Abstract
An enzymatic method for synthesizing various γ-d-glutamyl compounds efficiently and stereospecifically involving bacterial γ-glutamyltranspeptidase (EC 2.3.2.2) with d-glutamine as a γ-glutamyl donor was developed. With d-glutamine as a γ-glutamyl donor instead of l-glutamine in γ-glutamyltaurine synthesis, by-products such as γ-glutamylglutamine and γ-glutamyl-γ-glutamyltaurine were not synthesized and the yield of γ-glutamyltaurine dramatically increased from 25 to 71%. It was also shown that the purification could be simplified without these γ-glutamyl by-products. The possibility of synthesizing various γ-d-glutamyl compounds was also shown.
γ-Glutamyltranspeptidase (GGT) (EC 2.3.2.2) catalyzes the hydrolysis of γ-glutamyl compounds such as glutathione and the transfer of their γ-glutamyl moieties to other amino acids and peptides (27). The reactions catalyzed by GGT proceed via a γ-glutamyl enzyme intermediate involving the hydroxyl group of Thr-391 (9). If the intermediate is subjected to nucleophilic attack by water, the reaction is hydrolytic, releasing glutamic acid. If the intermediate is subjected to nucleophilic attack by amino acids and peptides, the reaction is a transpeptidation yielding new γ-glutamyl compounds. By employing various γ-glutamyl acceptors, we can synthesize various γ-glutamyl compounds using GGT. The pH optima for these reactions are very different (25). Therefore, by adjusting the pH of the reaction mixture, we can make GGT catalyze the transpeptidation reaction selectively. γ-Glutamyl compounds are very attractive, because (i) compounds that are not very soluble in water become much more soluble with γ-glutamylization (5); (ii) the γ-glutamyl linkage is resistant to peptidases in serum, and some γ-glutamyl compounds can possibly be used as prodrugs specific for the organs that express GGT (8, 10, 18, 30); and (iii) some γ-glutamyl compounds taste good and thus can be used as food additives (21, 22). We have already developed and reported efficient methods for synthesizing various γ-l-glutamyl compounds using Escherichia coli GGT (13-17, 21, 22, 26). The characteristics of our methods are as follows. (i) l-Glutamine, which is less expensive than glutathione, can be used as a γ-glutamyl donor. (ii) No energy source, such as ATP, is required because GGT is a transferase and not a synthetase. (iii) Since E. coli GGT exhibits a broad substrate specificity for γ-glutamyl acceptors, various γ-glutamyl compounds can be synthesized. (iv) Since E. coli GGT can be purified from overproducing strains by means of a simple two-step method (1, 23), a large amount of GGT is readily available.
During the development of a method for the enzymatic production of various γ-glutamyl compounds, we found that the yield of a γ-glutamyl compound with a γ-glutamyl acceptor such as taurine was not very high. This was mainly because nonnegligible amounts of by-products, such as γ-glutamylglutamine and γ-glutamyl-γ-glutamyltaurine, were formed (26). It has been reported that GGT cannot utilize d-amino acids as γ-glutamyl acceptors (27-29). Therefore, by using d-glutamine as a γ-glutamyl donor instead of l-glutamine, we expected that neither γ-d-glutamyl-d-glutamine nor γ-d-glutamyl-γ-d-glutamyltaurine would be synthesized and that the yields of the expected compounds would increase. Moreover, there is a latent demand for γ-d-glutamyl compounds. For example, l-theanine (γ-l-glutamylethylamide) is known as the major “umami” component of Japanese green tea (20). It is known that there is a positive correlation between a grade of Japanese green tea and its concentration of theanine (4, 19). Ekborg-Ott et al. (3) reported that d-theanine also exists in tea leaves and that it has a taste similar to that of l-theanine. However, they did not compare the threshold concentrations of l- and d-theanine for perceiving the taste. If d-theanine tastes as strong as l-theanine, d-theanine could be used instead of l-theanine to improve the taste of tea. It has also been reported that γ-d-glutamyl amino acids, such as γ-d-glutamyltaurine, have an antagonistic effect against excitatory amino acids (2, 11). We speculate that the relaxing effect of l-theanine (12) might possibly be observed for d-theanine with a much lower dosage. Therefore, an effective method for producing γ-d-glutamyl compounds is necessary to obtain a large amount of these γ-d-glutamyl compounds for testing on animals. It was advantageous to develop an enzymatic method involving bacterial GGT for synthesizing γ-d-glutamyl compounds.
