Abstract
Acid resistance of Mycobacterium paratuberculosis was examined as a function of growth conditions (i.e., in vitro growth medium and pH). M. paratuberculosis was cultured in either fatty acid-containing medium (7H9-OADC) or glycerol-containing medium (WR-GD or 7H9-GD) at two culture pHs (pHs 6.0 and 6.8). Organisms produced in these six medium and pH conditions were then tested for resistance to acetate buffer at pHs 3, 4, 5, and 6 at 20°C. A radiometric culture method (BACTEC) was used to quantify viable M. paratuberculosis cell data at various acid exposure times, and D values (decimal reduction times, or the times required to kill a 1-log10 concentration of bacteria) were determined. Soluble proteins of M. paratuberculosis grown under all six conditions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to identify proteins that may be associated with acid resistance or susceptibility. The culture medium affected growth rate and morphology: thin floating sheets of cells were observed in 7H9-OADC versus confluent, thick, waxy, and wrinkled pellicles in WR-GD. Culture medium pH affected growth rate (which was highest at pH 6.0), but it had little or no effect on D values for M. paratuberculosis at any test pH. When grown in 7H9-OADC, M. paratuberculosis was more acid resistant at all test pHs (higher D values) than when grown in WR-GD. Glycerol appeared to be the culture medium component most responsible for lower levels of M. paratuberculosis acid resistance. When glycerol was substituted for OADC in the 7H9 medium, D values were significantly lower than those of 7H9-OADC-grown M. paratuberculosis and were approximately the same as those for M. paratuberculosis grown in WR-GD medium. Comparison of the SDS-PAGE protein profiles for M. paratuberculosis cultures grown in 7H9-OADC, WR-GD, or 7H9-GD medium revealed that increased expression of 34.2- and 14.0-kDa proteins was associated with higher levels of acid resistance of M. paratuberculosis grown in 7H9-OADC medium and that 56.6- and 41.3-kDa proteins were associated with lower levels of acid resistance. This is the first report showing that in vitro culture conditions significantly affect growth characteristics, acid resistance, and protein expression of M. paratuberculosis, and the results emphasize the importance of culture conditions for in vitro susceptibility studies.
Mycobacterium paratuberculosis, also known as Mycobacterium avium subsp. paratuberculosis, is the cause of Johne's disease, a widespread chronic granulomatous inflammatory bowel disease of dairy cattle and other ruminants (5, 6, 40). Several reports indicate a significant association of M. paratuberculosis with a similar inflammatory bowel disease of humans called Crohn's disease (2, 7, 11, 16, 23, 28). Whether this is a causal association remains controversial (17, 41).
There are a number of routes for human exposure to M. paratuberculosis. Foods of animal origin (and dairy products in particular) have received the most attention. Consequently, the ability of M. paratuberculosis to resist heat (such as during pasteurization) and chemical factors important in food preservation (such as low pH and high salt concentration) has become important in the field of M. paratuberculosis research (13-15, 19, 30, 34, 36, 37).
We previously reported rates of survival of M. paratuberculosis in a soft white Hispanic-style cheese made from M. paratuberculosis-spiked milk (37). The decimal reduction times (D values [i.e., times required for a 1-log10 reduction in viable counts]) were 36.5 and 59.9 days for cheese made from heat-treated and non-heat-treated M. paratuberculosis-spiked milk, respectively. Similar D values for M. paratuberculosis in Swiss hard (27.8 days) and semihard (45.5 days) cheeses were reported by Spahr and Schafroth (34).
Low pH appeared to be one of the factors in cheese production that are important in affecting M. paratuberculosis survival (37). The present study was undertaken to further characterize acid resistance of M. paratuberculosis and determine the degree to which in vitro culture conditions affect the outcome of pH resistance studies.
MATERIALS AND METHODS
M. paratuberculosis strains.
Three bovine M. paratuberculosis strains were used: two bovine clinical strains (JTC 114 and JTC 303) and one bovine laboratory-adapted strain (ATCC 19698). The clinical strains were isolated in our laboratory from Holstein cows with paratuberculosis and verified by PCR for presence of IS900 and dependency for growth on mycobactin, an iron-chelating agent.
Culture media.
The three liquid culture media used in this study were those commonly used for mycobacterial growth and were modified by the addition of 0.0002% (wt/vol) mycobactin J (Allied Monitor Inc., Fayette, Mo.) to support the growth of mycobactin-dependent M. paratuberculosis. Table 1 lists the composition of each medium in detail, but the primary difference among them was the carbon source: oleic acid (C17H33COOH) for the 7H9-OADC medium and glycerol for the WR-GD and 7H9-GD media. The 7H9-OADC medium was Middlebrook 7H9 medium (Difco, Detroit, Mich.) enriched with 10% (vol/vol) oleic acid-albumin-dextrose-catalase (OADC; Difco), and the WR-GD medium was modified Watson-Reid medium (29). The 7H9-GD medium was Middlebrook 7H9 medium (Difco) enriched with 6.3% (vol/vol) glycerol and 1.0% (wt/vol) d-dextrose.
