information about carrier status that is irrelevant to the infant's health. The consensus to date is that this information should be disclosed to parents—indeed, that to withhold such information would be unethical.
In our view, the current practice of automatic disclosure of incidental carrier results reflects a bad marriage between clinical bioethics and public health praxis. It combines clinical bioethics' emphasis on autonomy and distaste for paternalism with the determination of public health to exercise collective authority and entertain justifiable compulsion. We seek to shed new light on this enduring dilemma. We considered the moral significance of reproductive risk information as well as the interests of the child to explore why automatic disclosure to parents might be morally troubling. We then considered how the application of distinctive ethical principles of public health could guide the development of sound policy on this issue.
THE STATE OF THE DEBATE
In the extensive literature on newborn screening, remarkably little attention has focused on the ethics of carrier status identification. Interest in the issue emerged with the introduction of newborn screening for sickle cell disorders in the United States in the 1970s and 1980s, because all screening protocols reliably detect most carriers.2 The issue has resurfaced with renewed urgency as other countries have moved to include sickle cell disorders on the newborn screening panel and as newborn screening by DNA analysis has emerged for cystic fibrosis and a range of other conditions.3,4 These developments have prompted research addressing both the immediate needs of families and the longer-term implications of disclosure practices.4,5
Empirical evidence about the benefits and harms of disclosing carrier status information is both limited in quantity and equivocal in the policy guidance it provides.4,5 The primary benefit is that parents and future adults may be made aware of the reproductive risks that they face. Some also suggest that individuals may benefit from “ownership” of genetic information.6(p407),7 The primary harms include (1) misunderstanding the meaning of carrier status, leading to overmedicalization, vulnerable child syndrome, stigmatization, and discrimination,8–10 and (2) the detection of misattributed paternity.4,6,7
Ethical discussion is also limited. In 1994, the US Institute of Medicine Committee on Assessing Genetic Risks concluded that information about an infant's carrier status generated through newborn screening should be disclosed only if informed consent is obtained.6 This recommendation proved controversial, given the general preference for mandatory screening in the United States.11 Indeed, the chair of the Institute of Medicine committee argued against the majority position, and the Committee on Bioethics of the American Academy of Pediatrics sided with it.12 The British Medical Association argued that “unavoidable incidental information” about carrier status generated through newborn screening should be disclosed, although it called for prior notification to parents of this possibility.13(p116) With few exceptions (e.g., Horn et al.14), commentators have presumed the necessity of disclosing carrier information to parents.4,5,15,16 Some argue that ethics, law, and custom require that anyone testing positive, including carriers, be notified and counseled.16 Others bluntly aver that “withholding information from parents is not justified.”7(p410)
The argument in favor of disclosure is that withholding data constitutes paternalism: judging individuals incapable of managing important health information and violating their autonomous right to knowledge about their own person. This normative stance encodes the principles of traditional, clinically oriented bioethics. Newborn screening, however, is a classic public health intervention that is pursued largely in the absence of informed consent.11,17 Thus, another euphemism is also relevant to the disclosure of carrier results: requiring—that is, requiring parents to possess information about their infant that is primarily relevant to the identification and management of reproductive risks. Although we may be ethically concerned about withholding such information from parents, we argue that requiring parents to receive it is also ethically worrisome.
REQUIRING PARENTS TO KNOW
Although individuals' right to access information is widely accepted, there is growing discussion of a corollary right: a right to ignorance based on an interest in not knowing medical information about oneself—particularly medical genetic information.18–22 Although limited by the need to avoid harm to others, and disputed in its entirety by some scholars,23,24 the right not to know is increasingly recognized in ethical and legal instruments.25,26 Such a right has greatest support where the state of not knowing is chosen by the individual in question,27 but it may extend to situations where the individual's preferences are unknown,21 relying on the “individual's privacy interests in not being subjected to unwarranted information about themselves.”19(p129)
Where the preferences of the screened individual are unknown, this right requires those with authority to identify “compelling reasons” to disclose.28(p39) Avoidance of harm or service of a legitimate public interest provides such a warrant19 and justifies the disclosure of screening results that identify remediable health risks in infants to unknowing and unconsenting parents. However, a similar warrant may not hold in the case of carrier screening results.