MATERIALS AND METHODS
Chemicals and enzyme.
γ-d-Glutamyltaurine and l-theanine were purchased from Tocris Cookson (St. Louis, Mo.) and Tokyo Kasei (Tokyo, Japan), respectively, and used as standards. γ-l-Glutamyl-l-glutamine was from Sigma Chemical (St. Louis, Mo.), and d-glutamine, taurine, and ethylamine were from Nacalai Tesque (Kyoto, Japan). E. coli K-12 strain SH642 (23), which harbors pUC18 with the E. coli GGT gene, was grown at 20°C (6, 24) in Luria-Bertani broth containing 100 μg of ampicillin/ml. Its periplasmic fraction was prepared (24), and the overproduced GGT was purified to homogeneity by ammonium sulfate fractionation and chromatofocusing as described previously (23).
Measurement of GGT activity.
GGT activity was measured as described previously (25) by using γ-l-glutamyl-p-nitroanilide and glycylglycine as substrates. One unit of enzyme was defined as the amount of the enzyme that released 1 μmol of p-nitroaniline per min from γ-l-glutamyl-p-nitroanilide through the transpeptidation reaction.
Measurement of amino acids and γ-glutamyl compounds.
Amino acids and γ-glutamyl compounds were measured by high-pressure liquid chromatography (HPLC) as described previously (21, 26). Briefly, γ-glutamyltaurine and γ-glutamyl-γ-glutamyltaurine were measured with an HPLC instrument (model L-7100; Hitachi, Tokyo, Japan) equipped with a Cosmosil SC18-AR column (10 by 250 mm) (Nacalai Tesque). Both γ-glutamyltaurine and γ-glutamyl-γ-glutamyltaurine were eluted with water containing 0.05% trifluoroacetic acid at a flow rate of 2.5 ml/min and detected by measuring the absorbance at 213 nm. Amino acids and other γ-glutamyl compounds were measured with an HPLC instrument (model LC-9A; Shimadzu, Kyoto, Japan) equipped with a Shim-pack amino Na column (Shimadzu), with gradient elution at 60°C at a flow rate of 0.6 ml/min. The gradient of the mobile phase was formed with buffer A (66.6 mM citrate, 1% perchloric acid, 7% ethanol, pH 2.8) and buffer B (200 mM citrate, 200 mM boric acid, 0.12 N NaOH, pH 10). The concentration of buffer B was kept at 0% until 9 min. It was linearly increased to 7% from 9 to 13 min, to 8% from 13 to 17.2 min, and then to 11%. o-Phthalaldehyde was used as the detection reagent, and the fluorescence was detected with a fluorescence detector (model RF-535; Shimadzu) as the absorbance at 450 nm, with excitation at 348 nm.
Large-scale production and purification of γ-d-glutamyltaurine and d-theanine.
γ-d-Glutamyltaurine and d-theanine were synthesized and purified as described previously (21, 26) with slight modification. Briefly, the synthesis of γ-d-glutamyltaurine was carried out with 15 ml of the reaction mixture comprising 200 mM Gln, 200 mM taurine, and 0.2 U of GGT/ml, pH 10, at 37°C for 5 h. The reaction mixture was applied to a column (30 ml) of Dowex 1 × 8, which had been prepared as the CH3COO− form. The column was washed with 200 ml of water and 200 ml of 5 N acetic acid. γ-Glutamyltaurine was eluted with a mixture of formic acid, acetic acid, and water (1:2:16 by volume). The fractions that contained only γ-glutamyltaurine were saved and lyophilized. The synthesis of d-theanine was carried out with 22-ml of the reaction mixture comprising 150 mM Gln, 150 mM ethylamine, and 0.2 U of GGT/ml, pH 10, at 37°C for 5 h. The reaction mixture was applied to a column (50 ml) of Dowex 50W X 8, which had been prepared as the Ca2+ form. Theanine was eluted with water, and the fractions containing theanine were collected and lyophilized. Theanine was dissolved with water and applied to a column (30 ml) of Dowex 1 X 8, which had been prepared as the Cl− form. Theanine was eluted with water, and the fractions containing only theanine were collected and lyophilized.