TABLE 1.
Ingredients of 7H9-OADC, WR-GD, and 7H9-GD media
| Ingredient | % of indicated ingredient in:
|
||
|---|---|---|---|
| 7H9-OADC | WR-GD | 7H9-GD | |
| Monopotassium phosphate | 1.0 | 2.0 | 1.0 |
| Ferric ammonium citrate | 0.04 | 0.075 | 0.04 |
| Magnesium sulfate | 0.05 | 1.0 | 0.05 |
| Sodium chloride | 0.85 | 2.0 | 0.85 |
| Zinc sulfate | 0.001 | 0.01 | 0.001 |
| Calcium chloride | 0.0005 | 0.02 | 0.0005 |
| Mycobactin J | 0.0002 | 0.0002 | 0.0002 |
| Cobalt chloride | 0.002 | ||
| Ammonium hydrogen citrate | 2.0 | ||
| l-Asparagine | 5.0 | 5.0 | |
| l-Glutamic acid | 0.5 | 0.5 | |
| Disodium phosphate | 2.5 | 2.5 | |
| Copper sulfate | 0.001 | 0.001 | |
| Ammonium sulfate | 0.5 | 0.5 | |
| Sodium citrate | 0.1 | 0.1 | |
| Pyridoxine | 0.001 | 0.001 | |
| Biotin | 0.0005 | 0.0005 | |
| Dextrose | 2.0 | 10.0 | 10.0 |
| Glycerol | 63 | 63 | |
| Oleic acid (1% [wt/vol]) | 0.05 | ||
| Bovine serum albumin | 5.0 | ||
M. paratuberculosis seedlots.
All M. paratuberculosis strains were grown for 4 months at 37°C in 7H9-OADC (pH 6.8), WR-GD (pH 6.0), or 7H9-GD (pH 6.8) medium. The cultures were then centrifuged at 30,000 × g for 10 min, and the cell pellets were washed three times with 30 ml of 10 mM PBS (pH 6.8) by centrifugation (30,000 × g for 10 min). The M. paratuberculosis cell suspensions were homogenized with an overhead stirrer (Wheaton Instruments, Millville, N.J.) for 4 min on ice to break up large clumps of cells. The resulting suspensions were predominantly composed of small clumps and single cells of M. paratuberculosis (36). The homogenized cell suspensions were enumerated by a radiometric culture method (BACTEC) as explained below, stored in multiple small aliquots at −70°C, and used as the seedlots for subsequent production of M. paratuberculosis cells under the specified culture conditions studied.
Growth curves.
Growth curves for M. paratuberculosis were obtained for cultures grown in 7H9-OADC or WR-GD medium at pH 6.0 or 6.8 and adjusted using either 1 N HCl or 1 N NH4OH. Each medium (35 ml) was placed in a 250-ml tissue culture flask (Costar, Cambridge, Mass.) (75-cm2 canted neck), and 0.1 ml of M. paratuberculosis seedlot, originating from the same type of culture medium, was inoculated in the flasks (starting concentration, approximately 104 cells/ml). Culture flasks were sealed and incubated at 37°C without shaking. Two culture flasks were taken at each sampling time to measure the optical density (OD). To do this and avoid problems caused by extensive clumping of M. paratuberculosis cells, M. paratuberculosis was harvested and washed three times with 30 ml of 10 mM PBS (pH 6.8) by centrifugation (30,000 × g for 10 min). The washed cell pellets were homogenized as explained above and resuspended to the original culture medium volume (35 ml) with 10 mM PBS (pH 6.8). The OD was then measured at 630 nm. Growth curves were obtained using nonlinear regression analysis (Prism version 3.0; GraphPad Software, Inc., San Diego, Calif.) by plotting the OD values versus incubation time in days.
Menstruum.
Acetate buffer (50 mM) was adjusted (using 10 mM acetic acid and 10 mM sodium acetate) to four different test pH levels (3.0, 4.0, 5.0, and 6.0). These were used as working menstruums under acidic conditions for the M. paratuberculosis survival experiments. Changes in menstruum pH after autoclaving were made in units of <0.1 pH. Each working menstruum (9 ml) was placed in sterilized Wheaton glass vials (3 by 6 cm). During acid resistance tests the pH values of each menstruum were monitored (pH meter 125; Corning, Charlotte, N.C.) throughout the trial period.
Survival of M. paratuberculosis in acidic conditions.
Early-stationary-phase M. paratuberculosis cells from each of the six sets of culture medium conditions (i.e., 7H9-OADC, WR-GD, or 7H9-GD medium at pH 6.0 or 6.8) were harvested, washed, and homogenized. They were then exposed to each of four pH levels. Specifically, 1 ml of M. paratuberculosis cells was added to the Wheaton vials containing 9 ml of the working menstruum to achieve a final concentration of approximately 106 cells/ml. The Wheaton vials were sealed with sterile rubber septa and incubated at 20°C. After 0, 7, 14, 28, 56, and 85 days of incubation, two Wheaton vials from each treatment were selected and vortexed for 1 min. Duplicate aliquots (0.25 ml) were then obtained from each vial to enumerate viable M. paratuberculosis cells.
