Abstract
Six luminous bacteria were isolated from migrating salmon in the Yukon River, Alaska. All isolates were identified as Photobacterium phosphoreum. Previous studies suggest that P. phosphoreum is an exclusively marine bacterium, while our Alaskan isolates are from salmon which migrated up to 1,228 km from the marine environment.
Luminous bacteria have been extensively studied and are well described phylogenetically and ecologically. Compared to the broad distribution and high abundance in the marine environment (17), only one luminous species has been isolated from fresh water (17, 29, 30) and another has been isolated from soil (19). Luminous bacteria have been observed living in many ecological niches including planktonic (23, 25, 26, 30, 32), saprophytic (16), symbiotic (6, 7, 12, 13, 22-24), and parasitic (16) niches. Some species inhabit more than one niche (10). Despite several studies describing the distribution and abundance of luminous bacteria, details regarding population dynamics, ecological function, and especially niche relationships remain poorly understood.
Photobacterium phosphoreum has been well described relative to their light organ symbioses with several families of marine fish inhabiting cold and deep ocean waters (11). Free-living P. phosphoreum also has been isolated by direct plating of seawater (20). Aside from the free-living forms and symbioses formed with marine fish, P. phosphoreum has been described as living saprophytically and parasitically (16). Recent reports implicate P. phosphoreum as an important factor in spoilage of cold-cured salmon and cod from the north Atlantic Ocean (2, 3). P. phosphoreum is considered an exclusively marine bacterium because of its specific requirement for sodium in growth medium (20).
Identification of luminous environmental isolates traditionally has relied on a set of nutritional versatility tests to quickly and reliably distinguish among luminous bacterial groups (20). More recently, PCR primers which are suitable for the amplification and sequencing of luxA have been used. The gene product of luxA, α-luciferase, is necessary for the light-emitting reaction of all known luminous bacteria (14, 31).
We tested whether P. phosphoreum was responsible for bioluminescence from migrating salmon harvested up to 1,228 km from the marine environment. The identification of the luminous isolates as P. phosphoreum was accomplished by the use of three complementary methods: tests to assess nutritional versatility and DNA sequence analysis of luxA and of the 16S rRNA gene.
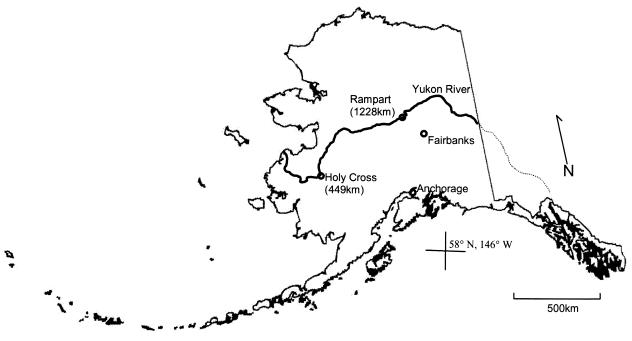
Luminous bacterial strains were isolated from whole chum salmon, Oncorhynchus kisutch, harvested from the Yukon River near the village of Rampart, Alaska (Fig. 1; Table 1). Whole chum salmon were transported to Fairbanks, Alaska, within 6 h of harvest and partially submerged in artificial seawater (0.4 M NaCl, 0.1 M MgSO4 · 7H2O, 0.02 M KCl, 0.02 M CaCl2 · 2H2O) as previously described (15). The partially submerged salmon were stored at 10°C for 10 days and inspected visually for the presence of luminous areas daily. Luminous areas were swabbed and transferred to seawater complete (SWC) broth and later purified into pure culture. One additional isolate (AK-8) was received in pure culture from the Pathology Laboratory of the Alaska Department of Fish and Game from a partially smoked chum salmon caught near Holy Cross, Alaska (Fig. 1; Table 1).
FIG. 1.
Sample locations along the Yukon River in Alaska where salmon with bioluminescent bacteria were caught. Distances indicate kilometers from the mouth of the Yukon River.