NMR and polarimeter analysis.
Purified γ-d-glutamyltaurine and d-theanine were subjected to 1H nuclear magnetic resonance (NMR) and polarimeter analysis as described previously (21, 26). Purified γ-d-glutamyltaurine (5 mg) and d-theanine (5 mg) were dissolved in 0.5 ml of D2O and analyzed with a Bruker 500-MHz spectrometer, and then the spectrum was compared with that obtained with γ-d-glutamyltaurine and l-theanine purchased from commercial sources. Purified γ-d-glutamyltaurine (5 mg) and d-theanine (10 mg) were dissolved in distilled water (1.2 and 1.0 ml, respectively) and then analyzed with a Jasco DIP-1000 polarimeter (Nippon Bunko, Tokyo, Japan).
RESULTS AND DISCUSSION
Effect of the use of d-glutamine on the yield of γ-glutamyltaurine.
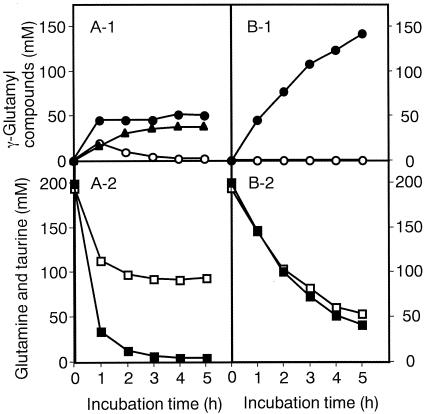
The optimum reaction conditions for the synthesis of γ-l-glutamyltaurine were 200 mM l-glutamine, 200 mM taurine, and 0.2 U of GGT/ml, pH 10 (26). Therefore, the yields of enzymatic synthesis of γ-glutamyltaurine using l-glutamine and d-glutamine were compared under these conditions. The reaction mixture (total volume, 1 ml) was incubated at 37°C, an aliquot of it being withdrawn every hour to measure the concentrations of amino acids and γ-glutamyl compounds. When l-glutamine was used as the γ-glutamyl donor (Fig. 1A), it decreased greatly after 1 h of incubation and about 45 mM γ-glutamyltaurine accumulated. In addition to γ-glutamyltaurine, γ-glutamyl-γ-glutamyltaurine and γ-glutamylglutamine were formed. The concentration of γ-glutamyltaurine remained almost constant thereafter. That of γ-glutamylglutamine decreased in 1 h, while that of γ-glutamyl-γ-glutamyltaurine increased gradually until glutamine and γ-glutamylglutamine had been completely consumed. Since a significant amount of the by-product γ-glutamyl-γ-glutamyltaurine was formed after 5 h of incubation and since glutamine was wasted when l-glutamine was used as the γ-glutamyl donor (Fig. 1A), the rate of conversion of l-glutamine to γ-glutamyltaurine was unsatisfactory (Table 1).
FIG. 1.
Enzymatic synthesis of γ-glutamyltaurine with l-glutamine (A) or d-glutamine (B) as the γ-glutamyl donor. The reaction mixture for the synthesis of γ-l-glutamyltaurine comprised 200 mM l-glutamine, 200 mM taurine, and 0.2 U of GGT/ml at pH 10 and 37°C. That for the synthesis of γ-d-glutamyltaurine comprised 200 mM d-glutamine, 200 mM taurine, and 0.2 U of GGT/ml at pH 10 and 37°C. Samples were withdrawn at the indicated times, and the concentrations of γ-glutamyl compounds and amino acids were determined. Solid circles, γ-glutamyltaurine; open circles, γ-glutamylglutamine; solid triangles, γ-glutamyl-γ-glutamyltaurine; solid squares, glutamine; open squares, taurine.