Enumeration methods.
Viable M. paratuberculosis cell numbers were estimated using a radiometric culture method (BACTEC) as previously described (36, 37). At each sampling time, the aliquots (0.25 ml) were inoculated into commercial BACTEC 12B bottles (Becton Dickinson Microbiologic Systems, Sparks, Md.) containing 1.0 ml of egg yolk suspension (Difco) and 0.1 ml of mycobactin J solution (Allied Monitor Inc.) (40 μg/ml). The bottles were incubated at 37°C without agitation and read on a BACTEC 460 instrument without CO2 daily for 80 days. The BACTEC 460 instrument measures 14CO2 gas produced by the metabolism of [14C]palmitate in the medium of the commercial BACTEC 12B bottles. From the total amount of CO2 gas produced (expressed as the cumulative growth index), the number of M. paratuberculosis cells inoculated into each vial is estimated by comparison to a standard growth model described by the following equation (22):
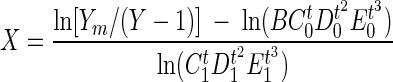 |
where Y is the cumulative growth response in units of 14CO2 released, Ym is a fixed value bounded by the maximum cumulative growth response (which is 12,950), X is the inoculum size (i.e., the number of viable M. paratuberculosis cells), t is the incubation time (in days), and letters B through E are regression coefficient constants determined as having the following values: B, 10,340; C0, 1.2217; C1, 0.84345; D0, 0.98959; D1, 1.004644; E0, 1.00008339; and E1, 0.99996559.
D value.
The D value was calculated from the slope of the best-fit line as graphically determined by plotting the log10 of the number of M. paratuberculosis survivors per milliliter versus exposure time for each pH tested, as previously described (36, 37). The best-fit lines were calculated by a linear regression analysis (Prism version 3.0; GraphPad Software, Inc.).
Statistical analysis.
Linear regression of inactivation curves for D value were determined on the basis of the concepts presented by Chatterjee and Price (3) and Draper and Smith (8). Differences among slopes of inactivation curves were analyzed using Prism software (GraphPad Software, Inc.) as previously described (36, 37). P values of <0.05 were considered significant.
Soluble proteins extracted from the cell pellet.
Early-stationary-phase M. paratuberculosis cells from the three culture media at two pH levels were pelleted and washed three times by centrifugation at 30,000 × g for 10 min with 30 ml of phosphate-buffered saline (10 mM, pH 6.8) containing 0.05% (vol/vol) protease inhibitor cocktail (Sigma, St. Louis, Mo.). Cell pellets were disrupted using glass beads (Glasperlen [0.10 to 0.11 mm in diameter]) and a cell homogenizer (model MSK; Braun Instruments, Burlingame, Calif.). Homogenization was performed for 5 min with discontinuous cooling by liquid CO2. After homogenization, the glass beads were recovered by centrifugation at 1,500 × g for 10 min. The homogenate was then transferred to another sterile tube and centrifuged at 30,000 × g for 1 h at 4°C. The supernatant of soluble proteins was concentrated (using Centriprep centrifuge PM-3 concentrators; Amicon, Beverly, Mass.) and filter sterilized (using Millipore filters [0.2-μm pore size]). Protein concentrations were determined with a bicinchoninic acid assay (Protein Assay kit; Pierce, Rockford, Ill.) (32, 33). Soluble protein extracts were stored at −70°C.
SDS-PAGE.
The soluble protein extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with commercial precast gels (Jule Biotechnologies, Inc., Milford, Conn.) under reducing conditions as described by Laemmli (21). Protein samples (30 μg) were mixed with Laemmli sample buffer (Bio-Rad, Hercules, Calif.) containing 200 mM dithiothreitol, boiled for 10 min, and loaded to each lane of the gradient gels. The gradient gels (10 to 20% [wt/vol] polyacrylamide; 1.5 mm thick; 16 by 18 cm) were placed in a vertical electrophoresis apparatus (Hoefer SE600; Amersham Pharmacia Biotech, Piscataway, N.J.). Gels were electrophoresed for 15 h at 15 mA per gel, and the protein bands were visualized by Coomassie brilliant blue staining.
Analysis and documentation of gels.
The Coomassie brilliant blue-stained SDS-PAGE gels were digitized (Digital Imaging System EDAS 290; Kodak, Rochester, N.Y.) to generate the protein band profiles. Each protein band was quantified for molecular net intensity, and the molecular weights were established (using computer software [1D image analysis software; Kodak, N.Y.]) on the basis of mobility compared to that of standard molecular weight markers (Bio-Rad).