TABLE 1.
P. phosphoreum isolates used in this study
| Strain | Host fish | Location on fish | Location | Date of isolation |
|---|---|---|---|---|
| AK-1 | Chum salmon | Head | Rampart | August 1997 |
| AK-5 | Chum salmon (female) | Gut content | Rampart | September 2001 |
| AK-6 | Chum salmon (male) | Slime | Rampart | September 2001 |
| AK-7 | Chum salmon (male) | Liquid around fisha | Rampart | September 2001 |
| AK-8 | Chum salmon | Flesh | Holy Cross | August 2001 |
| AK-9 | Chum salmon | Liquid around fisha | Rampart | September 2002 |
Brownish discharge from fish after fish was partially submerged in artificial seawater for several days.
The Yukon River flows at a rate of approximately 6 to 12 km/h (9). Due to the glacial origin of some of its tributaries, the Yukon River is silty in summer and clear in winter. The climate of the Yukon River watershed is characterized by long, cold winters and brief, warm summers. Air temperatures below freezing are common in September, and the Yukon River is generally frozen from late October until May (9).
Holy Cross and Rampart are located 449 and 1,228 km from the mouth of the Yukon River, respectively (Fig. 1). Migration of chum salmon in the Yukon River averages 35 to 40 km/day (R. Brown, personal communication); consequently, the migration times are approximately 11 days to Holy Cross and 30 days to Rampart.
All isolates of luminous bacteria were grown in SWC medium (0.38 M NaCl, 0.02 M MgCl2 · 6H2O, 0.25 M MgSO4 · 7H2O, 8 mM KCl, 0.5% peptone, 0.3% yeast extract, 0.3% glycerol). All Alaskan luminous isolates were grown at 15°C. The reference strain, P. phosphoreum strain NZ-11-D (provided by one of us [C.F.W.]) (24) was grown and maintained under the same conditions as Alaskan isolates. Long-term storage of strains was at −80°C in SWC medium containing 15% glycerol. Luminous isolates of Alaskan origin are designated AK strains.
We isolated DNA from 100 ml of exponentially growing cultures with a standard genomic DNA isolation protocol (1). RNA was degraded by 1-h incubation at 37°C with 10 μg of RNase A (Promega, Madison, Wis.)/ml. We determined yield, quality, and concentration of DNA isolations by gel electrophoresis.
All PCRs were performed with PCR Core System II (Promega) in a GeneAmp PCR System 2400 (Perkin-Elmer, Norwalk, Conn.). PCRs were generally performed with 50-μl mixtures containing (concentrations are final) 1× PCR buffer, 1.5 mM MgCl2, 200 μM (each) deoxynucleoside triphosphate, 1 μM (each) primer, 1.25 U of Taq polymerase, and 50 to 500 ng of genomic DNA template. PCR conditions for 16S rRNA reactions were 1 cycle of denaturation for 5 min at 94°C; 25 amplification cycles consisting of denaturation (94°C for 30 s), primer annealing (49°C for 30 s), and primer extension (72°C for 90 s); and a final extension of 7 min at 72°C. PCR conditions for luxA were the same except for 30 amplification cycles and a primer annealing temperature of 45°C.
Primers used to amplify the 16S rRNA gene from genomic DNA were 16S-11f (5′ GTTTGATCCTGGCTCAG 3′) and 16S-1512r (5′ ACGGYTACCTTGTTACACTT 3′) (28). All oligonucleotide primers were synthesized by Integrated DNA Technologies (Coralville, Iowa). 16S rRNA amplicons were gel purified with a QIAquick gel cleanup kit (Qiagen, Valencia, Calif.) and directly sequenced. We amplified luxA by PCR with the primers luxA127f (5′ GAICAICAITTIACIGAGTTTGG 3′) and luxA1007r (5′ ATTTCITCTTCAGIICCATTIGCTTCAAAICC 3′) (27), with genomic DNA as the template. luxA amplicons were gel purified with Freeze ′N Squeeze DNA gel extraction spin columns (Bio-Rad, Hercules, Calif.). Following gel purification, luxA PCR products were ligated into the pCRII-TOPO vector and TOP10 cells were transformed (Invitrogen, Carlsbad, Calif.). Ligations and transformations were done by following the manufacturer's instructions. Clones were screened by PCR for the presence of the luxA insert. The plasmids from one positive clone were isolated on a DNA-Pure plasmid miniprep kit (CPG, Lincoln Park, N.J.) by following the manufacturer's instructions and used as the template in cycle sequencing reactions.
