Abstract
Vibrio cholerae serotype O1 is autochthonous to estuarine and coastal waters. However, its population dynamics in such environments are not well understood. We tested the proliferation of V. cholerae N16961 during a Lingulodinium polyedrum bloom, as well as other seawater conditions. Microcosms containing 100-kDa-filtered seawater were inoculated with V. cholerae or the 0.6-μm-pore-size filterable fraction of seawater assemblages. These cultures were diluted 10-fold with fresh 100-kDa-filtered seawater every 48 h for four cycles. Growth rates ranged from 0.3 to 14.3 day−1 (4.2 day−1 ± 3.9) for V. cholerae and 0.1 to 9.7 day−1 (2.2 ± 2.8 day−1) for bacterial assemblage. Our results suggest that dissolved organic matter during intense phytoplankton blooms has the potential to support explosive growth of V. cholerae in seawater. Under the conditions tested, free-living V. cholerae was able to reach concentrations per milliliter that were up to 3 orders of magnitude higher than the known minimum infectious dose (104 cell ml−1) and remained viable under many conditions. If applicable to the complex conditions in marine ecosystems, our results suggest an important role of the growth of free-living V. cholerae in disease propagation and prevention during phytoplankton blooms.
Vibrio cholerae is a facultative human pathogen found in coastal waters that causes the acute gastrointestinal disease cholera, a major health threat in poor nations that frequently results in mortality. Consequently, over the past two decades much work has been done to understand the ecology of V. cholerae. This effort is due not only to V. cholerae's current role in disease but also to changes anticipated in the incidence and distribution of infectious diseases in general in response to ecosystem perturbations such as global climate change (7, 12, 13).
Despite these efforts the proliferation and population dynamics of V. cholerae in the marine environment are still not well understood. The pandemic periodicity (6) and spread of cholera suggest a strong link between coastal ecosystem processes and the occurrence of disease (see, for example, references 18, 30, 33, and 35). Much of the research emphasis has been on the importance of attachment and growth of V. cholerae to pelagic plankton, especially copepod zooplankters. In areas of outbreak a temporal association between phytoplankton blooms, subsequent copepod blooms and V. cholerae has been proposed as a regulating factor for cholera outbreaks (see references 23 and 30). Furthermore, it is thought that V. cholerae growth is inhibited by the combined effect of high salinity and low temperature (in temperate waters) and that the organism usually persists in a viable, nonculturable but potentially dormant state (see references 10, 23, and 27 and references therein). Hence, in combination with attachment studies (24, 25), this observation has resulted in the implication of copepods as a vector for V. cholerae and disease outbreaks (see, for example, references 11 and 23). To date, few studies have focused on the importance of dissolved organic material (DOM) for V. cholerae growth in the natural environment, although DOM is known to support the growth of natural assemblages of marine bacteria (2, 17).
Proliferation of marine bacteria is dependent on the structure and dynamics of coastal ecosystems (see references 38 and 41). For instance, during different phytoplankton bloom stages, DOM concentration and water composition undergo dramatic changes. These variations in available organic matter are accompanied by strong shifts in bacterial abundance, growth, ectoenzymatic activity, and particle colonization (see, for example, reference 42). Furthermore, bacterial growth rates, particle colonization, and ectoenzymatic activity have been shown to increase during the decline phase of phytoplankton blooms (42). The concentrations and types of organic substrates available through the various stages of a phytoplankton bloom influence the growth of individual members of the bacterial community, as well as the expression of ecologically relevant biochemical activities (see, for example, references 37 and 38). If V. cholerae is capable of phenotypic plasticity similar to that of other marine and estuarine bacteria with respect to DOM-stimulated growth, then DOM availability may play an important role in regulating V. cholerae's population dynamics in the natural environment.
We hypothesized that V. cholerae could both persist and proliferate in the free-living state at the sole expense of the DOM pool present during an algal bloom. To address this hypothesis, we performed a series of microcosm experiments exploring growth in filtered seawater (100 kDa) and the effects of amendment with organic and inorganic nutrients on populations of the toxigenic V. cholerae O1 N16961 and compared these data with the response of the natural marine assemblage under the same conditions.
MATERIALS AND METHODS
Strain and inoculum preparation.