RESULTS
Growth of M. paratuberculosis in 7H9-OADC and WR-GD media.
When grown in 7H9-OADC medium, cultures of M. paratuberculosis started as submerged granules. After roughly 20 days of incubation, log-phase growth began and the size of the submerged granules increased. At the commencement of the stationary-growth phase (at approximately 60 days of incubation), thin floating sheets of M. paratuberculosis cells, resembling sheets of ice on a lake, appeared on the surface of the broth cultures containing the submerged granules. A shorter lag phase, a higher growth rate, and larger M. paratuberculosis cell masses were observed in 7H9-OADC medium at pH 6.0 than at 6.8 (Fig. 1A). The beige color of M. paratuberculosis cultures growing in 7H9-OADC medium differed little regardless of medium pH or growth phase (Fig. 1C).
FIG. 1.
The growth curves for M. paratuberculosis JTC114 were obtained from cultures grown in 7H9-OADC (A) or WR-GD (B) medium at pHs 6.0 and 6.8. The photographs present surface morphologies in liquid culture flasks of M. paratuberculosis JTC114 at the stationary phase in 7H9-OADC (C) or WR-GD (D) medium at pH 6.0.
The appearance and rate of growth of M. paratuberculosis in WR-GD medium were remarkably different from those in 7H9-OADC, but the lag phase was longer: 40 and 60 days in WR-GD at pH 6.0 and 6.8, respectively. M. paratuberculosis again formed submerged granules during the lag phase; however, in this medium yellow floating cells began forming in the early log phase of growth. The stationary-growth phase in WR-GD medium at pH 6.0 and 6.8, respectively, was reached at 110 and 130 days (Fig. 1B). At this stage of growth a confluent, thick, wax-like, yellow, floating pellicle covered the surface of the medium (Fig. 1D). As was seen with 7H9-OADC medium, when the medium was at pH 6.0 versus 6.8 M. paratuberculosis cultures grew faster during log-phase growth and produced a larger cell mass.
Acid resistance.
Fig. 2 shows (as a representative example) the inactivation curves for M. paratuberculosis strain JTC 303 in acetate buffer (pH 5.0) when grown in 7H9-OADC, WR-GD, or 7H9-GD medium at pH 6.0 (panel A) or 6.8 (panel B). All inactivation curves for M. paratuberculosis strains tested were linear (r2 > 0.85), with narrow 95% confidence intervals in all test pH conditions. When organisms were produced in 7H9-OADC medium, the D values for the laboratory-adapted ATCC strain of M. paratuberculosis were significantly (P < 0.05) different from those of the two clinical M. paratuberculosis strains: D values for the ATCC strain were higher at test pHs 3 and 6 but lower for test pHs 4 and 5 (data not shown). However, when M. paratuberculosis was cultured in WR-GD medium, no significant (P > 0.1) difference in acid resistance was found between the ATCC and the clinical strains (data not shown).
FIG. 2.
Inactivation curves for M. paratuberculosis strain JTC 303 in acetate buffer (50 mM; pH 5) at 20°C. This strain was grown in 7H9-OADC, WR-GD, or 7H9-GD medium at pH 6.0 (A) or 6.8 (B) to reach the early stationary phase.
Culture medium pH had no significant effects on acid resistance of M. paratuberculosis. That is, although M. paratuberculosis exhibited faster growth and more abundant cell mass production at pH 6.0 than at 6.8 in both 7H9-OADC and WR-GD media (Fig. 1), the D values for M. paratuberculosis bovine clinical strains grown at pH 6.0 or 6.8 in all media used were not significantly different at any test pH (P > 0.1) except in 7H9-OADC medium at test pH 3 (P < 0.05) (data not shown).
Average D values for the clinical strains of M. paratuberculosis are shown in Table 2. M. paratuberculosis survival was shorter at lower test pH levels, with D values ranging from 2.5 days at test pH 3 to 25.9 days at test pH 6. As the test pH increased, so did the D values in 7H9-OADC and 7H9-GD media but not in WR-GD medium: a 1-U increase in test pH in 7H9-OADC and 7H9-GD media resulted in an approximate doubling of D values. M. paratuberculosis produced in WR-GD medium consistently had lower D values than did M. paratuberculosis produced in 7H9-OADC medium regardless of the growth medium pH.
TABLE 2.
