Abstract
The adhesion of nonflagellated Escherichia coli strain K-12 to polystyrene (PS) latex spheres or glass capillaries has been observed by using several techniques. Attention was focused on the orientation of the rod-shaped bacteria as they adhered to the surfaces in 100 mM phosphate-buffered saline. Data show that PS particles adhered to the ends of the bacteria more than 90% of the time. Moreover, the PS particles adhered to one end only, never to both. Similarly, for experiments with bacteria adhering to glass, the bacteria adhered on their ends. In order to determine whether the end of a bacterium had a different charge density from that of the middle, rotational electrophoresis experiments were used. These experiments indicated no measurable charge nonuniformity. In order to examine how strongly adhered the bacteria were to the PS particles, differential electrophoresis was used. Almost always, bacteria were found to be irreversibly adhered to the PS spheres. The cause of the oriented adhesion is not likely due to surface lipopolysaccharides (LPS), since the three strains of K-12 that were used, each having a different length of LPS, showed similar behavior. The results are discussed in terms of bacterial cell polarity. The data indicate that nanodomains on the bacterial ends are important for adhesion and that the time scale for irreversible adhesion is short.
Bacterial adhesion and biofilms are a critical problem for in situ bioremediation (25, 12), heat exchanger fouling (18), biomaterial infections (9), and ship hull drag increase (8, 18). Prior to the anchoring of a bacterium onto a surface (28), a number of initial adhesion events must occur as a bacterium approaches a surface. First, the bacterium must overcome the repulsive electrostatic forces (40, 36). This is at least partly done by the attractive van der Waals forces (27, 35), although the hydrophobic forces, which are less well understood, are often important in causing attractive forces between bacteria and surfaces (47). These initial adhesion events occur over length scales of the order of 100 nm [i.e., O(100 nm)] and time scales of O(seconds), rather than over a few nanometers and hours, like long-term adhesion processes (39). Not until these initial events have occurred can long-term events such as surface conformational changes (11), extracellular polymeric substance production (44, 53), DNA production (52), and cell signaling (14) dominate the adhesion process.
In this work we show that bacterial surface nonuniformities play an important role in adhesion. Such nonuniformities could result from bacterial polarity, which means that specialized structures or dynamic molecular localization occurs at or near the ends of the cell (42). While bacterial polarity has been shown to be important in cell division (24) and cell life cycle (2), bacterial polarity has been studied with regard to adhesion primarily for specific adhesion events (29), not for physical forces only. The mechanism of how bacterial polarity affects adhesion is not known. One possibility is surface charge nonuniformity, which is the localization of charged regions on a length scale of O(10 nm) or O(100 nm). Charge nonuniformity has been shown to affect adhesion in colloidal systems (17), and it could arise on bacterial surfaces if molecules at the bacterial surface localized on a larger-than-molecular scale.
Techniques to measure and observe initial bacterial adhesion events are usually macroscopic in nature, and they are better suited to examining populations than probing effects of individual bacterial cell surface polarity. Incubation and rinse (19, 46), column flow (4, 38), and flow chamber experiments (21, 45) cannot be used to study bacteria on a submicrometer level. The technique of atomic force microscopy (AFM) is a powerful tool that has been used to study individual bacteria and bacterial adhesion, and researchers have recently examined polymer and extracellular polymeric substance attachment strength related to bacterial adhesion (7, 41). However, the method has not been used to characterize the adhesion of the ends of rod-shaped bacteria (6, 37). There are several challenges to using AFM to study bacterial adhesion in more depth. (i) The adhered portion of the bacterium faces away from the AFM tip and is therefore very difficult to probe. (ii) Only the central region of the bacterium can be studied, since the tip slides off the edges. (iii) Bacterial measurements on soft surfaces are difficult to conduct and to interpret (50, 51).