Each 16S rRNA amplicon and luxA plasmid insert was bidirectionally sequenced on an ABI 3100 automated sequencer (Applied Biosystems, Foster City, Calif.). Cycle sequencing conditions for all reactions involved 40 to 60 ng of template DNA, 3.2 pmol of primer, 4 μl of Big Dye (Applied Biosystems), and water to a final volume of 20 μl. 16S rRNA reactions were primed with primers 16S-11f and 16S-1512r. The following internal primers were used to ensure overlapping sequences for analysis of 16S rRNA sequences: 16S-515f (5′ GTGCCAGCMGCCGCGGTAA 3′), 16S-1100f (5′ CAACGAGCGCAACCCT 3′), 16S-519r (5′ GWATTACCGCGGCKGCTG 3′), and 16S-907r (5′ CCGTCAAATCCTTTRAGTTT 3′) (28). Cycle sequencing reactions for luxA plasmid inserts were primed with SP6 and T7 promoter primers (Promega). Cycle sequencing reactions consisted of 25 amplification cycles that included denaturation (96°C for 30 s), primer annealing (49°C for 15 s), and primer extension (60°C for 4 min). Cycle sequencing conditions for luxA were the same, except for a primer annealing temperature of 45°C. Extension products were submitted for sequencing at the University of Alaska's Core Facility for Nucleic Acid Analysis.
We used a set of tests to assess the ability of Alaskan isolates and the reference strain, P. phosphoreum NZ-11-D, to utilize each of 12 compounds as a sole carbon source in minimal media. As an additional component of this analysis, we assayed for the presence of three extracellular enzymes (15). Our P. phosphoreum isolates required the addition of 40 μg of l-methionine per ml of minimal medium for growth (19, 20). Optimal growth temperature was determined by inoculating log-phase cells into SWC medium and observing growth at 4, 10, 15, and 20°C.
Bidirectional contigs of 16S rRNA and luxA sequences were assembled with Sequencher, version 4.0.5 (Gene Codes, Ann Arbor, Mich.). We imported contigs into ClustalX and aligned them with representative 16S rRNA and luxA sequences obtained from GenBank. Aligned sequences were imported into PAUP* 4.0b10 (D. Swafford, Phylogenetic analysis using parsimony and other methods), ed. 4.0, Sinauer Associates, Sutherland, Mass., 2000), where maximum-likelihood analysis was performed and phylograms were generated. Maximum-likelihood analysis included 100 bootstrap replicates. Only bootstrap support values of >50 are displayed.
GenBank accession numbers for sequences used in 16S rRNA sequence analyses are AE000474 for Escherichia coli K-12 strain MG1655, X82248 for Photorhabdus luminescens DSM 3368, X82132 for Shewanella hanedai CIP 103207T, X74706 for Vibrio harveyi ATCC 14126, Z21729 for Vibrio fischeri MJ-1, X74686 for Photobacterium leiognathi ATCC 22551T, and X74687 for P. phosphoreum ATCC 11004T. Accession numbers chosen as representative for luxA sequence analyses are X58791 for V. harveyi CTP5 luxB (used as the outgroup), M57416 for Photorhabdus luminescens ATCC 29999, X58791 for V. harveyi CTP5, X08036 for P. leiognathi 554, X55458 for P. phosphoreum NCMB 844, AB058949 for S. hanedai ATCC 33224, and AF170104 for V. fischeri MJ-1.