TABLE 1.
Comparison of the yields of γ-glutamyltaurine and theanine after a 5-h reaction with l- or d-glutamine as the γ-glutamyl donora
| Product | γ-Glutamyl donor | Yield of γ-glutamyl compound (mM, g/liter) | Conversion rate of glutamine (%) |
|---|---|---|---|
| γ-Glutamyltaurine | l-Glutamine | 50, 12.7 | 25 |
| d-Glutamine | 142, 36.1 | 71 | |
| Theanine | l-Glutamine | 121, 21.1 | 61 |
| d-Glutamine | 147, 25.6 | 74 |
On the other hand, when d-glutamine was used as the γ-glutamyl donor (Fig. 1B), no by-product, such as γ-glutamyl-γ-glutamyltaurine or γ-glutamylglutamine, was formed. As a consequence, the yield of γ-glutamyltaurine increased dramatically (Table 1).
Effect of the use of d-glutamine on the yield of theanine.
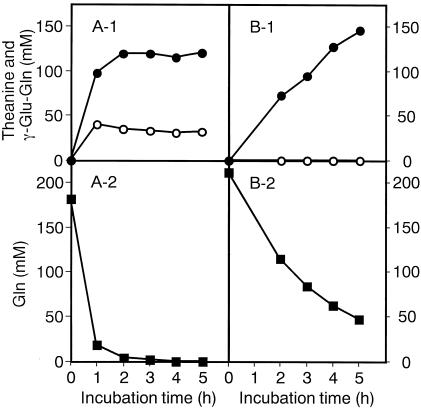
The optimum reaction conditions for the synthesis of l-theanine were 200 mM l-glutamine, 1,500 mM ethylamine, and 0.4 U of GGT/ml, pH 10 (21). The yields of enzymatic synthesis of theanine with l-glutamine and d-glutamine were compared under these conditions. When l-glutamine was used as the γ-glutamyl donor (Fig. 2A), it decreased to 1/10 of the original level after 1 h of incubation and about 100 mM theanine accumulated, which increased to 120 mM after 2 h of incubation. But its concentration remained unchanged thereafter. In this case, γ-glutamylglutamine was formed as a by-product. When d-glutamine was used as the γ-glutamyl donor (Fig. 2B), no by-product was formed and the yield of theanine increased (Table 1). However, the yield of d-theanine was only slightly higher than that of l-theanine, possibly because the yield of l-theanine was much higher than that of γ-l-glutamyltaurine. The rate of synthesis of theanine with d-glutamine was slower than that with l-glutamine, and about 50 mM d-glutamine remained, even after 5 h of incubation, and thus the yield may improve with longer incubation.
FIG. 2.
Enzymatic synthesis of theanine with l-glutamine (A) or d-glutamine (B) as the γ-glutamyl donor. The reaction mixture for the synthesis of l-theanine comprised 200 mM l-glutamine, 1,500 mM ethylamine, and 0.4 U of GGT/ml at pH 10 and 37°C. That for the synthesis of d-theanine comprised 200 mM d-glutamine, 1,500 mM ethylamine, and 0.4 U of GGT/ml at pH 10 and 37°C. Samples were withdrawn at the indicated times, and the concentrations of γ-glutamyl compounds and amino acids were determined. Solid circles, theanine; open circles, γ-glutamylglutamine; solid squares, glutamine.
Purification of γ-d-glutamyltaurine and d-theanine.
γ-d-Glutamyltaurine and d-theanine were synthesized and purified as described in Materials and Methods. When γ-d-glutamyltaurine was purified, the purification step with reverse-phase HPLC was omitted. This was because γ-d-glutamyltaurine had already been isolated in the purification with a Dowex 1 X 8 column. γ-Glutamyl-γ-glutamyltaurine was not produced when d-glutamine was used as the γ-glutamyl donor, and thus it was not necessary to separate it from γ-glutamyltaurine by reverse-phase HPLC. We emphasize that this is another merit of utilizing d-glutamine as a γ-glutamyl donor.