The literature contains several references concerning oriented bacterial adhesion to particles or surfaces. Several researchers have examined the role of flagella, which can cause oriented bacterial adhesion. Marshall et al. observed the preferential orientation, dependent upon motility, of a flagellated Pseudomonas strain attaching to a surface (28). A Pseudomonas strain was observed by Fletcher to attach to hydrophilic surfaces via flagella, while assuming random orientations on hydrophobic surfaces (20). McClaine and Ford have found that flagellar rotation increases attachment rates of Escherichia coli bacteria to glass (30, 31), although it could not be ascertained whether the bacteria were adhering by their cell bodies or by their flagella. Other researchers have observed polar (oriented) adhesion due to specific mechanisms (5), such as adhesin-mediated adherence to fibronectin (10), lectin-mediated adherence to Sepharose beads covalently derivatized with lactose (26), or pilus adherence to tracheal cells (54). Using a synthesized wettability gradient surface for nonflagellated bacteria, Ellen et al. have shown that Treponema denticola adheres flat onto hydrophobic surfaces but adheres on its ends to hydrophilic surfaces (15). More recently, Haruff et al. caused rod-shaped Klebsiella pneumoniae organisms to adhere in specific orientations by positioning them with a laser trap (22). In the present study, we examined the oriented adhesion of nonflagellated E. coli to PS latex spheres that have only sulfate charge groups and that were not treated to promote adhesion.
We combined four methods for examining bacterial adhesion. (i) Video microscopy orientation experiments reveal not only whether a bacterium is adhered to a particle or a surface, but they also reveal the precise location on a bacterium where the adherence occurs. (ii) Differential electrophoresis allows us to measure bacterium-particle attractive forces with subpiconewton resolution, up to a force of about 50 pN. This technique can thus discern irreversible adhesion. (iii) Rotational electrophoresis enables us to determine whether the bacteria are uniformly charged, which might be important since we are considering bacterial polarity. (iv) Shear swaying experiments enable us to visualize whether bacteria adhere to a flat surface by their ends or by their entire surface.
MATERIALS AND METHODS
Bacteria.
Three strains of Escherichia coli K-12—D21f2 and D21 (obtained from the E. coli Genetic Stock Center [Department of Biology, Yale University, New Haven, Conn.]) and JM109 (donated by Shahriar Mobashery [Wayne State University, Detroit, Mich.])—were studied. The wild type is D21. The D21f2 strain has a shorter lipopolysaccharide (LPS) chain on the surface, while the JM109 strain has a longer LPS chain. All bacteria were grown in a shaker incubator at 150 rpm and 37°C in Miller's Luria broth, and they were harvested in mid-exponential growth. All three strains were nonmotile and nonflagellated. While E. coli K-12 organisms have peritrichous flagella under many conditions (1), we saw no motility in our experiments other than Brownian motion, and neither transmission electron micrographs nor scanning electron micrographs revealed flagella on the bacteria under our growth conditions (M. Elimelech, personal communication).
Doubling times were determined from growth curves conducted in our laboratory. These were 39 min for JM109, 35 min for D21, and 28 min for D21f2. Cells were prepared for experimentation by washing them in the suspending media three times with centrifugation (Sorvall Biofuge Primo) at 5,000 rpm (3,466 × g) for 10 min. The bacterial solution was then prepared for experimentation. All three strains were found to be >90% viable after 30 min (the approximate time required for our experiments) in solution by using a Live/Dead BacLight (kit L-7007, lot 02A1-3; Molecular Probes, Eugene, Oreg.). The temperature during the experiments was typically 20 to 25°C, with the temperature holding constant to within 0.5°C during each experiment.
Experimental techniques.
A Nikon TE 300 Eclipse inverted optical microscope coupled with video equipment (a Cohu 4010 charge-coupled device VHS camera) was used for all experiments with a ×100 magnification oil objective and differential interference contrast for visualization of the bacteria. Experiments were conducted in 100 mM phosphate-buffered saline (PBS) solutions consisting of potassium hydrogen phosphate and potassium dihydrogen phosphate dissolved in Milli-Q water. Glassware for the experiments was cleaned by sonication, soaked in 16 N nitric acid for 24 h, and rinsed with Milli-Q water.