The primary benefit to be gained by disclosure of carrier results is guidance for reproductive decisionmaking. Individuals have a clear right to elect to receive such information, but a preference to possess such information cannot be presumed. Nor can a failure to possess such information be presumed to generate harm, as the desire to know or manage reproductive risks is not universal.19,22,29,30 Indeed, as Takala has argued,
“Since it is by no means clear what the prospective parents should, morally speaking, do with genetic information it is unclear why the reasonable choice would be to acquire the information.”31(p488)
This line of reasoning has fostered nondirectiveness in genetic counseling: clinicians help clients make decisions for themselves, without directing them toward an outcome.32 Indeed, state screening programs that require parents to receive carrier status information might be accused of a subtle form of eugenics.19
CHILDREN'S INDEPENDENT INTERESTS
Even if it can be presumed that parents want carrier status information about their infant, the infant's interests may differ. Indeed, an extensive policy and scholarly literature suggests that generating genetic information in childhood is inappropriate unless it is required to address health needs that emerge in childhood.12,33 The provision of carrier or predictive genetic testing is seen to infringe on the child's autonomy and right to confidentiality because it forecloses on the child's right to decide whether to seek this information and to whom it should be disclosed.34 In a recent systematic review, Borry et al. identified 2 guidance statements that addressed the incidental generation of carrier screening results in minors outside the context of newborn screening34; these statements suggest that such information should be stored and withheld until the child reaches a state of maturity.35
Few discussions about the disclosure of carrier results in the newborn screening context acknowledge the infringement on the infant's autonomy that may result from disclosure (see, for example, Wheeler et al.36) or the extent to which the practice of disclosure conflicts with accepted policy on genetic testing in childhood (see Wilfond and Rothenberg37 and Campbell and Ross38). Yet this is becoming increasingly salient. Indeed, Parsons et al. highlighted the double-message problem, whereby parents provided with carrier information about their infants through newborn screening programs are refused similar genetic testing for their other children.3 As Borry et al. argue, these contradictory policies are unsustainable.39
It might be suggested that nondisclosure of carrier status information about the infant poses harm to the parents and, further, that the interests of parents in learning their own reproductive risks trumps any potential interest that the infant may have in nondisclosure. But if a harm cannot be presumed to arise when information to guide reproductive decisionmaking in the screened individual is not disclosed to that individual, the argument that harm might arise to third parties (in this case parents) from a failure to disclose genetic information that might guide reproductive decisionmaking is very weak indeed.19 This is even more so in the case of identifying infant carrier status, because such information is not required for the parents to learn their own status (as is the case, for example, with tests to identify hereditary cancer risk). If parents wish to know their reproductive risks, they may seek such information independently, motivated by their own values and priorities.
In recent years, several authors have suggested that the blanket prohibition on childhood genetic testing that does not confer clinical benefit is too strict because it ignores the child's emerging autonomy and the right of parents to make important decisions for their children.40–43 These authors suggest that, at least under some circumstances, the informed choice of parents or mature minors in selecting such testing should be respected. Yet even if accepted, permitting parents to acquire this information when they request it clinically does not mean that parents and infants must receive this unsolicited information in the context of newborn screening.
PUBLIC HEALTH ETHICS
The disposition of infant carrier screening results generates competing moral infringements: to withhold information or to require its possession. Failure to recognize this fact reflects the incompatibility between clinical bioethics and public health praxis. Resolving this dilemma demands consideration of a distinctive body of public health ethics.