Average D values for two bovine clinical M. paratuberculosis strains cultured in 7H9-OADC, WR-GD or 7H9-GD medium at two initial culture medium pHs and tested at pHs 3, 4, 5, and 6
| Test pH | Culture pH | Average D value (days) ± SD
|
||
|---|---|---|---|---|
| 7H9-OADC | WR-GD | 7H9-GDa | ||
| 3 | 6.0 | 4.8 ± 0.3b | 4.7 ± 0.5 | 2.5 |
| 6.8 | 6.0 ± 1.1b | 4.8 ± 0.2 | 2.6 | |
| 4 | 6.0 | 11.8 ± 0.9 | 5.8 ± 0.7 | 5.0 |
| 6.8 | 11.4 ± 0.5 | 6.0 ± 0.6 | 5.4 | |
| 5 | 6.0 | 15.7 ± 0.3 | 7.2 ± 0.6 | 10.1 |
| 6.8 | 18.9 ± 0.4 | 7.4 ± 0.4 | 10.2 | |
| 6 | 6.0 | 23.1 ± 0.6 | 19.8 ± 0.9 | NTc |
| 6.8 | 25.9 ± 2.3 | 19.9 ± 0.8 | NT | |
D values in 7H9-GD medium were calculated on the basis of results for a single bovine clinical strain (M. paratuberculosis JTC 303).
D values were significantly different at initial culture pHs.
NT, not tested.
As described above, glycerol and dextrose (GD) were substituted for OADC in the 7H9 formulation, resulting in 7H9-GD medium. The D values for M. paratuberculosis strain JTC 303 in 7H9-GD were unaffected by the culture medium pH (Table 2). The D values for M. paratuberculosis strain JTC303 produced in 7H9-GD in two culture pH conditions were significantly (P < 0.02) lower than those for the strain in 7H9-OADC medium in all test pH conditions. However, the D values in 7H9-GD medium were significantly lower at test pH 3 (P < 0.02), the same at test pH 4 (P > 0.2), and higher at test pH 5 (P < 0.05) compared to those of M. paratuberculosis grown in WR-GD medium.
SDS-PAGE protein profiles.
Multiple protein expression differences were seen. These differences were correlated with the type of M. paratuberculosis culture medium. WR-GD medium supported the highest number of protein bands: 45, 55, and 40 bands were found when stationary-growth-phase M. paratuberculosis strain JTC303 was cultured in 7H9-OADC, WR-GD, and 7H9-GD media, respectively (Fig. 3).
FIG. 3.
(Upper panel) SDS-PAGE soluble protein profiles for M. paratuberculosis strain JTC303 grown at pH 6.0 or 6.8 in 7H9-OADC, WR-GD, or 7H9-GD medium. The amount of total soluble proteins applied per lane was 30 μg. STD, standard. (Lower panel) Relative intensity levels of the protein bands for the strain grown in glycerol-containing media (WR-GD and 7H9-GD) versus 7H9-OADC medium.
Three prominent proteins (70.0, 23.1, and 11.3 kDa) were seen in stationary-growth-phase M. paratuberculosis cells produced in all media at both culture pHs 6.0 and 6.8. No notable differences in protein expression profiles between culture medium pHs were observed, except that expression for the 34.2-kDa protein in 7H9-OADC medium was higher at pH 6.8 than at 6.0.
Expression of two proteins (56.5 and 41.3 kDa) appeared to be up-regulated in both glycerol-containing media (WR-GD and 7H9-GD) compared to that seen with 7H9-OADC medium. The relative intensities for another two proteins (28.6 and 19.1 kDa) were about 1.5-fold higher in 7H9-OADC and 7H9-GD media than in WR-GD medium. One protein of low molecular mass (14.0 kDa) was highly expressed in the fatty acid-based medium (7H9-OADC) compared to the expression levels seen with the glycerol-based media (WR-GD and 7H9-GD). Expression (higher at culture pH 6.8 than at 6.0 in 7H9-OADC medium) of a 34.2-kDa protein was down-regulated in the glycerol-based media (WR-GD and 7H9-GD).
DISCUSSION
The growth rate, quantity, and visual appearance of M. paratuberculosis cultured in 7H9-OADC were strikingly different from those observed when the organism was grown in WR-GD medium (Fig. 1). These observations correlate well with reports on other mycobacteria; for the growth of Mycobacterium phlei (39) and Mycobacterium tuberculosis (25), glycerol-containing media supported higher levels of pellicle formation than did media in which glucose was the sole carbon source. Defining the optimal ingredients and pH for a growth medium for the cultivation of an organism depends upon the objective. Common objectives include early growth detection (important for infection diagnosis by culture), maximum growth rate during log phase, and maximum cell yield (important for vaccine or antigen production). We found that 7H9-OADC medium at pH 6.0 was optimal for diagnosis by cultures, since among the culture conditions we tested, these supported the fastest growth rate for M. paratuberculosis. However, since WR-GD medium at pH 6.0 supported the highest level of cell mass production, it may represent the optimal conditions (when the necessary antigens are present in the medium) for vaccine and antigen production of M. paratuberculosis.
