Abstract
A defined solid and liquid minimal medium, HTM, which contained methionine and cysteine as the sole amino acids, was developed for Listeria monocytogenes. Complex broth-grown L. monocytogenes had to adapt to HTM by inducing amino acid biosyntheis. HTM is the simplest minimal medium available for growth of L. monocytogenes.
Listeria monocytogenes grows well on complex bacteriological media such as brain heart infusion (BHI) broth (Difco Laboratories, Detroit, Mich.). Several defined chemical media containing different amino acids and vitamins for L. monocytogenes strains A4413, NCTC7973, Scott A, and 19303 were reported (2, 6, 10, 11, 12, 13, 14). None of them were able to support growth of all strains. Liquid culture is a poor criterion for definition of a minimal medium. A much more rigorous test is the ability to form colonies on a solidified minimal medium. All the defined media above were solidified and examined for their ability to support colonies of L. monocytogenes strain 10403 (serotype 1/2a), but none were capable.
MOPS is an efficient pH buffer.
Modified Welshimer's broth (MWB) (Table 1) (10) was reported to support some growth of 10403 in liquid culture (8) but failed to serve as solid medium. MWB contains a high concentration of phosphate salts for buffering and as a phosphate source, and this could be toxic to the bacterium. We used 100 mM MOPS (3-N-[morpholino]propanesulfonic acid; pH 7.4) as a buffering agent and reduced the phosphate concentration to 1/10 the original level. Growth in liquid modified MWB was dramatically improved (30°C, agitation at 250 rpm), and the solidified medium could support good colony formation by strain 10403 at 37°C.
TABLE 1.
L. monocytogenes minimal mediuma
| Compound | Concn in:
|
|
|---|---|---|
| MWB | HTM | |
| MOPS pH 7.4 | 100.00 mM | |
| KH2PO4 | 48.2 mM | 4.82 mM |
| Na2HPO4 | 115.5 mM | 11.55 mM |
| MgSO4 | 1.70 mM | 1.70 mM |
| Ferric citrate | 360 μM | |
| Glucose | 55.00 mM | 55.00 mM |
| Thiamine | 2.96 μM | 2.96 μM |
| Riboflavin | 1.33 μM | 1.33 μM |
| Biotin | 2.05 μM | 2.05 μM |
| Lipoic acid | 24.00 pM | 24.00 pM |
| Histidine | 0.1 mg ml−1 | |
| Tryptophan | 0.1 mg ml−1 | |
| Leucine | 0.1 mg ml−1 | |
| Isoleucine | 0.1 mg ml−1 | |
| Valine | 0.1 mg ml−1 | |
| Arginine | 0.1 mg ml−1 | |
| Cysteine | 0.1 mg ml−1 | 0.1 mg ml−1 |
| Methionine | 0.1 mg ml−1 | 0.1 mg ml−1 |
| Glutamine | 0.6 mg ml−1 | |
| (NH4)2SO4 | 0.6 mg ml−1 | |
| Agarose | 15 g liter−1 | |
Supplements were purchased from BDH, Poole, Dorset, United Kingdom, or Sigma-Aldrich Chemical Co. Ltd., Poole, Dorset, United Kingdom. Amino acids and vitamins were filter sterilized (0.22-μm pore size), while the others were autoclaved. MOPS was adjusted to pH 7.4. Solid cultures were incubated at 30°C, while liquid cultures were incubated at 30°C with agitation (250 rpm).
Only cysteine and methionine are essential amino acids for 10403.