Bacteria were mixed by hand-shaking them with 1.5-μm sulfated PS particles (batch no. 6951; Interfacial Dynamics Corporation, Portland, Oreg.). The bacteria and PS particles were then able to undergo Brownian aggregation in a 0.2-by-2.0-mm (inside dimensions) capillary tube (Vitrocom, Mountain Lakes, N.J.). The capillary was mounted on a standard 25-by-75-mm microscope slide, and we observed the resulting couplets by using video microscopy, recording whether the PS particles adhered to the middle or ends of the bacteria (Fig. 1). A motorized stage (Prior) allowed a linear progression across the length of the capillary tube so that couplets were not recorded twice.
FIG. 1.
Bacterium-particle couplets. (a) One of the rare couplets for which the PS latex particles adhere to the center region of the bacterium. (b) One sphere adhered to the end of a bacterium. (c) Two spheres adhered to one end of a bacterium. (d) Three spheres adhered to the end of a bacterium. Note that particles never adhere to both ends.
For observations of bacteria adhering to glass surfaces, solutions containing only bacteria in PBS were placed in the glass microelectrophoresis cell (48) and allowed to contact the glass surface. A low-intensity electric field (∼1 V/cm) was applied and then reversed; the electro-osmotic flow within the electrophoresis cell produced a gentle shear field that caused many bacteria to sway back and forth. Thus, we could determine whether the bacteria were adhered, and moreover, whether they were adhered on one end.
For the D21 strain, adhesion forces were measured between the bacterial cells and the PS sphere in many couplets by using differential electrophoresis. Differential electrophoresis is a technique that exploits differences in zeta (ζ) potential of colloidal species to measure the attractive forces between the two moieties (3, 43, 48). Since the bacterium and the PS sphere composing the couplet have different ζ potentials, they tend to move at different velocities in an applied electric field (E0). As the magnitude of E0 increases, a stronger tension force is applied to the couplet. In our experiments, we located a particle-bacterium couplet, applied an electric field (E0), and incrementally increased its strength. The couplet was then monitored visually. This process was continued until either the couplet broke or the couplet escaped the viewing plane. Figure 2 shows the only particle-bacterium couplet that broke in all our experiments with PS particles. From the value of E0 and the ζ potentials of the two parts of the couplet, a value for the attractive force (F) holding together the bacterium and particle can be estimated by using the equation (described previously in reference 48) F = 8.76πaɛ|ζ2 − ζ1|E0, where a is an average particle radius; ɛ is the fluid permittivity for water, and the subscript 1 and 2 represent the bacterium and the particle, respectively. This equation was derived for two spherical particles and can therefore give only estimates for the bacterium-PS sphere couplets. Importantly, this relationship does not depend upon any particular model for interparticle forces. In order to interpret our experiments, we chose an a value of 0.75 μm. The ζ potentials on the bacteria and the PS particles were measured with a Brookhaven Zeta PALS analyzer.
FIG. 2.
Differential electrophoresis image of PS particle-bacterium couplet breaking. The images are taken approximately 0.5 s apart. Of the 25 couplets that we have observed, this is the only couplet that broke.
The fact that bacteria adhere by one end indicated that the bacteria were perhaps nonuniformly charged, and so we used the technique of rotational electrophoresis to measure the charge nonuniformity on the bacterial surfaces (16, 17, 49). The essence of this technique is that particles that are uniformly charged do not rotate by electrophoresis, regardless of shape (34). However, nonuniformly charged particles will rotate by electrophoresis, and we have recently developed the experimental and theoretical tools with which to measure and interpret charge nonuniformity (16, 17, 49). Thus, if we see a bacterium rotating in an applied electric field, we know that this bacterium is nonuniformly charged, and we can interpret the angular velocity in terms of a standard deviation of ζ potential over the bacterial surface.
RESULTS
Orientation observations.