Isolates from Alaskan salmon used in this study were short rods, oxidase negative and gram negative (8), and required l-methionine for growth in minimal media. Additionally, all isolates from Alaska grew at 4°C; however, optimal growth occurred at 15°C and growth diminished at >20°C. Compared to other species of luminous bacteria, all AK strains can be assigned to the P. phosphoreum group based on published data on nutritional versatility (Table 2). We verified our results by including a reference strain, P. phosphoreum NZ-11-D (18), in our test (Table 2).
TABLE 2.
Phenotypic characteristics of Alaskan isolates
| Characteristic | Result from:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Published reference datad for:
|
This study for strain:
|
|||||||||||
| V. harveyia | V. fischeria | P. leiognathia | P. phosphoreuma,b | P. phosphoreum NZ-11-Dc | NZ-11-D | AK-1 | AK-5 | AK-6 | AK-7 | AK-8 | AK-9 | |
| Growth on: | ||||||||||||
| Maltose (0.2%) | + | + | − | + | + | + | + | + | + | + | + | + |
| Cellobiose (0.2%) | + | + | − | − | − | − | − | − | − | − | − | − |
| Glucuronate (0.1%) | + | − | − | (+) | (+) | + | − | − | − | − | − | − |
| Mannitol (0.1%) | + | + | − | − | (−) | − | − | − | − | − | − | − |
| Proline (0.1%) | + | + | + | (−) | (−) | − | − | − | − | − | − | − |
| Lactate (0.2%) | + | − | + | (−) | − | − | − | − | − | − | − | − |
| Pyruvate (0.1%) | + | − | + | − | − | − | − | − | − | − | − | − |
| Acetate (0.05%) | + | − | + | − | − | − | − | − | − | − | − | − |
| Propionate (0.05%) | + | − | − | − | − | − | − | − | − | − | − | − |
| Heptanoate (0.05%) | + | − | − | − | − | − | − | − | − | − | − | − |
| d-α-Alanine (0.05%) | + | (−) | − | − | − | − | − | − | − | − | − | − |
| l-tyrosine (0.4%) | + | − | − | − | − | − | − | − | − | − | − | − |
| Production of: | ||||||||||||
| Lipase | + | − | − | − | − | − | − | − | − | − | − | − |
| Gelatinase | + | − | − | − | − | − | − | − | − | − | − | − |
| Amylase | + | − | − | − | − | − | − | − | − | − | − | − |
| Optimal growth temp (°C) | 20 | 22 | 15 | 15 | 15 | 15 | 15 | 15 | ||||
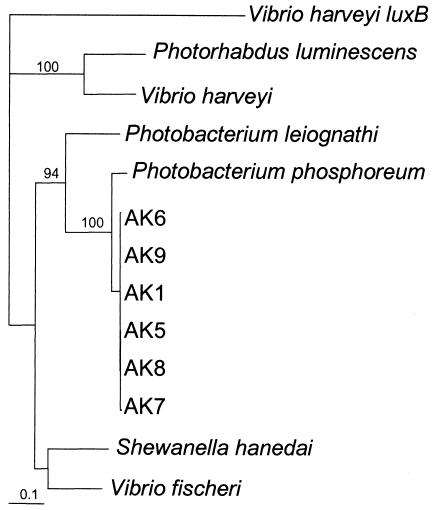
Gene sequences of luxA of the seven AK isolates were aligned with six representative sequences from other luminous bacteria. The alignment produced a consensus sequence 554 bp in length shared by all 13 taxa. Maximum-likelihood analysis of the alignment by PAUP* 4.0b10 revealed that all AK isolates clustered closely with P. phosphoreum (Fig. 2).
FIG. 2.
Phylogeny of Alaskan luminous bacteria based on maximum-liklihood analysis using PAUP* 4.0b10 with luxA sequences. All strains with the prefix AK are from salmon harvested from the Yukon River. V. harveyi luxB was used as the outgroup in the maximum-likelihood analysis of luxA genes. The bar represents substitutions per site.