V. cholerae O1 N16961 was kindly provided by J. Mekalanos, Harvard University. Before experiments, T1N1 agar plates (1% tryptone, 1% sodium chloride, 1.5% agar) were inoculated with V. cholerae N16961 and incubated overnight at 37°C. A loopful of the resulting growth was resuspended in 150 ml of autoclaved 0.2 μm filtered seawater amended with 0.1% (wt/vol) tryptone and incubated at room temperature (23 ± 1°C) overnight. Cells were then harvested by centrifugation (3,100 × g, 10 min, room temperature), the supernatant was aspirated, and the remaining pellet was resuspended in 0.2-μm-filter-sterilized, autoclaved phosphate-buffered saline (pH 7.4). This washing process was repeated five times in order to prevent the carryover of medium nutrients. Finally, the pellet was resuspended in phosphate-buffered saline for subsequent inoculation of microcosms. To generate an inoculum representing the natural assemblage of bacteria from the marine environment, seawater was filtered through a 0.6-μm-pore-size polycarbonate filter (Osmonics), and the filtrate used as an inoculum. Epifluorescence microscopy of DAPI (4′,6′-diamidino-2-phenylindole)-stained inoculum showed no detectable protists or algae. Most viruses present in the seawater sample presumably passed into the inoculum, but we did not attempt to detect them. The DAPI method does not stain viruses intensely enough for detection.
Experimental design.
Microcosm experiments were performed by using the seawater collected: (i) during a bloom of the dinoflagellate Lingulodinium polyedrum (this experiment was started on 19 July 2001); (ii) during nonbloom conditions (this experiment was started on 13 April 2002); and (iii) during a Prorocentrum bloom (this experiment was started on 30 May 2002). Water was obtained from approximately the upper 0.5 m of surface waters off Scripps Pier (32°53′N, 117°15′W). Water temperature and salinity were as follows: 19.6°C, 33.6 psu (19 July); 16.4°C, 33.7 practical salinity units (psu) (13 April); 17.6°C, 33.8 psu (30 May) (data courtesy of the Scripps Shore Station Program). Seawater for all experiments was prefiltered by tangential flow through a 100-kDa filter (Pall Filtron). The microcosms were amended with inoculums of either V. cholerae (see Table 1 for final concentrations) or the marine assemblage (at 10% [vol/vol] of the total microcosm volume; see Table 1 for the final concentrations) prepared as described above. For a summary of the experimental setup see Table 1.
TABLE 1.
Amendment and cell concentrations, as well as total volume for each microcosm treatment at the onset of the experiments
| Microcosm and treatment | Amendment (final concn) | Mean cell concna ± SD (105 ml−1) | Vol (ml) |
|---|---|---|---|
| Microcosm A | |||
| VCRT | None | 16 ± 0.7 | 1,000 |
| MART | None | 7.9 ± 0.16 | 1,000 |
| Microcosm B | |||
| VCTA | 2.0 mg of tryptone liter−1 | 1.4 ± 0.31 | 450 |
| VCNA | None | 1.3 ± 0.01 | 450 |
| MATA | 2.0 mg of tryptone liter−1 | 0.45 ± 0.01 | 450 |
| MANA | None | 0.54 ± 0.42 | 450 |
| Microcosm C | |||
| B | None | 2.8 ± 0.13 | 450 |
| C | 2.0 mg of Casamino Acids liter−1 | 2.6 ± 0.14 | 450 |
| CFV | 2.0 mg of Casamino Acids liter−1; 10 ng l−1 FeCl3 liter−1 vitamins (concn as given below) | 2.2 ± 0.01 | 450 |
| F | 10 ng of FeCl3 liter−1 | 2.2 ± 0.20 | 450 |
| GNP | 1 mg of glucose liter−1; 59 μM ammonium nitrate; 725 nM phosphate | 2.2 ± 0.05 | 450 |
| T | 2.0 mg of tryptone liter−1 | 2.5 ± 0.10 | 450 |
| TM | F/100b | 2.2 ± 0.07 | 450 |
| V | 2 μg of B1 liter−1; 10 ng of B6 liter−1; 10 ng of B12 liter−1 | 2.4 ± 0.20 | 450 |
| GNPFV | GNP + F + V (concn as given above) | 2.8 ± 0.06 | 450 |
Average initial cell concentration after inoculation at the onset of the respective experiment (T0 sampling point) for duplicated treatments.
As defined by Guillard (16).
In microcosm experiment A, four acid-cleaned 2.8-liter glass flasks (Pyrex) each received 1 liter of 100-kDa filtrate of seawater taken during the bloom of L. polyedrum. Duplicate microcosms were inoculated with V. cholerae N16961 (VCRT). The other two microcosms were inoculated with the marine assemblage as described above (MART).