Initially on the basis of observations with differential electrophoresis, the bacteria (E. coli D21) were found to adhere to the colloidal PS particles most often in an end-on fashion (Fig. 1b through d). Only rarely did the PS particles adhere to the central section of a bacterium (Fig. 1a). Because of the size comparison of the particles to that of the bacteria, adhesion to the ends of the bacteria versus the middle was visually unambiguous. In the systematic studies, we found that an overwhelming majority (>90%) of all three strains of bacteria adhered by their ends to the PS particles (Table 1). Furthermore, only one of the ends of the bacteria adhered to the PS particles; out of 246 couplets observed, no bacterium was found with particles adhered to both ends. In fact, for many couplets, several particles were adhered to one end of a bacterium (Fig. 1c and d) but never to both.
TABLE 1.
Adhesion statistics for E. coli K-12 to PS latex spheresa
| Strain | No. of PS spheres adhering to E. coli bacteria
|
||||||
|---|---|---|---|---|---|---|---|
| Middle | Single PS on end | Multiple PS on end | PS on both ends | Total on end | Total couplets | % On end | |
| D21 | 9 | 122 | 18 | 0 | 140 | 149 | 93.3 |
| D21f2 | 4 | 47 | 3 | 0 | 50 | 54 | 92.6 |
| JM109 | 3 | 39 | 1 | 0 | 40 | 43 | 93.0 |
The columns indicate whether the PS latex sphere adhered to the middle of the bacterium or on the ends. Furthermore, the table distinguishes between having one sphere adhered on the end versus multiple spheres. Note that in no case did PS spheres ever adhere on both ends.
Attachment of the bacteria to the glass capillary was also observed to occur in an end-on geometry. This geometry was observed by applying a small electric field and observing the bacteria swaying in the electroosmotic flow. Figure 3a shows two bacteria attached to a glass surface in the absence of flow, while Fig. 3b and c show the same bacteria in the presence of a flow field. The bacteria are anchored by one end but otherwise sway in the flow.
FIG. 3.
Swaying experiment images. (a) Bacteria attached to glass surface under static conditions. (b) Bacteria beginning to sway in a flow field. (c) Bacteria aligned with the flow field (to the right). The image is cropped and enlarged to make the bacterial orientations clear.
Force measurements.
Differential electrophoresis was done on 25 additional bacterium (D21)-PS couplets in order to quantify adhesion forces. The technique can apply roughly 50 pN or less, which is more than sufficient for measuring the weak, reversible (often subpiconewton) forces associated with adhesion in a Derjaguin-Landau-Verwey-Overbeek (DLVO) secondary energy minimum (23). Although the literature does not define irreversible bacterial adhesion in quantitative terms, we define irreversible here as an adhesion force greater than 10 pN, based on calculations from classical colloid theory. However, during experiments on 25 couplets, only one couplet broke. This strong adhesion is not typical of DLVO forces but rather of much stronger forces (e.g., hydrophobic forces and specific interactions). Zeta potential values in 100 mM PBS, used in calculating the applied force of differential electrophoresis, were −26 ± 1 mV for D21 and −73 ± 2 mV for PS.
Charge nonuniformity.
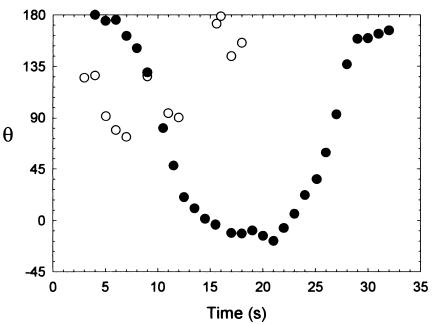
All three E. coli strains were tested for surface charge nonuniformity with rotational electrophoresis. No rotation was found for any strain, indicating that charge nonuniformity was too small to be measurable with this technique. That is, the angular velocities could not be distinguished from the Brownian motion of the bacteria. Figure 4 shows the rotation angles of a strain D21f2 bacterium in an electric field compared to a Bacillus subtilis bacterium with a polar flagellum. While B. subtilis rotated with the electric field to a steady angle and then rotated back when the field was reversed at 20 s, E. coli exhibited only small, random changes in angle associated with Brownian motion. B. subtilis had a quantifiable surface charge nonuniformity based on its angular velocity, while E. coli did not. The experiments represented in Fig. 4 were conducted in 1 mM Tris buffer. However, for all three E. coli strains, these experiments were repeated in 100 mM PBS, and again no measurable charge nonuniformity was observed.