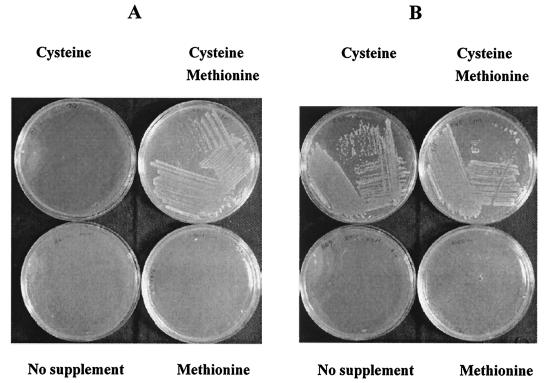
Previous reports indicated that L. monocytogenes could not synthesize all 20 amino acids (2, 10, 11, 12, 13, 14). We systematically deleted the amino acids from modified MWB and found that only methionine and cysteine were essential (Fig. 1A). Cysteine can be synthesized by the reduction of sulfate to sulfide, which is condensed with O-acetylserine to form cysteine. However, a genome survey indicated that genes for reducing sulfate to sulfide equivalent are absent from L. monocytogenes strain EGD-e (3; http://genolist.pasteur.fr/ListiList/). Alternatively, thiosulfate can be condensed with O-acetylserine to form sulfocysteine, which is reduced to cysteine. We found that thiosulfate could not replace cysteine.
FIG. 1.
Essential amino acid requirements for strain 10403 (A) and strain EGD-e (B). Amino acid-free HTM was supplemented with 0.1 mg of each amino acid ml−1.
L. monocytogenes can use inorganic nitrogen sources.
It was reported that L. monocytogenes could not use inorganic nitrogen sources (10), but we found that we could replace glutamine with ammonium [(NH4)2SO4 and (NH4)NO3] but not nitrate (NaNO3), implying a lack of nitrate reductase.
Biotin, lipoic acid, riboflavin, and thiamine are essential.
The requirement for all vitamins in MWB was examined. We removed each of the four vitamins in turn from the medium and streaked any resultant colonies on the same medium to ensure complete vitamin depletion. Riboflavin and lipoic acid were found to be essential, and thiamine and biotin were required for healthy colony formation. There may have been trace amounts of biotin and thiamine in the seaweed-derived agar (4). Examination of the EGD-e genome revealed a lack of the genes encoding the pathways of synthesis of the four cofactors (http://genolist.pasteur.fr/ListiList/).
L. monocytogenes carbohydrate catabolism appears limited.
The following filter-sterilized (0.22-μm pore size) carbon sources were tested at 1%: glycerol, arabinose, ribose, xylose, glucose, fructose, mannose, mannitol, fucose, galactose, rhamnose, maltose, sucrose, lactose, starch, and Casamino Acids. Only glucose, glycerol, fructose, and mannose could support growth. The pH of the media, as shown by incorporating 1% BDH universal pH 4.0 to 11.0 indicator, was reduced from between 7.0 and 7.5 to 4.5, indicating that fermentation rather than respiration occurred. The failure of Casamino Acids to be used, as previously noted by Premaratne et al. (10), indicates that amino acid catabolic pathways are not present in this bacterium. The final minimal medium was named HTM (Hsiang-Ning Tsai medium; Table 1).
HTM can support the growth of L. monocytogenes strains 10403, EGD-e, and L028 and Listeria innocua 33090.
Strain EGD-e could grow without methionine (Fig. 1B) and again failed to use thiosulfate as a cysteine substitute, while strain L028 could grow, albeit poorly, in the absence of methionine. HTM could support the growth of L. innocua 33090 but not strain Scott A. Strain EGD-e, as expected, contains all the met genes, but it cannot synthesize cysteine from methionine (Fig. 1B), indicating the lack of the trans-sulfuration pathway (5). The requirement for methionine by 10403 implies a lesion(s) in a met gene(s).
Adaptation to HTM.
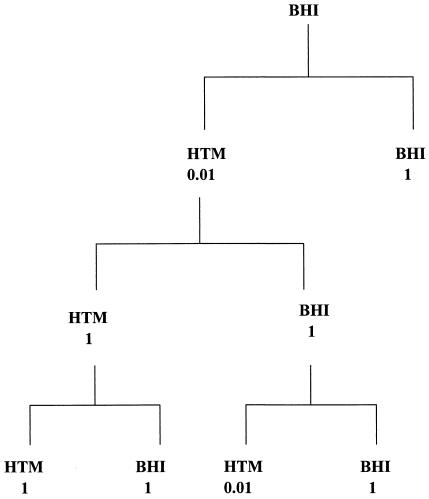
When a culture of BHI-grown cells was diluted in phosphate-buffered saline (PBS) or liquid HTM, the efficiency of plating (eop) on solid HTM was reduced 100-fold compared to that on BHI agar. When the HTM-grown colonies were resuspended in PBS or HTM and diluted, the eop on solid BHI and HTM was the same. This showed the cells had adapted to growth on HTM. To test if this adaptation was physiological or genetic, the cells that had previously grown on HTM and then grown on BHI were resuspended, diluted, and spread on HTM agar again. The eop was the same as the first time they were presented with HTM, i.e., 100-fold less than on BHI agar (Fig. 2). This showed that the cells had not retained the ability to grow on HTM during subculture on BHI; thus the adaptation was physiological and not genetic.