In microcosm experiment B, 450 ml of 100-kDa-filtered seawater was distributed into each of eight acid-cleaned 500-ml glass (Pyrex) flasks. These flasks were then amended as follows: V. cholerae inoculum and tryptone amendment (see Table 1 for concentrations), hereafter referred to as VCTA; marine assemblage inoculum and tryptone amendment (MATA); V. cholerae inoculum with no amendment (VCNA); and marine assemblage inoculum with no amendment (MANA). Each of the four different treatments was performed in duplicate, yielding a total of eight experimental microcosms.
In microcosm experiment C, nine treatments were performed with 450 ml of 100-kDa-filtered seawater per flask collected during a Prorocentrum bloom (all treatments were performed in duplicate). This experiment was designed to test the growth performance of V. cholerae under different nutritional scenarios and was inoculated with V. cholerae only. The amendments (see Table 1 for concentrations) were tryptone (T); Casamino Acids (C); Casamino Acids plus Fe plus vitamins (CFV); glucose plus ammonium nitrate plus phosphate (GNP); GNP plus Fe plus vitamins (GNPFV); Fe (F); trace metals (TM); vitamins (V); and a nonamended control (B).
Microcosms were incubated for four 48-h cycles at 23 ± 1°C under ambient laboratory light conditions (warm white fluorescent bulbs) on the lab bench. After 48 h, 1 volume of the cycle 1 microcosm was added to 9 volumes of 100-kDa-filtered seawater from the same initial source. This cycle 2 microcosm was sampled for another 48 h (cycle 2). The process was repeated two more times (cycles 3 and 4) so that the total duration of the experiments was 192 h. Each microcosm was subsampled periodically for DAPI-based cell counts, V. cholerae immunofluorescence counts, and for all V. cholerae microcosms, except VCRT, culturability assay (CFU plate counts).
Bacterial abundance.
Samples were fixed with formalin (2% final). Tween 80 (10 μg ml−1; Sigma) was then added, and the samples were sonicated for 60 s and stained for 10 min with DAPI at a final concentration of 1 to 5 μg ml−1 (47). Aliquots were then filtered onto 0.2-μm-pore-size black polycarbonate filters (Poretics), and the total bacteria were enumerated by epifluorescence microscopy (Olympus BX-51).
V. cholerae abundance.
V. cholerae cells were enumerated by both DAPI (as described above) and a fluorescent-antibody (19) techniques in separate preparations for which samples were fixed and filtered as described above. In brief, filters were placed on a glass slide, and a drop of 100% methanol was added. After they were air dried, the filters were incubated with a lyophilized monoclonal antibody-fluorescein isothiocyanate conjugate (Cholera-DFA kit; New Horizon Diagnostics Corp.), containing the monoclonal antibody to the O antigen of V. cholerae O1, in a dark moisture chamber at 37°C for 30 min. Samples were observed immediately or stored at −20°C and observed within 1 to 2 days by epifluorescence microscopy.
CFU.
T1N1 agar plates were spread with 0.1-ml portions of sample dilutions ranging from 100 to 10−5. Colonies were counted after incubation for 12 h at 37°C in the dark.
Statistics and growth rate calculations.
Means, standard deviations, and standard errors were calculated by combining values for duplicate microcosms. Regression analysis was used to assess the dependence of CFU on cell abundance. Significance was determined as highly significant (P < 0.001) and significant (P < 0.05); P values of >0.05 were considered not significant. The growth rate was calculated as that occurring in the first 24 h of each cycle based on changes in cell abundance between endpoint samples (i.e., T0 and T24), based on the assumption of exponential growth, using the equation: μ (day−1) = ln(T24/T0).
RESULTS
Microcosm experiment A. (i) Bacterial abundance.
In the red tide-derived 100-kDa-filtered seawater, V. cholerae abundance increased modestly within the first two cycles (1- to 4-fold) and by up to 25-fold (cycle 3) in later cycles (Fig. 1). Antibody counts performed on a subset of samples confirmed that increase in DAPI-based cell counts was due to antibody-positive V. cholerae N16961. The marine assemblage also increased modestly in the initial cycles (4- to 6-fold) but 28- and 57-fold in cycles 3 and 4, respectively.
FIG. 1.
Cell abundance of the marine assemblage and V. cholerae in microcosm A as determined by direct counts. Error bars represent the standard deviations of counts from duplicate treatments.
(ii) Growth rate.
V. cholerae did not show net growth within the first cycle, but in subsequent cycles it achieved growth rates up to 2.6 ± 0.3 day−1 (cycle 3) (Fig. 2). The marine assemblage grew at comparable rates to V. cholerae through the first three cycles and then reached a maximum rate of 2.7 ± 0.0 day−1 in cycle 4.