Culture medium composition clearly affects the physiology of M. paratuberculosis and influences acid resistance in vitro. No previous study has shown the impact of culture medium on acid or thermal resistance of M. paratuberculosis. The study of Spahr and Schafroth investigating M. paratuberculosis survival in cheese used Middlebrook 7H9-OADC medium supplemented with 0.5% (vol/vol) glycerol (34). The thermal resistance of M. paratuberculosis had been previously reported but with widely different results for growth of the organism in different types of medium such as 7H9 medium (35), 7H9-ADC medium (30), 7H9-OADC medium (19, 36, 37), 7H9-OADC medium supplemented with 0.5% (vol/vol) glycerol (13), and WR-GD medium (18). Klijn et al. (20) and Lund et al. (26) reviewed possible reasons for the differences between these results and those from previously published thermal resistance studies of M. paratuberculosis. Although they delineated many critical factors influencing results of the thermal resistance studies (such as application of heat, preparation of inoculum, disruption of clumping, and recovery methods after heat treatment), the effect of culture medium on the production of M. paratuberculosis was not mentioned. In the present report, we have shown that in the vitro culture medium significantly influenced the growth rate, the acid resistance, and the cellular protein expression of M. paratuberculosis.
Results indicate that laboratory-adapted strains such as M. paratuberculosis ATCC 19698 may behave somewhat differently from low-passage-number clinical strains when cultured in 7H9-OADC medium and thus may not be the best strains to use for in vitro studies designed to predict the survival rates of organisms subjected to various food processing methods. Differences in the thermal tolerance of laboratory and clinical M. paratuberculosis strains were previously observed (4, 36). D values for the two clinical strains in the present study were not significant at any test pH.
Excepting the results seen with 7H9-OADC medium at test pH 3, the culture medium pH did not significantly affect survival of M. paratuberculosis subjected to low pH regardless of the culture medium used to produce the organism for testing. Instead, the medium composition affected pH survival characteristics.
M. paratuberculosis cultured in WR-GD medium was more susceptible to the lethal effects of low pH (lower D values) than was the organism after growth in 7H9-OADC medium. The presence of glycerol in the medium apparently affected the acid resistance of M. paratuberculosis. This finding differs from what would be expected, in that glycerol has been reported to increase the acid-fast staining intensity, cell wall lipid content, and antimicrobial resistance of mycobacteria, presumably by fostering production of a thicker, more impervious cell wall (9, 10, 25, 31, 39).
M. paratuberculosis was relatively resistant to killing by acidic conditions. The average D values for bovine clinical M. paratuberculosis strains grown in 7H9-OADC medium and tested at pHs 4, 5, and 6 were 11.6 ± 0.6, 17.3 ± 1.9, and 24.5 ± 2.1 days, respectively. These D values are very similar to those we reported previously for 7H9-OADC medium (pH 6.8)-grown M. paratuberculosis (37) but higher than those previously reported for other bacterial pathogens. Acid survival D values for Yersinia enterocolitica O:3 were from 0.4 to 0.6 days at test pH 4 (24), and D values reported for Listeria monocytogenes were 0.5, 1.2, and 3.6 days at test pH 4, 5, and 6, respectively (1).
Protein profiles of M. paratuberculosis in the present study suggest a possible association of certain up-regulated proteins and acid resistance. The association of protein expression with acid resistance has been observed in other bacteria, including mycobacteria (12, 27, 29, 38). For example, Brucella melitensis, an intracellular pathogen, exhibited 15 up- and 18 down-regulated protein spots on two-dimensional gels when exposed to pH 5.5 (38). In addition, O'Brien et al. (29) showed that after adapting Mycobacterium smegmatis to pH 5.0, survival after challenge with a lethal pH of 3.5 was dependent on protein synthesis.
It is not clear whether M. paratuberculosis also possesses the adaptive acid resistance response, since the organism (when cultured at pHs 6.0 and 6.8 in both 7H9-OADC and WR-GD media) had statistically the same D values at all test pHs. Instead, medium composition differences between the 7H9-OADC and WR-GD media used for production of M. paratuberculosis cells appeared to be the largest single determinant of organism acid resistance, with 7H9-OADC-grown M. paratuberculosis being the most acid resistant.
Comparison of SDS-PAGE protein profiles of M. paratuberculosis grown in 7H9-OADC and WR-GD media revealed four up-regulated proteins in 7H9-OADC (34.2, 28.6, 19.1, and 14.0 kDa). Substitution of GD for OADC in the 7H9 medium formulation reduced M. paratuberculosis resistance to the same low level as that seen with M. paratuberculosis grown in WR-GD medium. Two of the four aforementioned 7H9-OADC up-regulated proteins (namely, 28.1 and 19.1 kDa) were also up-regulated in the 7H9-GD medium. The 34.2- and 14.0-kDa proteins may be associated with higher acid resistance of M. paratuberculosis and are deserving of further study. Two other proteins up-regulated in glycerol-containing media (56.6 and 41.3 kDa) may be associated with lower acid resistance.
In summary, M. paratuberculosis is quite resistant to acidic environments, but the level of resistance is affected by in vitro culture conditions. Acid resistance of low-passage-number clinical strains may be more reflective of naturally occurring M. paratuberculosis than of laboratory-adapted strains. Additionally, acid resistance measurements in vitro may not reliably predict the survival curves of M. paratuberculosis in foods that depend on acidic conditions to control microbial growth, as we observed previously (37). Hence, multiple factors must be considered when designing in vitro acid resistance studies in hopes of extrapolating results to food. Lastly, this work provides interesting leads for specific M. paratuberculosis proteins that may be involved in acid resistance or susceptibility.