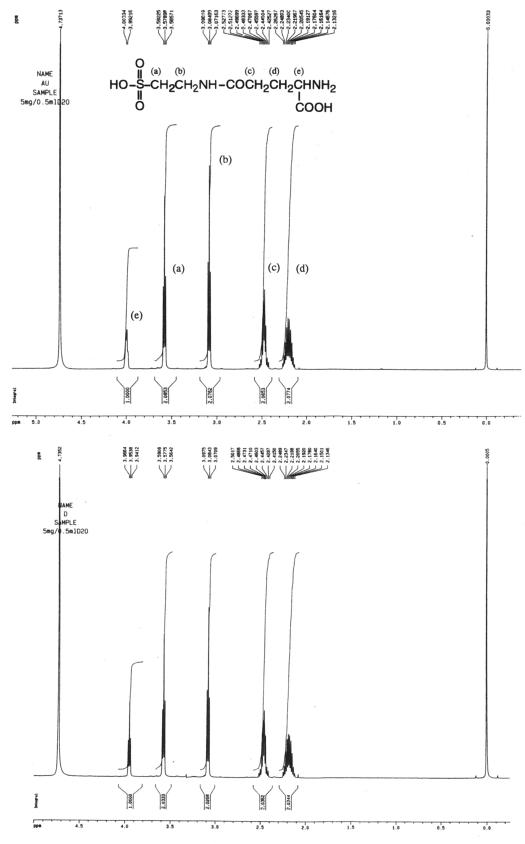
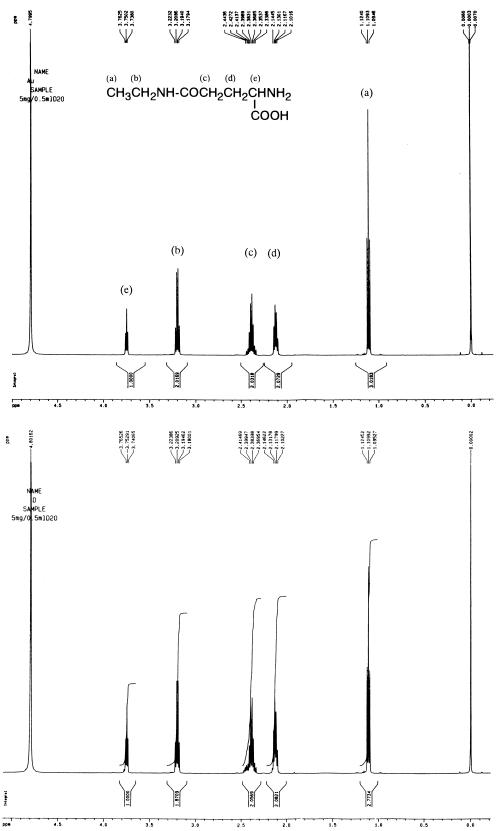
Purified samples were identified as γ-d-glutamyltaurine and d-theanine by 1H NMR and polarimeter analysis as shown in Fig. 3 and 4 and Table 2.
FIG. 3.
1H NMR spectra of γ-d-glutamyltaurine obtained from a commercial source (top) and that synthesized and purified in this study (bottom). They were measured in D2O with a Bruker 500-MHz spectrometer. Each peak is assigned to a proton of γ-glutamyltaurine and denoted by a letter.
FIG. 4.
1H NMR spectra of l-theanine obtained from a commercial source (top) and d-theanine synthesized and purified in this study (bottom). They were measured in D2O with a Bruker 500-MHz spectrometer. Each peak is assigned to a proton of theanine and denoted by a letter.
TABLE 2.
Results of polarimeter analysis
| γ-Glutamyl compound | Source or reference | Optical rotation | cd |
|---|---|---|---|
| γ-Glutamyltaurine | |||
| γ-d-Glutamyltaurine | Synthesizeda | [α]23d = −17.7 | 0.417 |
| Purchasedb | [α]23d = −18.2 | 0.417 | |
| 7 | [α]d = −17.3 | 1 | |
| γ-l-Glutamyltaurine | 26 | [α]26.5d = +16.4c | 0.417 |
| 7 | [α]d = +18.6 | 1 | |
| Theanine | |||
| d-Theanine | Synthesizeda | [α]31d = −4.0 | 1 |
| l-Theanine | Purchasedb | [α]31d = +7.3 | 1 |
| 31 | [α]30d = +6.1 | 2 |
Samples synthesized and purified in this study.