FIG. 2.
Growth rate (as calculated in Materials and Methods) for the marine assemblage (MART) and V. cholerae (VCRT) in microcosm A for each experiment cycle. Error bars represent the standard deviations for growth estimates from duplicate treatments.
Microcosm experiment B. (i) Bacterial abundance.
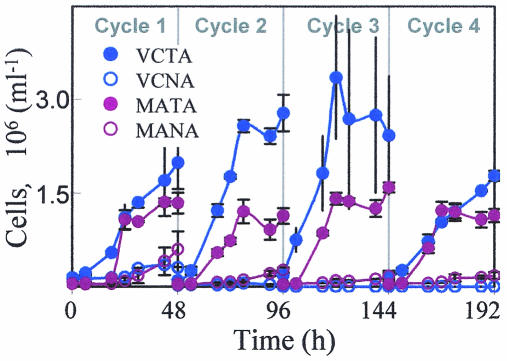
V. cholerae and the marine assemblage showed different treatment-dependent responses in terms of abundance changes over the course of this experiment (Fig. 3). Tryptone enrichment resulted in abundance increases of 14- and 24-fold for V. cholerae and the marine assemblage, respectively, between T0 (initial) to T192 (final). In the nonamended treatments V. cholerae declined by 2,000-fold, whereas the marine assemblage increased by 3-fold over the course of the experiment.
FIG. 3.
Cell abundance of the marine assemblage and V. cholerae in both the nonamended and tryptone-amended treatments of microcosm B as determined by direct counts. Error bars represent the standard deviations of counts from duplicate treatments.
(ii) Growth rates.
V. cholerae achieved positive growth rates in all cycles of the tryptone-enriched treatment (Fig. 4). In the nonamended treatment, V. cholerae showed low or negative growth rates. The marine assemblage growth rate was on average slightly higher (1.36-fold) than that of V. cholerae in the tryptone amendment. In the nonamended treatment the marine assemblage always experienced positive growth, whereas V. cholerae did not.
FIG. 4.
Growth rate for the marine assemblage in nonamended (MANA) and tryptone-amended (MATA) treatments and the same for V. cholerae (VCNA and VCTA, respectively) in microcosm B for each experiment cycle. Error bars represent the standard deviations for growth estimates from duplicate treatments.
(iii) Culturability.
Like abundance by direct counts, CFU-based abundance increased within each cycle and over the entire course of the experiment in the tryptone enrichment (Fig. 5), yielding a high correlation between direct counts and CFU (r2 = 0.79, P < 0.0001). In contrast, CFU decreased dramatically (by 3 orders of magnitude) in the nonamended control.
FIG. 5.
CFU for V. cholerae in tryptone-enriched (VCTA) and nonamended seawater (VCNA) in microcosm B. Error bars represent the standard deviations for CFU determinations from duplicate treatments.
Microcosm experiment C. (i) Bacterial abundance.
Abundance in treatments that included DOC enrichment increased by more than an order of magnitude over the course of the experiment (Fig. 6). Within these treatments cell abundance frequently reached a plateau during the later part of each cycle. All other treatments, as well as the control, increased approximately twofold by the end of the experiment.
FIG. 6.
V. cholerae cell abundance changes in the different treatments used in microcosm C (as defined in Table 1) over each of the four 48-h cycles. (A) Carbon-amended treatments. Triangles: dark blue, treatment C; gray, treatment CFV; orange, treatment GNP; red, treatment T; light blue, treatment GNPFV. (B) Other treatments. Circles: dark green, treatment B; brown, treatment F; yellow, treatment TM; light green, treatment V. Values were determined by direct counts (see Table 1). Error bars represent the standard deviations of counts from duplicate treatments.
(ii) Growth rate.
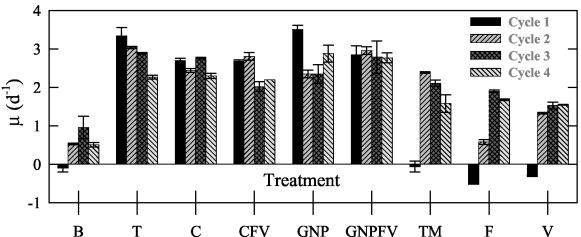
V. cholerae showed significant growth in the carbon-enriched treatments (Fig. 7) and, over the course of the experiments, it was capable of growth rates of up to 3.5 ± 0.1 day−1 (GNP, cycle 1). Other amended treatments (those with no additional C source), and the nonamended control experienced no or negative growth in the first cycle of the experiment. In subsequent cycles of these treatments, growth was positive, with rates up to 2.9 ± 0.0 day−1 (TM, cycle 2).