FIG. 2.
Adaptation on HTM medium. Numbers indicated the eops on HTM agar compared to that on BHI agar.
Amino acid supplements improved growth in liquid HTM.
Strain 10403 grew slowly with a long lag period in liquid HTM (Table 2). Neither washing and resuspending the inocula in HTM nor applying anaerobic conditions nor incubating at 37°C improved the growth. Addition of the trace elements ZnCl2 (80 μg liter−1), CuCl2 (20 μg liter−1), MnCl2 (20 μg liter−1), Na2B4O7 (20 μg liter−1), and Mo7O24 (20 μg liter−1) (9) did not improve growth, contrary to previous reports (1, 2, 7, 11).
TABLE 2.
Effect of iron and amino acid supplements on the growth of L. monocytogenes in liquid HTMc
| Medium and supplement (concn [μg ml−1]) | Lag period (h) | Doubling time (h)d | Highest yield (OD600) |
|---|---|---|---|
| BHI | <5 | 0.902 ± 0.016 | 1.502 |
| HTMa | |||
| None | 15 | 2.19 ± 0.26 | 0.967 |
| Ferric ammonium citrate (20) | 10 | 2.55 ± 0.17 | 0.733 |
| Ferric ammonium citrate (2.0) | 15 | 2.62 ± 0.39 | 0.900 |
| Ferric ammonium citrate (0.2) | 15 | 2.05 ± 0.23 | 0.777 |
| Ferric citrate (20) | 15 | 2.23 ± 0.13 | 1.031 |
| Ferric citrate (2.0) | 10 | 2.16 ± 0.62 | 1.217 |
| Ferric citrate (0.2) | 10 | 1.63 ± 0.09 | 1.040 |
| Hemin (20) | 35 | 2.20 ± 0.11 | 0.134 |
| Hemin (2.0) | 15 | 2.13 ± 0.11 | 0.263 |
| Hemin (0.2) | 10 | 1.38 ± 0.04 | 1.067 |
| HTM with amino acidsb | |||
| None | <5 | 1.65 ± 0.07 | 1.110 |
| Ferric ammonium citrate (0.2) | <5 | 1.94 ± 0.07 | 1.003 |
| Ferric citrate (0.2) | <5 | 1.76 ± 0.04 | 1.043 |
| Hemin (20) | 25 | 2.26 ± 0.18 | 0.581 |
| Hemin (0.2) | <5 | 1.80 ± 0.02 | 1.095 |
HTM with 1.6 mg of glutamine ml−1.
HTM with 0.1 mg each of alanine, arginine, asparagine, aspartic acid, glutamine, glutamate, glycine, histidine, isoleucine, leucine, lysine, phenylalanine, proline, serine, threonine, and valine ml−1.
Iron supplements were purchased from BDH or Sigma-Aldrich Chemical Co. Ltd., dissolved in hot water, adjusted to pH 7, and filter sterilized. A BHI culture was washed twice in distilled water, resuspended in PBS, diluted 100-fold, and used as an inoculum at an initial optical density at 600 nm (OD600) of 0.01. The culture was incubated at 30°C with agitation (250 rpm), and the OD was measured every 5 h until the stationary phase. Experiments were repeated three times.
P < 0.05. Values are means ± standard deviations.