FIG. 4.
Measuring charge nonuniformity on bacteria using rotational electrophoresis. Each open circle (○) represents an E. coli bacterium. θ is the angle in degrees between the applied electric field and the long axis of the bacterium. Since this bacterium is undergoing only random Brownian rotation, its charge nonuniformity is too small to be measured. On the other hand, the filled circles (•) represent a B. subtilis bacterium, which rotated in the electric field. When the field direction was switched at about 20 s, the rotation reversed since this bacterium was nonuniformly charged.
DISCUSSION
Table 1 clearly shows that E. coli adheres end-on to PS latex spheres. This result is complemented by the observations of bacteria adhered on end to bulk glass. One end of the bacterium clearly showed adhesion properties different from those of the rest of the bacterium. One explanation of the data is bacterial cell polarity (42). In E. coli, this bacterial polarity is caused by the localization of peptidoglycan (13), proteins (33), and phospholipids (32) on the surface of the bacterium. Differences in the surface composition and chemistry at the bacterium end could result in preferential adhesion at the end.
Nevertheless, the rotational electrophoresis experiments revealed no charge nonuniformity on the bacteria, and this finding remains puzzling. Although no charge nonuniformity could be discerned, localization of particular surface molecules must still be occurring. The small nanodomain where the PS latex particles adhere is a small fraction of the total bacterial surface area, and so based on geometry, it is highly improbable that 90% of the PS particles would adhere to the end of the bacteria. More importantly, the PS particles adhere to one end but never to both. Apparently, this bacterial polarity is not exhibited through charged functionalities, or at least the differences in charge across the surface are not detectable by rotational electrophoresis. To maintain the integrity of the membrane structure, it is unlikely that a large localization of molecules other than LPS occurs. However, the combination of several patches of molecules at a polar end could result in significant differences in adhesion properties.
Although the three strains of E. coli had differing lengths of LPS surface molecules, the adhesion orientation of these bacteria did not change, remaining at 90% end-on adhesion. This result indicates that LPS does not seem to be the important molecule involved in adhesion. Our differential electrophoresis experiments showed that the initial adhesion process was most often irreversible, consistent with the findings of Rijnaarts et al. (39). The one couplet (out of 25) that did break in our experiments had an attractive force that was roughly <10 pN.
Polar adhesion of E. coli bacteria was shown to occur to both PS latex particles and to standard glass microscope slides. Greater than 90% of the adhering bacteria adhered on their ends, which cannot be accounted for by geometric considerations. The adhesion of individual bacteria was found to occur at only a single end, indicating different functionalities in those regions. And yet rotational electrophoresis measurements revealed no charge nonuniformity on the E. coli organisms. Since the three strains of K-12, each with a different length of LPS, behaved similarly in the experiments, it is possible that the bacterial polarity due to protein localization causes the polar adhesion. This adhesion is essentially irreversible, since the classical colloidal force models cannot explain the large attractive forces measured using differential electrophoresis.
We are currently working to apply the techniques we used in this research—in particular, the electrophoresis and video microscopy techniques—to other bacteria. Bacteria adhere to surfaces by a variety of mechanisms, and by using these techniques, we will examine the generality or specificity of end-on adhesion for various strains of bacteria. As of yet we do not know, for instance, whether the polar adhesion results from the division process. But our aim is to isolate and identify the molecular composition that causes the adhesion to the “sticky” nanodomains, with the long-term goal of controlling the expression of these molecules.
Acknowledgments
This work was funded by the National Science Foundation through NSF CRAEMS grant no. CH3-0089156 and NSF CAREER grant no. CTS-9984443.