Chemicals purchased from commercial sources.
Sample synthesized and purified by us and reported previously.
c, concentrations of solutions used to mesaure optical rotations (in grams per cubic centimeter).
Substrate specificity of E. coli GGT for γ-glutamyl acceptors with d-glutamine as the γ-glutamyl donor.
The transpeptidation reaction was performed with various γ-glutamyl acceptors. The amounts of γ-glutamyl compounds formed versus time were measured by HPLC, and the initial velocities were calculated. The relative activity for γ-glutamyl acceptors is expressed as the relative initial velocity in Table 3. Under the reaction conditions used, l-tryptophan, taurine, l-methionine, l-phenylalanine, and l-histidine were good acceptors. Therefore, the acceptor specificity with d-glutamine as the γ-glutamyl donor is almost the same as that with γ-l-glutamyl-p-nitroanilide (25). When ethylamine was used as the acceptor, the production of theanine was not detectable under the reaction conditions used. This may be because the reaction pH, and the concentrations of GGT and ethylamine used for this assay were very different from those used for the production of d-theanine, with which the yield of d-theanine was reasonably good.
TABLE 3.
Substrate specificity for γ-glutamyl acceptors with d-glutamine as the γ-glutamyl donor
| Substratea | Relative activity (%)b |
|---|---|
| Gly-Gly | 100 |
| l-Ala | 6.2 |
| l-Cys | 19.3 |
| l-Glu | 8.1 |
| Gly | 20.1 |
| l-His | 116 |
| l-Met | 352 |
| l-Phe | 144 |
| l-Trp | 558 |
| l-Val | 8.1 |
| Taurine | 422 |
| Ethylamine | Not detectable |
γ-Glutamyl acceptors were used at 20 mM, and the pH of the reaction mixtures was adjusted to 8.73. As the γ-glutamyl donor, 20 mM d-glutamine was used.
The relative activities are expressed as relative initial velocities. The activity with Gly-Gly as an acceptor was taken as 100%.
Comparison of the affinities of GGT for d- and l-γ-glutamyl compounds.
The Km values for γ-l-glutamyl-p-nitroanilide, γ-d-glutamyl-p-nitroanilide, l-glutamine, and d-glutamine were 12.5, 137, 8.3, and 278 μM, respectively, as measured by means of the hydrolysis reaction. Although the Km values for γ-d-glutamyl compounds are much higher than those for γ-l-glutamyl compounds, they are small enough for the enzymatic production of γ-glutamyl compounds by means of our method, for which several hundred millimolar d-glutamine is used.
In conclusion, γ-d-glutamyl compounds can be synthesized efficiently and stereospecifically by employing bacterial GGT and d-glutamine. Furthermore, by using d-glutamine as the γ-glutamyl donor instead of l-glutamine, we can minimize the synthesis of by-products and possibly simplify the purification procedure.
Acknowledgments
We thank Kazuhiro Irie, Graduate School of Agriculture, Kyoto University, for the NMR and polarimeter analysis and for fruitful discussions.
This work was supported by a grant-in-aid for scientific research, no. 13660090, to H.S. from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by research funds from the Society for Research on Umami Taste to H.S.