Acknowledgments
We thank Elizabeth Manning, Department of Pathobiological Sciences, the University of Wisconsin—Madison, for valuable comments and critical review of the manuscript.
This research was funded in part by the USDA-NRI Competitive Grant Program (project WIS 04405).
REFERENCES
- 1.Buchanan, R. L., M. H. Golden, and R. C. Whiting. 1993. Differentiation of the effects of pH and lactic or acetic acid concentration on the kinetics of Listeria monocytogenes inactivation. J. Food Prot. 56:474-478. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlin, W., D. Y. Graham, K. Hulten, H. M. El Zimaity, M. R. Schwartz, S. Naser, I. Shafran, and F. A. El Zaatari. 2001. Review article: Mycobacterium avium subsp. paratuberculosis as one cause of Crohn's disease. Aliment. Pharmacol. Ther. 15:337-346. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee, S., and B. Price. 1991. Regression analysis by example, p. 107-116. John Wiley & Sons, New York, N.Y.
- 4.Chiodini, R. J., and J. Hermon-Taylor. 1993. The thermal resistance of Mycobacterium paratuberculosis in raw milk under conditions simulating pasteurization. J. Vet. Diagn. Investig. 5:629-631. [DOI] [PubMed] [Google Scholar]
- 5.Chiodini, R. J., H. J. Van Kruiningen, and R. S. Merkal. 1984. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 74:217-262. [PubMed] [Google Scholar]
- 6.Clarke, C. J. 1997. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J. Comp. Pathol. 116:217-261. [DOI] [PubMed] [Google Scholar]
- 7.Collins, M. T., G. Lisby, C. Moser, D. Chicks, S. Christensen, M. Reichelderfer, N. Hoiby, B. A. Harms, O. O. Thomsen, U. Skibsted, and V. Binder. 2000. Results of multiple diagnostic tests for Mycobacterium avium subsp. paratuberculosis in patients with inflammatory bowel disease and in controls. J. Clin. Microbiol. 38:4373-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draper, N. R. and H. Smith. 1981. Applied regression analysis, p. 241-257. John Wiley & Sons, New York, N.Y.
- 9.Dubos, R. J., and G. Middlebrook. 1947. Media for tubercle bacilli. Am. Rev. Tuberc. 56:334-345. [PubMed] [Google Scholar]
- 10.Dubos, R. J., and G. Middlebrook. 1948. The effect of wetting agents on the growth of tubercle bacilli. J. Exp. Med. 88:83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Zaatari, F. A. K., M. S. Osato, and D. Y. Graham. 2001. Etiology of Crohn's disease: the role of Mycobacterium avium paratuberculosis. Trends Mol. Med. 7:247-252. [DOI] [PubMed] [Google Scholar]
- 12.Foster, J. W. 2000. Microbial responses to acid stress, p. 99-115. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 13.Gao, A., L. Mutharia, S. Chen, K. Rahn, and J. Odumeru. 2002. Effect of pasteurization on survival of Mycobacterium paratuberculosis in milk. J. Dairy Sci. 85:3198-3205. [DOI] [PubMed] [Google Scholar]
- 14.Grant, I. R., H. J. Ball, and M. T. Rowe. 2002. Incidence of Mycobacterium paratuberculosis in bulk raw and commercially pasteurized cows' milk from approved dairy processing establishments in the United Kingdom. Appl. Environ. Microbiol. 68:2428-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant, I. R., E. I. Hitchings, A. McCartney, F. Ferguson, and M. T. Rowe. 2002. Effect of commercial-scale high-temperature, short-time pasteurization on the viability of Mycobacterium paratuberculosis in naturally infected cows' milk. Appl. Environ. Microbiol. 68:602-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green, E. P., M. L. V. Tizzard, M. T. Moss, J. Thompson, J. J. Winterbourne, J. J. McFadden, and J. Hermon-Taylor. 1989. Sequence and characteristics of IS900, an insertion element identified in a human Crohn's disease isolate of Mycobacterium paratuberculosis. Nucleic Acids Res. 17:9063-9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris, J. E., and A. M. Lammerding. 2001. Crohn's disease and Mycobacterium avium subsp. paratuberculosis: current issues. J. Food Prot. 64:2103-2110. [DOI] [PubMed] [Google Scholar]
- 18.Hope, A. F., P. A. Tulk, and R. J. Condron. 1996. Pasteurization of Mycobacterium paratuberculosis in whole milk, p. 377-382. In R. J. Chiodini, M. E. Hines II, and M. T. Collins (ed.), Proceedings of the Fifth International Colloquium on Paratuberculosis. International Association for Paratuberculosis, Rehoboth, Mass.