From the perspective of public health ethics, the pursuit of public health benefits may itself be a moral imperative, one that can—under specified circumstances—permit the infringement of individual rights. Indeed, as Kass notes, “Much of public health is inherently and unabashedly paternalistic.”44(p1778) Frameworks for public health ethics outline principles to justify the pursuit of public health benefits and to identify and mitigate potential moral infringements.44–46
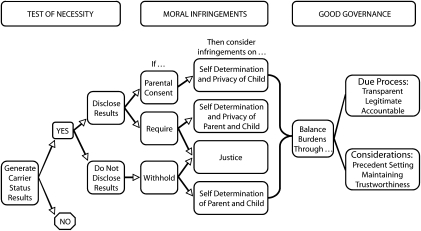
Figure 1 illustrates the example of incidental carrier results in light of 3 overarching principles of public health ethics (shown in Table 1). The test of necessity asks whether the goals of a public health initiative are sufficiently laudable and achievable to justify the exercise of collective authority. The generation of carrier results is incidental to the primary goal of newborn screening: to improve the health of infants. The application of the test of necessity to this incidental outcome depends on how carrier information is produced. Carrier results generated through confirmatory testing (e.g., for cystic fibrosis) are provided to parents alongside other positive results from the screening and distinguished from true positives in a clinical encounter. In this context, the test of necessity considers whether these results must be generated at all and asks that the infringements of carrier result disclosure be weighed and balanced with the effects (moral and clinical) of not generating such information (e.g., non-DNA screening protocols, no screening). The test of necessity for carrier results generated through the initial blood spot screen (e.g., for sickle cell disorders) asks whether these results must be generated and also considers the benefits and burdens of disclosure compared with nondisclosure (i.e., results masked or destroyed).
FIGURE 1.
Decision tree for considering the application of public health ethics principles to incidental carrier results generated through newborn screening.
TABLE 1.
Frameworks for Justifying Public Health Initiatives
| Ethical Principle | Justification | Requirements |
| Necessity | The public health intervention is demonstrably necessary. | Public health goals of the proposed program must be specified. |
| The policy must be necessary and effective in achieving its stated goals. | ||
| Minimal infringement | The intervention minimizes moral harms. | Known or potential burdens of the program should be specified, including risks to privacy and confidentiality, liberty and self-determination, and justice. |
| Action must be exercised in the least restrictive manner possible. | ||
| Burdens should be minimized and should be proportional to the health benefits to be achieved. | ||
| Good governance | The exercise of collective authority is worthy of the public's trust. | The benefits and burdens of a program must be fairly balanced. |
| The program must be implemented fairly. | ||
| Officials must explain and justify moral infringements to the public. | ||
| Decisions must be legitimated by comprehensive participation of relevant parties and transparent processes. | ||
| Consideration must be given to existing precedents and the precedents to be established. |
The second principle asks that the full range of moral infringements be identified and mitigated. We contend that infringements on individuals arise whether results are withheld or disclosed. Informed consent, which enables parents to decide whether to learn their infant's carrier status, may prove a partial remedy. Yet consent processes that maintain a high uptake of newborn screening (e.g., as in the programs examined by Faden et al.47 and Dhondt48) are unlikely to foster authentic choice about the receipt of carrier results. Conversely, consent processes that foster authentic choice may engender reduced uptake of newborn screening as a whole49 (although mixed mandatory and consent models might remedy this). Still, the child's autonomous rights regarding this information remain unresolved.
Threats to justice arise in the case of newborn screening because genetic risks are often unevenly distributed across ethnic communities. Thus, even when newborn screening is not targeted at specific ethnic groups, it may differentially affect such groups. Much of the negative response to the sickle cell disorders screening programs of the 1970s in the United States arose from a fear of racial genocide: a perceived attempt to reduce reproduction among African Americans.50 Programs that require or encourage the receipt of sickle cell carrier results might create particular moral burdens given the frequency of these alleles in specific ethnic populations, the relevance of carrier information almost exclusively for reproductive decisionmaking, and the legacy of mistrust created by earlier sickle cell–screening efforts. Conversely, to withhold carrier status results may be experienced as an ethnic-specific denial of care.
The weighing and balancing of moral infringements against one another, and against clinical and social benefits and harms, adheres to no specific calculus. Thus the third overarching principle of public health ethics concerns good governance. Benefits and burdens must be fairly balanced through due process in the interests of sound decisions about individual initiatives and sustainable programs of public health. Deliberations might consider the resource implications of carrier status disclosure, the precedent-setting nature of carrier status information policy for the management of future newborn screening technologies (e.g., see Green and Pass51), and whether the public health mandate is diminished or enhanced by policies governing carrier status results. In our view, the mechanisms employed to date to engage debate on the issue (e.g., see Consensus Development Panel2 and Andrews at al.6) have been insufficient to support current policy and practice or to sustain the authority and trustworthiness of future newborn screening initiatives.