REFERENCES
- 1.Adler, J., and B. Templeton. 1967. The effect of environmental conditions on the motility of Escherichia coli. J. Gen. Microbiol. 46:175-184. [DOI] [PubMed] [Google Scholar]
- 2.Alley, M. R. K., J. R. Maddock, and L. Shapiro. 1993. Requirement of carboxyl terminus of a bacterial chemoreceptor for its targeted proteolysis. Science 259:1754-1757. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, J. L., D. Velegol, and S. Garoff. 2000. Measuring colloidal forces using differential electrophoresis. Langmuir 16:3372-3384. [DOI] [PubMed] [Google Scholar]
- 4.Burks, G. A., S. B. Velegol, E. Paramonova, B. E. Lindenmuth, J. D. Feick, and B. E. Logan. 2003. Microscopic and nanoscale measurements of adhesion of bacteria with varying outer layer surface composition. Langmuir 19:2366-2371. [Google Scholar]
- 5.Busscher, H. J., and A. H. Weerkamp. 1987. Specific and non-specific interactions in bacterial adhesion to solid substrata. FEMS Microbiol. Rev. 46:165-173. [Google Scholar]
- 6.Camesano, T. A., and B. E. Logan. 2000. Probing bacterial electrosteric interactions using atomic force microscopy. Environ. Sci. Technol. 34:3354-3362. [Google Scholar]
- 7.Camesano, T. A., and N. I. Abu-Lail. 2002. Heterogeneity in bacterial surface polysaccharides, probed on a single-molecule basis. Biomacromolecules 3:661-667. [DOI] [PubMed] [Google Scholar]
- 8.Characklis, W G. 1990. Microbial fouling, p. 523-584. In W. G. Characklis and K. C. Marshall (ed.), Biofilms. John Wiley & Sons, Inc., New York, N.Y.
- 9.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 10.Dawson, J. R., and R. P. Ellen. 1990. Tip-oriented adherence of Treponema denticola to fibronectin. Infect. Immun. 58:3924-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson, J. R., and R. P. Ellen. 1994. Clustering of fibronectin adhesins toward Treponema denticola tips upon contact with immobilized fibronectin. Infect. Immun. 62:2214-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Flaun, M. F., C. J. Murray, W. Holben, T. Schaube, A. Mills, T. Ginn, T. Griffin, E. Majer, and J. L. Wilson. 1997. Preliminary observations on bacterial transport in a coastal plain aquifer. FEMS Microbiol. Rev. 20:473-487. [Google Scholar]
- 13.De Pedro, M. A., J. C. Quintela, J. V. Holtje, and H. Schwarz. 1997. Murein segregation in Escherichia coli. J. Bacteriol. 179:2823-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong, Y. H., L. H. Wang, J. L. Xu, H. B. Zhang, X. F. Zhang, and L. H. Zhang. 2001. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813-817. [DOI] [PubMed] [Google Scholar]
- 15.Ellen, R. P., M. Wikstrom, D. A. Grove, M. Song, and H. Elwing. 1998. Polar adhesion of Treponema denticola on wettability gradient surfaces. Colloids Surf. B 11:177-186. [Google Scholar]
- 16.Feick, J. D., and D. Velegol. 2000. The electrophoresis of spheroidal particles having a random distribution of zeta potential. Langmuir 16:10315-10321. [DOI] [PubMed] [Google Scholar]
- 17.Feick, J. D., and D. Velegol. 2002. Measurements of charge nonuniformity on polystyrene latex particles. Langmuir 18:3454-3458. [Google Scholar]
- 18.Flemming, H. C., and G. Schaule. 1996. Biofouling, p. 39-54. In E. Heitz, H. C. Flemming, and W. Sand (ed.), Microbially influenced corrosion of materials. Springer-Verlag, Berlin, Germany.