We examined the effects on growth of 0.2, 2, and 20 μg of ferric ammonium citrate, ferric citrate, and hemin ml−1. Ferric citrate and hemin at 0.2 μg ml−1 accelerated the growth rate (doubling time in hours) and decreased the lag period (in hours), with little effect on yield (Table 2). Hemin at 20 μg ml−1 greatly reduced the yield and increased the lag period, with little effect on the growth rate, demonstrating toxicity. A mixture of amino acids abolished the long lag phase and decreased the doubling time, with little increase in the yield (Table 2). Iron supplementation in addition to amino acid supplementation had little effect on growth, with the exception of hemin at 20 μg ml−1, which again inhibited growth.
The abolition of the lag phase with an amino acid mixture implied that there was no missing essential component(s) but rather greatly delayed initiation of amino acid biosynthetic pathways in HTM upon transfer from BHI. This also explained the poor plating efficiency and the physiological adaptation on solid HTM.
L. monocytogenes appears to be sensitive to extreme nutrient starvation and high phosphate levels, which implies a constitution less robust than those of many other bacteria. So far, HTM is the simplest minimal medium for L. monocytogenes and is currently the medium of choice for amino acid feeding for the proteome analysis of strain EGD-e by the European Listeria Genome Consortium (U. Kaerst, personal communication).
Acknowledgments
We thank Yvonne Paterson for great help in reviewing the paper and Tsan-Kuang Lee for help with the statistical analysis.
REFERENCES
- 1.Cowart, R. E., and B. G. Foster. 1985. Differential effects of iron on the growth of Listeria monocytogenes: minimum requirements and mechanism of acquisition. J. Infect. Dis. 151:721-730. [DOI] [PubMed] [Google Scholar]
- 2.Friedman, M. E., and W. G. Roessler. 1961. Growth of Listeria monocytogenes in defined media. J. Bacteriol. 82:528-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couve, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K. D. Entian, H. Fsihi, F. G. Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 4.Hodgson, D. A., and K. F. Chater. 1981. A chromosomal locus controlling extracellular agarase production by Streptomyces coelicolor A3(2) and its inactivation by chromosomal integration of plasmid SCP1. J. Gen. Microbiol. 124:339-348. [Google Scholar]
- 5.Hodgson, D. A. 2000. Primary metabolism and its control in streptomycetes: a most unusual group of bacteria. Adv. Microb. Physiol. 42:47-238. [DOI] [PubMed] [Google Scholar]
- 6.Jones, C. E., G. Shama, P. W. Andrew, I. S. Roberts, and D. Jones. 1995. Comparative study of the growth of L. monocytogenes in defined media and demonstration of growth in continuous culture. J. Appl. Bacteriol. 78:66-70. [DOI] [PubMed] [Google Scholar]
- 7.Kim, D. S., S. Thomas, and H. S. Fogler. 2000. Effects of pH and trace minerals on long-term starvation of Leuconostoc mesenteroides. Appl. Environ. Microbiol. 66:976-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marquis, H., H. G. Bouwer, D. J. Hinrichs, and D. A. Portnoy. 1993. Intracytoplasmic growth and virulence of Listeria monocytogenes auxotrophic mutants. Infect. Immun. 61:3756-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okanishi, M., K. Suzuki, and H. Umezawa. 1974. Formation and reversion of streptomycete protoplasts: cultural conditions and morphological study. J. Gen. Microbiol. 80:389-400. [DOI] [PubMed] [Google Scholar]
- 10.Premaratne, R. J., W. Lin, and E. A. Johnson. 1991. Development of an improved chemical defined minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 57:3046-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ralovich, B. S., M. Shahamat, and M. Woodbine. 1977. Further data on the characters of Listeria strains. Med. Microbiol. Immunol. 163:125-139. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqi, R., and M. A. Khan. 1989. Amino acid requirement of six strains of Listeria monocytogenes. Zentbl. Bakteriol. 271:146-152. [DOI] [PubMed] [Google Scholar]
- 13.Trivett, T. L., and E. A. Meyer. 1971. Citrate cycle and related metabolism of Listeria monocytogenes. J. Bacteriol. 107:770-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welshimer, H. J. 1963. Vitamin requirements of Listeria monocytogenes. J. Bacteriol. 85:1156-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]