CONCLUSIONS
The challenge of how to manage carrier status results generated incidentally through newborn screening has received insufficient attention from public health and ethics scholars. There has been a near-automatic reliance on the euphemism of withholding in guiding policy and practice. But it makes little sense to insist on routine disclosure of carrier status to avoid the taint of paternalism. Justified paternalism is central to the ethics of public health. Moreover, the euphemism of withholding operates without consideration of the legal and practical realities of newborn screening as a public health intervention—realities that suggest the relevance of a corollary euphemism of requiring. Viewed from this perspective, other infringements on individual rights are apparent, arising from the potential right not to know information whose primary function is to identify reproductive risks and from the potential discord between the rights of infants and of their parents regarding this knowledge.
Others may disagree with our interpretation of the nature and significance of the infringements arising from requiring knowledge of carrier status. We also expect debate over how to weigh and balance these burdens against others in determining how to manage carrier results. But traditional clinically oriented bioethics cannot provide adequate guidance. By contrast, distinctive ethics frameworks for public health highlight the moral imperatives associated with the exercise of collective authority in the pursuit of public health benefits and help to ensure that discussions are fulsome and rigorous. As Kass points out, different societies will reach different decisions even when applying the same ethical principles.44 Indeed, an end to the apparent consensus about the value of generating and the necessity of disclosing incidental carrier results will provide welcome evidence that the public health ethics of newborn screening is receiving full attention.
Acknowledgments
This project was supported by the Ontario Ministry of Health and Long-Term Care (grant 06358) and by the Institute of Health Services and Policy Research, Canadian Institutes of Health Research (New Investigator Award 80495 to F. A. Miller).
We thank members of the Ontario Advisory Committee on Newborn and Childhood Screening for entertaining these debates and Jessica Bytautas for her editorial and technical help.
Note. Sponsors' support of this work should not imply endorsement of the conclusions, for which the authors retain sole responsibility.
Human Participant Protection
No human participants were involved in this study.
References
- 1.Botkin JR. Research for newborn screening: developing a national framework. Pediatrics 2005;116:865–871 [DOI] [PubMed] [Google Scholar]
- 2.Consensus Development Panel Newborn screening for sickle cell disease and other hemoglobinopathies. JAMA 1987;258:1205–1209 [PubMed] [Google Scholar]
- 3.Parsons EP, Clarke AJ, Bradley DM. Implications of carrier identification in newborn screening for cystic fibrosis. Arch Dis Child Fetal Neonatal Ed 2003;88:F467–F471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliver S, Dezateux C, Kavanagh J, Lempert T, Stewart R. Disclosing to parents newborn carrier status identified by routine blood spot screening. Cochrane Database Syst Rev 2004Oct 18;(4):CD003859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver S, Lempert T, Stewart R, Kavanagh J, Dezateux C. Disclosing to parents newborn carrier status identified by routine blood spot screening. London: EPPI-Centre, University of London; 2004:1–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews LB, Fullarton JE, Holtzman NA, Motulsky AG. Assessing Genetic Risks: Implications for Health and Social Policy. Washington, DC: National Academy Press; 1994 [PubMed] [Google Scholar]