- 19.Fletcher, M., and J. H. Pringle. 1985. The effect of surface free energy and medium surface tension on bacterial attachment to solid surfaces. J. Colloid Interface Sci. 104:5-14. [Google Scholar]
- 20.Fletcher, M. 1996. Bacterial attachment in aquatic environments: a diversity of surfaces and adhesion strategies, p. 1-24. In M. Fletcher (ed.), Bacterial adhesion: molecular and ecological diversity. Wiley Interscience, New York, N.Y.
- 21.Goldstein, A. S., and P. A. DiMilla. 1998. Comparison of converging and diverging radial flow for measuring cell adhesion. AICHE Symp. Ser. 44:465-473. [Google Scholar]
- 22.Haruff, H. M., J. Munakata-Marr, and D. W. M. Marr. 2002. Directed bacterial surface attachment via optical trapping. Colloids Surf. B Biointerfaces 27:189-195. [Google Scholar]
- 23.Hermansson, M. 1999. The DLVO theory in microbial adhesion. Colloids Surf. B Biointerfaces 14:105-119. [Google Scholar]
- 24.Jacobs, C., and L. Shapiro. 1999. Bacterial cell division: a moveable feast. Proc. Natl. Acad. Sci. USA 96:5891-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, Q., and B. E. Logan. 1999. Enhancing bacterial transport for bioaugmentation of aquifers using low ionic strength solutions and surfactants. Water Res. 33:1090-1100. [Google Scholar]
- 26.Loh, J. T., S. C. Ho, A. W. de Feijter, J. L. Wang, and M. Schindler. 1993. Carbohydrate binding activities of Bradyrhizobium japonicum: unipolar localization of the lectin BJ38 on the bacterial cell surface. Proc. Natl. Acad. Sci. USA 90:3033-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahanty, J., and B. W. Ninham. 1976. Dispersion forces. Academic Press, New York, N.Y.
- 28.Marshall, K. C., R. Stout, and R. Mitchell. 1971. Mechanism of the initial events in the sorption of marine bacteria to surfaces. J. Gen. Microbiol. 68:337-348. [Google Scholar]
- 29.Matthysse, A. G. 1996. Adhesion in the rhizosphere, p. 129-153. In M. Fletcher (ed.), Bacterial adhesion: molecular and ecological diversity. Wiley Interscience, New York, N.Y.
- 30.McClaine, J. W., and R. M. Ford. 2002. Reversal of flagellar rotation is important in initial attachment of Escherichia coli to glass in a dynamic system with high- and low-ionic-strength buffers. Appl. Environ. Microbiol. 68:1280-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McClaine, J. W., and R. M. Ford. 2002. Characterizing the adhesion of motile and nonmotile Escherichia coli to a glass surface using a parallel-plate flow chamber. Biotechnol. Bioeng. 78:179-189. [DOI] [PubMed] [Google Scholar]
- 32.Mileykovskaya, E., and W. Dowhan. 2000. Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine orange. J. Bacteriol. 182:1172-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mileykovskaya, E., Q. Sun, W. Margolin, and W. Dowhan. 1998. Localization and function of early cell division proteins in filamentous Escherichia coli cells lacking phosphatidylethanolamine. J. Bacteriol. 180:4252-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison, F. A., Jr. 1970. Electrophoresis of a particle of arbitrary shape. J. Colloid Interface Sci. 34:210-214. [Google Scholar]
- 35.Parsegian, V. A. 1975. Long range van der Waals forces, p. 27-72. In H. van Olphen and Karol J. Mysels (ed.), Physical chemistry: enriching topics from colloid and surface science. Theorex, La Jolla, Calif.