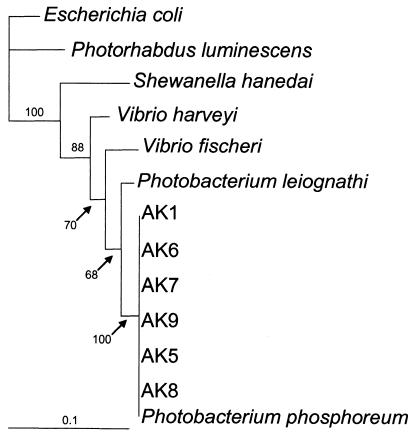
16S rRNA gene sequences of the seven AK isolates were aligned with six representative sequences from other luminous bacteria. The alignment produced a consensus sequence 1,159 bp in length shared by all 13 taxa. Maximum-likelihood analysis of the alignment by PAUP* 4.0b10 revealed that all AK isolates clustered closely with P. phosphoreum (Fig. 3).
FIG. 3.
Phylogeny of Alaskan luminous bacteria based on maximum-likelihood analysis using PAUP* 4.0b10 with 16S rRNA sequences from Alaskan isolates and representative sequences from GenBank. All strains with the prefix AK are from salmon harvested from the Yukon River. Escherichia coli was used as the outgroup in this analysis. The bar represents substitutions per site.
We positively identified P. phosphoreum isolated from migrating salmon, collected up to 1,228 km from the mouth of Yukon River, Alaska. Our data on nutritional versatility allow us to confidently place our Alaskan isolates into the P. phosphoreum group. Molecular data, both 16S rRNA and luxA sequence analysis, reinforce our identification by showing that our isolates cluster closely with representative P. phosphoreum 16S rRNA and luxA sequences. Our results are significant because of the scarcity of bioluminescent bacteria isolated outside of the marine environment and because all previous studies indicate that P. phosphoreum is an exclusively marine bacterium.
Our data indicate that P. phosphoreum is capable of remaining viable on the external surfaces of anadromous migrating salmon. Although it is possible that P. phosphoreum colonizes salmon while in the Yukon River, we believe that it is much more likely that the P. phosphoreum that we isolated has its origin in the marine environment. Preliminary attempts to cultivate P. phosphoreum from Yukon River water have been unsuccessful (unpublished data). Despite the absence of data on the distribution of luminous bacteria in the northern Pacific Ocean, we predict that P. phosphoreum is the primary species present because of increased abundance of P. phosphoreum in cold temperatures (24) and deep water (below 200 m) (21, 25).
Previous results (4, 5) suggest that P. phosphoreum is adapted for survival in low-salt environments, showing optimal growth in media with a salt (NaCl) concentration approximately 50% that of seawater. Preliminary studies in our laboratory suggest that Alaskan P. phosphoreum isolate AK-6 and the reference strain, NZ-11-D, are rendered nonviable after <1 day in river water; however, the viability of both strains is maintained in SWC medium prepared without NaCl for up to 5 days. We therefore hypothesize that P. phosphoreum remains viable in the freshwater environment of the Yukon River because the complex matrix of fish slime is of sufficient osmotic strength to protect bacterial cells from the very low osmotic conditions of freshwater. We believe that our P. phosphoreum isolates are of marine origin, forming a saprophytic association with migrating salmon while still in the ocean environment. When salmon migrate into freshwater, luminous bacteria on the salmon are protected by the slime of salmon until the fish are caught.
The description of our Alaskan strains of P. phosphoreum are nearly identical to other descriptions with respect to nutritional versatility and luxA and 16S rRNA sequences; however, our isolates appear to have a lower optimal growth temperature than the reference strain, P. phosphoreum NZ-11-D. Future investigations of the osmotic requirements and temperature tolerances of Alaskan P. phosphoreum may reveal adaptations specific to this unique niche.
Nucleotide sequence accession numbers.
GenBank accession numbers for luxA sequences derived in this study are AY345883 to AY345888; those for 16S rRNA sequences derived in this study are AY345889 to AY345894.