- 7.Laird L, Dezateux C, Anionwu EN. Neonatal screening for sickle cell disorders: what about the carrier infants? BMJ 1996;313:407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marteau TM, van Duijn M, Ellis I. Effects of genetic screening on perceptions of health: a pilot study. J Med Genet 1992;29:24–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mischler EH, Wilfond BS, Fost N, et al. Cystic fibrosis newborn screening: impact on reproductive behaviour and implications for genetic counseling. Pediatrics 1998;102:44–52 [DOI] [PubMed] [Google Scholar]
- 10.Ciske DJ, Haavisto A, Laxova A, Rock LZM, Farrell PM. Genetic counseling and neonatal screening for cystic fibrosis: an assessment of the communication process. Pediatrics 2001;107:669–705 [DOI] [PubMed] [Google Scholar]
- 11.Hiller EH, Landenburger G, Natowicz MR. Public participation in medical policy-making and the status of consumer autonomy: the example of newborn-screening programs in the United States. Am J Public Health 1997;87:1280–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson RM, Botkin JR, Kodish ED, et al. Ethical issues with genetic testing in pediatrics. Pediatrics 2001;107:1451–1455 [DOI] [PubMed] [Google Scholar]
- 13.British Medical Association Human Genetics: Choice and Responsibility. Oxford, England: Oxford University Press; 1998 [Google Scholar]
- 14.Horn MEC, Dick MC, Frost B, et al. Neonatal screening for sickle cell diseases in Camberwell: results and recommendations of a two year pilot study. BMJ 1986;292:737–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eboh W, Van den Akker O. Service provision for sickle cell disease. Br J Midwifery 1995;3:189–195 [Google Scholar]
- 16.Grossman L, K, Holtzman NA, Charney E, Schwartz AD. Neonatal screening and genetic counseling for sickle cell trait. Am J Dis Child 1985;139:241–244 [DOI] [PubMed] [Google Scholar]
- 17.Laberge C, Kharaboyan L, Avard D. Newborn screening, banking and consent. GenEdit 2004;2:1–15 [Google Scholar]
- 18.Chadwick R, Levitt M, Shickle D, eds. The Right to Know and the Right Not to Know. Aldershot, England: Ashgate; 1997. Avebury Series in Philosophy [Google Scholar]
- 19.Laurie G. In defense of ignorance: genetic information and the right not to know. Eur J Health Law 1999;6:119–132 [DOI] [PubMed] [Google Scholar]
- 20.Laurie G. Protecting and promoting privacy in an uncertain world: further defences of ignorance and the right not to know. Eur J Health Law 2000;7:185–191 [DOI] [PubMed] [Google Scholar]
- 21.Laurie G. Genetic Privacy: A Challenge to Medico-Legal Norms. Cambridge, England: Cambridge University Press; 2002 [Google Scholar]
- 22.Takala T, Hayry M. Genetic ignorance, moral obligations, and social duties. J Med Philos 2000;25:107–113 [DOI] [PubMed] [Google Scholar]
- 23.Rhodes R. Autonomy, respect, and genetic information policy: a reply to Tuija Takala and Matti Hayry. J Med Philos 2000;25:114–120 [DOI] [PubMed] [Google Scholar]
- 24.Rhodes R. Genetic links, family ties, and social bonds: rights and responsibilities in the face of genetic knowledge. J Med Philos 1998;23:10–30 [DOI] [PubMed] [Google Scholar]
- 25.International Bioethics Committee, UN Educational Scientific and Cultural Organization. Déclaration universelle sur le génome humain et les droits de l'homme. 1997. Available at: http://portal.unesco.org/en/ev.php-URL_ID=13177&URL_DO=DO_TOPIC&URL_SECTION=201.html. Accessed May 8, 2008
- 26.Council of Europe. Convention for the Protection of Human Rights and Dignity of the Human Being With Regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine. Oveido, 1997. Available at: http://conventions.coe.int/Treaty/Commun/QueVoulezVous.asp?NT=164&CL=ENG. Accessed May 8, 2008
- 27.Andorno R. The right not to know: an autonomy based approach. J Med Ethics 2005;30:435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurie G. Challenging medical-legal norms: the role of autonomy, confidentiality and privacy in protecting individual and familial group rights in genetic information. J Leg Med 2001;22:1–54 [DOI] [PubMed] [Google Scholar]