- 36.Poortinga, A. T., R. Bos, W. Norde, and H. J. Busscher. 2002. Electric double layer interactions in bacterial adhesion to surfaces. Surf. Sci. Rep. 47:1-32. [Google Scholar]
- 37.Razatos, A., Y. L. Ong, M. M. Sharma, and G. Georgiou. 1998. Molecular determinants of bacterial adhesion monitored by atomic force microscopy. Proc. Natl. Acad. Sci. USA 95:11059-11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rijnaarts, H. H. M., W. Norde, E. J. Bouwer, J. Lyklema, and A. J. B. Zehnder. 1993. Bacterial adhesion under static and dynamic conditions. Appl. Environ. Microbiol. 59:3255-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rijnaarts, H. H. M., W. Norde, E. J. Bouwer, J. Lyklema, and A. J. B. Zehnder. 1995. Reversibility and mechanism of bacterial adhesion. Colloids Surf. B Biointerfaces 4:5-22. [Google Scholar]
- 40.Russel, W. B., D. A. Saville, and W. R. Schowalter (ed.). 1989. Electrostatics, p. 88-126. In Colloidal dispersions. Cambridge University Press, Cambridge, United Kingdom.
- 41.Schellenburger, K., and B. E. Logan. 2002. Effect of molecular scale roughness of glass beads on colloidal and bacterial deposition. Environ. Sci. Technol. 36:184-189. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro, L., H. H. McAdams, and R. Losick. 2002. Generating and exploiting polarity in bacteria. Science 298:1942-1946. [DOI] [PubMed] [Google Scholar]
- 43.Thwar, P. K., and D. Velegol. 2002. Force measurements between weakly attractive polystyrene particles. Langmuir 18:7328-7333. [Google Scholar]
- 44.Vandevivere, P., and P. Baveye. 1992. Effect of bacterial extracellular polymers on the saturated hydraulic conductivity of sand columns. Appl. Environ. Microbiol. 58:1690-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Kooten, T. G., J. M. Schakenraad, H. C. Van der Mei, and H. J. Busscher. 1992. Development and use of a parallel-plate flow chamber for studying cellular adhesion to solid surfaces. J. Biomed. Mater. Res. 26:725-738. [DOI] [PubMed] [Google Scholar]
- 46.Van Loosdrecht, M. C. M., J. Lyklema, W. Norde, G. Schraa, and A. J. D. Zehnder. 1987. The role of bacterial cell wall hydrophobicity in adhesion. Appl. Environ. Microbiol. 53:1893-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Oss, C. J. 1995. Hydrophobicity of biosurfaces: origin, quantitative determination, and interaction energies. Colloids Surf. B Biointerfaces 5:91-110. [Google Scholar]
- 48.Velegol, D., J. L. Anderson, and S. Garoff. 1996. Determining the forces between polystyrene latex spheres using differential electrophoresis. Langmuir 12:4013-4110. [Google Scholar]
- 49.Velegol, D., J. D. Feick, and L. R. Collins. 2000. Electrophoresis of spherical particles with a random distribution of zeta potential or surface charge. J. Colloid Interface Sci. 230:114-121. [DOI] [PubMed] [Google Scholar]
- 50.Velegol, S. B., and B. E. Logan. 2002. Contributions of bacterial surface polymers, electrostatics, and cell elasticity to the shape of AFM force curves. Langmuir 18:5256-5262. [Google Scholar]
- 51.Velegol, S. B., S. Pardi, X. Li, D. Velegol, and B. E. Logan. 2003. AFM imaging artifacts due to bacterial cell height and AFM tip geometry. Langmuir 19:851-857. [Google Scholar]
- 52.Whitchurch, C. B., T. Tolker-Nielsen, P. C. Ragas, and J. S. Mattick. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295:1487. [DOI] [PubMed] [Google Scholar]
- 53.Wolfaardt, G. M., J. R. Lawrence, and D. R. Korber. 1999. Function of EPS, p. 171-200. In J. Wingender, T. R. Neu, and H. C. Flemming (ed.), Microbial extracellular polymeric substances: characterization, structure, and function. Springer-Verlag, Berlin, Germany.
- 54.Zoutman, D. E., W. C. Hulbert, B. L. Pasloske, A. M. Joffe, K. Volpel, M. K. Trebilcock, and W. Paranchych. 1991. The role of polar pili in the adherence of Pseudomonas aeruginosa to injured canine tracheal cells: a semiquantitative morphological study. Scanning Microsc. 5:109-126. [PubMed] [Google Scholar]