REFERENCES
- 1.Claudio, J. O., H. Suzuki, H. Kumagai, and T. Tochikura. 1991. Excretion and rapid purification of γ-glutamyltranspeptidase from Escherichia coli K-12. J. Ferment. Bioeng. 72:125-127. [Google Scholar]
- 2.Davies, J., R. H. Evans, A. W. Jones, D. A. Smith, and J. C. Watkins. 1982. Differential activation and blockade of excitatory amino acid receptors in the mammalian and amphibian central nervous systems. Comp. Biochem. Physiol. C. 72:211-224. [DOI] [PubMed] [Google Scholar]
- 3.Ekborg-Ott, K. H., A. Taylor, and D. W. Armstrong. 1997. Varietal differences in the total and enantiomeric composition of theanine in tea. J. Agric. Food Chem. 45:353-363. [Google Scholar]
- 4.Goto, T., Y. Yoshida, I. Amano, and H. Horie. 1996. Chemical composition of commercially available Japanese green tea. Foods Food Ingredients J. Jpn. 170:46-51. [Google Scholar]
- 5.Hara, T., Y. Yokoo, and T. Furukawa. 1992. Potential of γ-L-glutamyl-L-cystine and bis-γ-L-glutamyl-L-cystine as a cystine-containing peptide for parenteral nutrition, p. 607-611. In K. Takai (ed.), Frontiers and new horizons in amino acid research. Elsevier, Amsterdam, The Netherlands.
- 6.Hashimoto, W., H. Suzuki, K. Yamamoto, and H. Kumagai. 1997. Analysis of low temperature inducible mechanism of γ-glutamyltranspeptidase of Escherichia coli K-12. Biosci. Biotechnol. Biochem. 61:34-39. [DOI] [PubMed] [Google Scholar]
- 7.Higashiura, K., and K. Ienaga. 1992. Simple peptides. Part 7. The chemical conversions of C-terminal α-amino acids in peptides into unsubstituted or 2-substituted taurines via S-acetylthio- or halogeno-intermediates. J. Chem. Res. 1992(S):250-251, 1992(M):1901-1921.
- 8.Ichinose, H., A. Togari, H. Suzuki, H. Kumagai, and T. Nagatsu. 1987. Increase of catecholamines in mouse brain by systemic administration of γ-glutamyl L-3, 4-dihydroxyphenylalanine. J. Neurochem. 49:928-932. [DOI] [PubMed] [Google Scholar]
- 9.Inoue, M., J. Hiratake, H. Suzuki, H. Kumagai, and K. Sakata. 2000. Identification of catalytic nucleophile of Escherichia coli γ-glutamyltranspeptidase by γ-monofluorophosphono derivative of glutamic acid: N-terminal thr-391 in small subunit is the nucleophile. Biochemistry 39:7764-7771. [DOI] [PubMed] [Google Scholar]
- 10.Jeffrey, R. F., T. M. MacDonald, and M. R. Lee. 1988. A comparison of the renal actions of γ-glutamyl-L-tyrosine in normal man. Clin. Sci. 74:37-40. [DOI] [PubMed] [Google Scholar]
- 11.Jones, A. W., D. A. Smith, and J. C. Watkins. 1984. Structure-activity relations of dipeptide antagonists of excitatory amino acids. Neuroscience 13:573-581. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi, K., Y. Nagato, N. Aoi, L. R. Juneja, M. Kim, T. Yamamoto, and S. Sugimoto. 1998. Effects of L-theanine on the release of alpha-brain waves in human volunteers. Nippon Nogeikagaku Kaishi. 72:153-157. (In Japanese.) [Google Scholar]
- 13.Kumagai, H., T. Echigo, H. Suzuki, and T. Tochikura. 1989. Enzymatic synthesis of γ-glutamyl-L-histidine by γ-glutamyltranspeptidase from Escherichia coli K-12. Lett. Appl. Microbiol. 8:143-146. [Google Scholar]
- 14.Kumagai, H., T. Echigo, H. Suzuki, and T. Tochikura. 1989. Enzymatic synthesis of γ-glutamyltyrosine methyl ester from L-glutamine and L-tyrosine methyl ester with Escherichia coli K-12 γ-glutamyltranspeptidase. Agric. Biol. Chem. 53:1429-1430. [Google Scholar]
- 15.Kumagai, H., T. Echigo, H. Suzuki, and T. Tochikura. 1988. Synthesis of γ-glutamyl-DOPA from L-glutamine and L-DOPA by γ-glutamyltranspeptidase of Escherichia coli K-12. Agric. Biol. Chem. 52:1377-1382. [Google Scholar]