Acknowledgments
This publication is the result of research sponsored by Alaska Sea Grant with funds from the National Oceanic and Atmospheric Administration Office of Sea Grant, Department of Commerce, under grant no. NA 86RG0050 (project no. RR/01-05 and GC/02-01), and from the University of Alaska with funds appropriated by the state. Additional support was provided by the Alaska Natural Resources Fund, the University of Alaska Fairbanks Water and Environmental Research Center, and a student grant from the University of Alaska Fairbanks Center for Global Change.
We are grateful for the assistance offered by Randy Brown of the U.S. Fish and Wildlife Service.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1989. Short protocols in molecular biology. Greene Publishing Associates, New York, N.Y.
- 2.Dalgaard, P., O. Mejlholm, T. J. Christiansen, and H. H. Huss. 1997. Importance of Photobacterium phosphoreum in relation to spoilage of modified atmosphere-packed fish products. Lett. Appl. Microbiol. 24:373-378. [Google Scholar]
- 3.Dalgaard, P., L. G. Munoz, and O. Mejlholm. 1998. Specific inhibition of Photobacterium phosphoreum extends the shelf life of modified-atmosphere-packed cod fillets. J. Food Prot. 61:1191-1194. [DOI] [PubMed] [Google Scholar]
- 4.Dunlap, P. V. 1984. Ph.D. thesis. University of California, Los Angeles.
- 5.Dunlap, P. V. 1985. Osmotic control of luminescence and growth in Photobacterium leiognathi from ponyfish light organs. Arch. Microbiol. 141:44-50. [DOI] [PubMed] [Google Scholar]
- 6.Dunlap, P. V., K. Kita-Tsukamoto, J. B. Waterbury, and S. M. Callahan. 1995. Isolation and characterization of a visibly luminous variant of Vibrio fischeri strain ES114 from the Sepiolid squid Eurypna scolopes. Arch. Microbiol. 164:194-202. [Google Scholar]
- 7.Fidopiastis, P. M., S. V. Boletzky, and E. G. Ruby. 1998. A new niche for Vibrio logei, the predominant light organ symbiont of squids in the genus Sepiola. J. Bacteriol. 180:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerhardt, P. (ed.). 1994. Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 9.Gordon, J. A., S. P. Klosiewski, T. J. Underwood, and R. J. Brown (ed.). 1998. Estimated abundance of adult fall chum salmon in the upper Yukon River, Alaska, 1996. Alaska fisheries technical report 45. U.S. Fish and Wildlife Service, Fairbanks, Alaska.
- 10.Hastings, J. W. 1983. Biological diversity, chemical mechanisms, and the evolutionary origins of bioluminescent systems. J. Mol. Evol. 19:309-321. [DOI] [PubMed] [Google Scholar]
- 11.Hastings, J. W., and K. H. Nealson. 1981. The symbiotic luminous bacteria, p. 1322-1345. In M. Starr, H. Stolp, H. Trüper, A. Balows, and H. Schlegel (ed.), The prokaryotes. A handbook on habitats, isolation, and identification of bacteria. Springer-Verlag, Berlin, Germany.
- 11a.Holt, J. G., N. R. Kreig, P. H. A. Sneath, J. T. Stanley, and S. T. Williams (ed.). 1994. Bergey’s manual of determinative bacteriology, 9th ed. Williams & Wilkins, Baltimore, Md.
- 12.Lee, K.-H., and E. G. Ruby. 1994. Competition between Vibrio fischeri strains during initiation and maintenance of a light organ symbiosis. J. Bacteriol. 176:1985-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leisman, G., D. H. Cohn, and K. H. Neason. 1980. Bacterial origin of luminescence in marine animals. Science 208:1271-1273. [DOI] [PubMed] [Google Scholar]
- 14.Makemson, J. C., N. R. Fulayfil, W. Landry, L. M. V. Ert, C. F. Wimpee, E. A. Widder, and J. F. Case. 1997. Shewanella woodyi sp. nov., an exclusively respiratory luminous bacterium isolated from the Alboran Sea. Int. J. Syst. Bacteriol. 47:1034-1039. [DOI] [PubMed] [Google Scholar]
- 15.Nealson, K. 1978. Isolation, identification, and manipulation of luminous bacteria, p. 153-166. In M. A. Deluca (ed.), Bioluminescence and chemiluminescence, vol. LVII. Academic Press, New York, N.Y.