- 19.Keswani, J., and J. F. Frank. 1998. Thermal inactivation of Mycobacterium paratuberculosis in milk. J. Food Prot. 61:974-978. [DOI] [PubMed] [Google Scholar]
- 20.Klijn, N., A. A. Herrewegh, and P. de Jong. 2001. Heat inactivation data for Mycobacterium avium subsp. paratuberculosis: implications for interpretation. J. Appl. Microbiol. 91:697-704. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Lambrecht, R. S., J. F. Carriere, and M. T. Collins. 1988. A model for analyzing growth kinetics of a slowly growing Mycobacterium sp. Appl. Environ. Microbiol. 54:910-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lisby, G., J. Andersen, K. Engbaek, and V. Binder. 1994. Mycobacterium paratuberculosis in intestinal tissue from patients with Crohn's disease demonstrated by a nested primer polymerase chain reaction. Scand. J. Gastroenterol. 29:923-929. [DOI] [PubMed] [Google Scholar]
- 24.Little, C. L., M. R. Adams, and M. C. Easter. 1992. The effect of pH, acidulant and temperature on the survival of Yersinia enterocolitica. Lett. Appl. Microbiol. 14:148-152. [Google Scholar]
- 25.Long, E. R., and L. L. Finner. 1927. The relation of glycerol in culture media to the growth and chemical composition of tubercle bacilli. Am. Rev. Tuberc. 16:523-529. [Google Scholar]
- 26.Lund, B. M., G. W. Gould, and A. M. Rampling. 2002. Pasteurization of milk and the heat resistance of Mycobacterium avium subsp. paratuberculosis: a critical review of the data. Int. J. Food Microbiol. 77:135-145. [DOI] [PubMed] [Google Scholar]
- 27.Merrell, D. S., and A. Camilli. 2002. Acid tolerance of gastrointestinal pathogens. Curr. Opin. Microbiol. 5:51-55. [DOI] [PubMed] [Google Scholar]
- 28.Naser, S. A., D. Schwartz, and I. Shafran. 2000. Isolation of Mycobacterium avium subsp. paratuberculosis from breast milk of Crohn's disease patients. Am. J. Gastroenterol. 95:1094-1095. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien, L. M., S. V. Gordon, I. S. Roberts, and P. W. Andrew. 1996. Response of Mycobacterium smegmatis to acid stress. FEMS Microbiol. Lett. 139:11-17. [DOI] [PubMed] [Google Scholar]
- 30.Pearce, L. E., H. T. Truong, R. A. Crawford, G. F. Yates, S. Cavaignac, and G. W. de Lisle. 2001. Effect of turbulent-flow pasteurization on survival of Mycobacterium avium subsp. paratuberculosis added to raw milk. Appl. Environ. Microbiol. 67:3964-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratledge, C. 1976. The physiology of the mycobacteria. Adv. Microb. Physiol. 13:115-244. [DOI] [PubMed] [Google Scholar]
- 32.Redinbaugh, M. G., and R. B. Turley. 1986. Adaptation of the bicinchoninic acid protein assay for use with microtiter plates and sucrose gradient fractions. Anal. Biochem. 153:267-271. [DOI] [PubMed] [Google Scholar]
- 33.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 34.Spahr, U., and K. Schafroth. 2001. Fate of Mycobacterium avium subsp. paratuberculosis in Swiss hard and semihard cheese manufactured from raw milk. Appl. Environ. Microbiol. 67:4199-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stabel, J. R., E. M. Steadham, and C. A. Bolin. 1997. Heat inactivation of Mycobacterium paratuberculosis in raw milk: are current pasteurization conditions effective? Appl. Environ. Microbiol. 63:4975-4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung, N., and M. T. Collins. 1998. Thermal tolerance of Mycobacterium paratuberculosis. Appl. Environ. Microbiol. 64:999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung, N., and M. T. Collins. 2000. Effect of three factors in cheese production (pH, salt, and heat) on Mycobacterium avium subsp. paratuberculosis viability. Appl. Environ. Microbiol. 66:1334-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teixeira-Gomes, A. P., A. Cloeckaert, and M. S. Zygmunt. 2000. Characterization of heat, oxidative, and acid stress responses in Brucella melitensis. Infect. Immun. 68:2954-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tepper, B. S. 1968. Differences in the utilization of glycerol and glucose by Mycobacterium phlei. J. Bacteriol. 95:1713-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valentin-Weigand, P., and R. Goethe. 1999. Pathogenesis of Mycobacterium avium subspecies paratuberculosis infections in ruminants: still more questions than answers. Microbes Infect. 1:1121-1127. [DOI] [PubMed] [Google Scholar]
- 41.Van Kruiningen, H. J. 1999. Lack of support for a common etiology in Johne's disease of animals and Crohn's disease in humans. Inflamm. Bowel Dis. 5:183-191. [DOI] [PubMed] [Google Scholar]