- 16.Kumagai, H., H. Suzuki, T. Echigo, and T. Tochikura. 1990. Syntheses of γ-glutamyl peptides by γ-glutamyltranspeptidase from E. coli. Ann. N. Y. Acad. Sci. 613:647-651. [DOI] [PubMed] [Google Scholar]
- 17.Kumagai, H., H. Suzuki, M. Shimizu, and T. Tochikura. 1989. Utilization of the γ-glutamyltranspeptidase reaction for glutathione synthesis. J. Biotechnol. 9:129-138. [Google Scholar]
- 18.Misicka, A., I. Maszczynska, A. W. Lipkowski, D. Stropova, H. I. Yamamura, and V. J. Hruby. 1996. Synthesis and biological properties of gamma-glutamyl-dermorphin, a prodrug. Life Sci. 58:905-911. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa, M. 1970. Constituents in tea leaf and their contribution to the taste of green tea liquor. Jpn. Agric. Res. Q. 5:43-47. [Google Scholar]
- 20.Sakato, Y. 1949. Studies on the chemical constituents of tea. Part III. On a new amide theanine. Nippon Nogeikagaku Kaishi. 23:262-267. (In Japanese.) [Google Scholar]
- 21.Suzuki, H., S. Izuka, N. Miyakawa, and H. Kumagai. 2002. Enzymatic production of theanine, an “umami” component of tea, from glutamine and ethylamine with bacterial γ-glutamyltranspeptidase. Enzyme Microb. Technol. 31:884-889. [Google Scholar]
- 22.Suzuki, H., Y. Kajimoto, and H. Kumagai. 2002. Improvement of the bitter taste of amino acids through the transpeptidation reaction of bacterial γ-glutamyltranspeptidase. J. Agric. Food Chem. 50:313-318. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki, H., H. Kumagai, T. Echigo, and T. Tochikura. 1988. Molecular cloning of Escherichia coli K-12 ggt and rapid isolation of γ-glutamyltranspeptidase. Biochem. Biophys. Res. Commun. 150:33-38. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki, H., H. Kumagai, and T. Tochikura. 1986. γ-Glutamyltranspeptidase from Escherichia coli K-12: formation and localization. J. Bacteriol. 168:1332-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki, H., H. Kumagai, and T. Tochikura. 1986. γ-Glutamyltranspeptidase from Escherichia coli K-12: purification and properties. J. Bacteriol. 168:1325-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki, H., N. Miyakawa, and H. Kumagai. 2002. Enzymatic production of γ-L-glutamyltaurine through the transpeptidation reaction of γ-glutamyltranspeptidase from Escherichia coli K-12. Enzyme Microb. Technol. 30:883-888. [Google Scholar]
- 27.Tate, S. S., and A. Meister. 1981. γ-Glutamyl transpeptidase: catalytic, structural and functional aspects. Mol. Cell. Biochem. 39:357-368. [DOI] [PubMed] [Google Scholar]
- 28.Tate, S. S., and A. Meister. 1974. Interaction of γ-glutamyl transpeptidase with amino acids, dipeptides, and derivatives and analogs of glutathione. J. Biol. Chem. 249:7593-7602. [PubMed] [Google Scholar]
- 29.Thompson, G. A., and A. Meister. 1977. Interrelationships between the binding sites for amino acids, dipeptides, and γ-glutamyl donors in γ-glutamyl transpeptidase. J. Biol. Chem. 252:6792-6798. [PubMed] [Google Scholar]
- 30.Wilk, S., H. Mizoguchi, and M. Orlowski. 1977. γ-Glutamyl dopa: kidney-specific dopamine precursor. J. Pharmacol. Exp. Ther. 206:227-232. [PubMed] [Google Scholar]
- 31.Yamada, Y., M. Sakurai, and Y. Tsuchiya. 1966. The synthesis of γ-alkylamides of L-glutamic acid. The reactions of metallic salts of L-pyrrolidonecarboxylic acid with primary alkylamines. Bull. Chem. Soc. Jpn. 39:1999-2000. [Google Scholar]