- 29.Robert JS. Moral truthfulness in genetic counseling. Bus Prof Ethics J 1998;17:73–93 [DOI] [PubMed] [Google Scholar]
- 30.Takala T. The right to genetic ignorance confirmed. Bioethics 1999;13:288–293 [DOI] [PubMed] [Google Scholar]
- 31.Takala T. Genetic ignorance and reasonable paternalism. Theor Med Bioeth 2001;22:485–491 [DOI] [PubMed] [Google Scholar]
- 32.Elwyn G, Gray J, Clarke A. Shared decision making and non-directiveness in genetic counseling. J Med Genet 2000;37:135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Society for Human Genetics/Advisory Council on Medical Genetics. Points to considerethical, legal and psychosocial implications of genetic testing in children and adolescents. Am J Hum Genet 1995;57:1233–1241 [PMC free article] [PubMed] [Google Scholar]
- 34.Borry P, Fryns J-P, Schotsmans P, Dierickx K. Carrier testing in minors: a systematic review of guidelines and position papers. Eur J Hum Genet 2006;14:133–138 [DOI] [PubMed] [Google Scholar]
- 35.American Medical Association Council on Ethical and Judicial Affairs. Testing children for genetic status. 1995. Available at: http://www.ama-assn.org/ama1/pub/upload/mm/369/ceja_4a95.pdf. Accessed May 7, 2008
- 36.Wheeler PG, Smith R, Dorkin HL, Parad RB, Comeau AM, Bianchi DW. Genetic counseling after implementation of statewide cystic fibrosis newborn screening: two years' experience in one medical center. Genet Med 2001;3:411–415 [DOI] [PubMed] [Google Scholar]
- 37.Wilfond B, Rothenberg LS. Ethical issues in cystic fibrosis newborn screening: from data to public health policy. Curr Opin Pulm Med 2002;8:529–534 [DOI] [PubMed] [Google Scholar]
- 38.Campbell E, Ross LF. Parental attitudes regarding newborn screening of PKU and DMD. Am J Med Genet 2003;120A:209–214 [DOI] [PubMed] [Google Scholar]
- 39.Borry P, Nys H, Dierickx K. Carrier testing in minors: conflicting views. Nat Rev Genet 2007;8:828. [DOI] [PubMed] [Google Scholar]
- 40.Ross LF. Predictive testing for conditions that present in childhood. Kennedy Inst Ethics J 2002;12:225–244 [DOI] [PubMed] [Google Scholar]
- 41.Cohen CB. Wrestling with the future: should we test children for adult-onset genetic conditions? Kennedy Inst Ethics J 1998;8:111–130 [DOI] [PubMed] [Google Scholar]
- 42.Pelias MZ. Duty to disclose in medical genetics: a legal perspective. Am J Med Genet 1991;39:347–354 [DOI] [PubMed] [Google Scholar]
- 43.Pelias MK. Genetic-testing of children for adult-onset diseases: is testing in the child's best interests? J Mt Sinai Hosp N Y 2006;73:605–608 [PubMed] [Google Scholar]
- 44.Kass NE. An ethics framework for public health. Am J Public Health 2001;91:1776–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Childress JF, Faden RR, Gaare RD, et al. Public health ethics: mapping the terrain. J Law Med Ethics 2002;30:170–178 [DOI] [PubMed] [Google Scholar]
- 46.Upshur REG. Principles for the justification of public health intervention. Can J Public Health 2002;93:101–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faden R, Chwalow AJ, Holtzman NA, Horn SD. A survey to evaluate parental consent as public policy for neonatal screening. Am J Public Health 1982;72:1347–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhondt JL. Implementation of informed consent for a cystic fibrosis newborn screening program in France: low refusal rates for optional testing. J Pediatr 2005;147(3 suppl):S106–S108 [DOI] [PubMed] [Google Scholar]
- 49.Brown AJ, MacKenzie J, Fitch M, Estell A, Aitken D. Impact of obtaining signed consent for newborn screening tests in Scotland [abstract]. Poster presented at: Newborn Screening and Genetic Testing Symposium, Atlanta, GA, May 3–6, 2004. [Google Scholar]
- 50.Wailoo K. Genetic marker of segregation: sickle cell anemia, thalassemia, and racial ideology in American medical writing 1920–1950. Hist Philos Life Sci 1996;18:305–320 [PubMed] [Google Scholar]
- 51.Green NS, Pass KA. Neonatal screening by DNA microarray: spots and chips. Nat Rev Genet 2005;6:147–151 [DOI] [PubMed] [Google Scholar]