- 16.Nealson, K., and J. W. Hastings. 1992. The luminous bacteria, p. 625-639. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes: a handbook for the biology of bacteria. Ecophysiology, isolation, identification, applications, 2nd ed., vol. 1. Springer-Verlag, Berlin, Germany.
- 17.Nealson, K. H., and J. W. Hastings. 1979. Bacterial bioluminescence: its control and ecological significance. Microbiol. Rev. 43:496-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nealson, K. H., B. Wimpee, and C. Wimpee. 1993. Identification of Vibrio splendidus as a member of the planktonic luminous bacteria from the Persian Gulf and Kuwait region with luxA probes. Appl. Environ. Microbiol. 59:2684-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poinar, G. O., and G. Thomas. 1979. Growth and luminescence of the symbiotic bacteria associated with the terrestrial nematode, Heterorhabdus bacteriophora. Soil Biol. Biochem. 12:5-10. [Google Scholar]
- 20.Reichelt, J. L., and P. Baumann. 1973. Taxonomy of the marine, luminous bacteria. Arch. Microbiol. 94:283-330. [Google Scholar]
- 21.Ruby, E. G., E. P. Greenberg, and J. W. Hastings. 1980. Planktonic marine luminous bacteria: species distribution in the water column. Appl. Environ. Microbiol. 39:302-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruby, E. G., and K.-H. Lee. 1998. The Vibrio fischeri-Euprymna scolopes light organ association: current ecological paradigms. Appl. Environ. Microbiol. 64:805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruby, E. G., and J. G. Morin. 1979. Luminous enteric bacteria of marine fishes: a study of their distribution, densities, and dispersion. Appl. Environ. Microbiol. 38:406-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruby, E. G., and J. G. Morin. 1978. Specificity of symbiosis between deep-sea fishes and psychrotrophic luminous bacteria. Deep-Sea Res. 25:161-167. [Google Scholar]
- 25.Ruby, E. G., and K. H. Nealson. 1978. Seasonal changes in the species composition of luminous bacteria in nearshore seawater. Limnol. Oceanogr. 23:530-533. [Google Scholar]
- 26.Shilo, M., and T. Yetinson. 1979. Physiological characteristics underlying the distribution patters of luminous bacteria in the Mediterranean Sea and the Gulf of Elat. Appl. Environ. Microbiol. 38:577-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VanErt, L. M. 2001. Ph.D. dissertation. University of Wisconsin, Milwaukee.
- 28.Weisberg, W., S. Barns, D. Pelletier, and D. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.West, P. A., and J. V. Lee. 1982. Ecology of Vibrio species, including Vibrio cholerae, in natural waters of Kent, England. J. Appl. Bacteriol. 52:435-448. [DOI] [PubMed] [Google Scholar]
- 30.West, P. A., J. V. Lee, and T. N. Bryant. 1983. A numerical taxonomic study of species of Vibrio isolated from the aquatic environment and birds in Kent, England. J. Appl. Bacteriol. 55:263-282. [DOI] [PubMed] [Google Scholar]
- 31.Wimpee, C. F., T.-L. Nadeau, and K. H. Nealson. 1991. Development of species-specific hybridization probes for marine luminous bacteria by using in vitro DNA amplification. Appl. Environ. Microbiol. 57:1319-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yetinson, T., and M. Shilo. 1979. Seasonal and geographic distribution of luminous bacteria in the eastern Mediterranean Sea and the Gulf of Elat. Appl. Environ. Microbiol. 37:1230-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]