FIG. 7.
V. cholerae growth rates for each cycle of the different microcosm C treatments (as defined in Table 1) as calculated from direct counts (see Materials and Methods). Error bars represent the standard deviations for growth estimates from duplicate treatments.
(iii) Culturability.
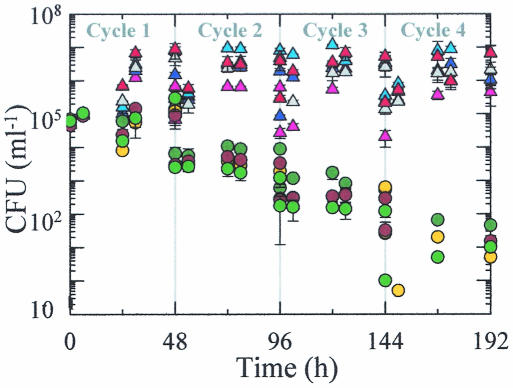
V. cholerae CFU increased within each cycle of carbon enriched treatments but decreased (by 4 orders of magnitude) within treatments that did not receive carbon enrichment (Fig. 8 and 9). Direct cell count and CFU were significantly correlated within some treatments but not others (Table 2). The strength of correlation and level of significance varied. Treatments GNPFV, C, CFV, and GNP exhibited the strongest relationships between these two parameters at the highest significance level.
FIG. 8.
Changes in V. cholerae CFU in microcosm C carbon-amended treatments—C (dark blue triangle), CFV (gray triangle), GNP (orange triangle), T (red triangles), and GNPFV (light blue triangles)—and in other treatments—B (dark green circles), F (brown circles), TM (yellow circles), and V (light green circles). Error bars represent the standard deviations for CFU determinations from duplicate treatments.
FIG. 9.
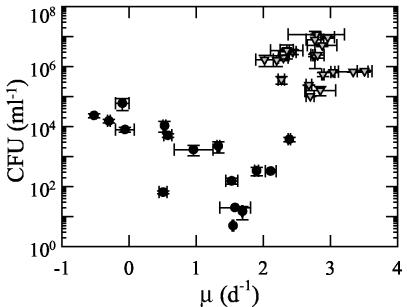
Relationship between growth rate and CFU count for V. cholerae in carbon-amended treatments (▿) and other treatments (•) in microcosm C. Growth rate values were determined for the initial 24 h of each cycle from direct counts (as in Materials and Methods), and the CFU values are for the 24-h time point in each cycle.
TABLE 2.
Dependence of CFU on direct cell counts in microcosm C
| Treatment | r2 | Pa |
|---|---|---|
| B | 0.0003 | NS |
| C | 0.68 | <0.001 |
| CFV | 0.66 | <0.001 |
| F | 0.244 | <0.05 |
| GNP | 0.50 | <0.001 |
| T | 0.30 | <0.05 |
| TM | 0.0048 | NS |
| V | 0.008 | NS |
| GNPFV | 0.82 | <0.001 |
That is, the significance of linear regression. P < 0.005, significant; P < 0.001, highly significant; P > 0.05, not significant (NS).
DISCUSSION
Significance of DOM for V. cholerae growth in the marine environment.
The identification of marine plankton as a vector for V. cholerae has been an important advance in understanding the mechanisms of environmental persistence and proliferation of this organism (see references 9 and 22). Laboratory incubation of V. cholerae with zooplankton, phytoplankton, and detrital particles results in attachment of V. cholerae to these “particles” (21, 25, 43). Furthermore, by using a V. cholerae/V. mimicus oligonucleotide probe, the attachment of V. cholerae/V. mimicus to zooplankton has been demonstrated in the natural environment, although a significant quantity of free-living V. cholerae/V. mimicus can also be present (20). The environmental concentrations of potential vectors (e.g., zooplankton) are generally intensified during phytoplankton blooms and dynamically change at different stages of bloom development and demise (28, 38, 42). These considerations have led to the proposal that phytoplankton blooms, and the patterns of bloom progression, are useful predictors of cholera outbreaks. For instance, two potential vectors—copepods and detritus—generally increase late in blooms. We considered that, in addition to the particulate phase vectors, DOM could also play a role in the persistence and “blooms” of free-living V. cholerae as it does for other marine bacteria (2, 17). Testing this hypothesis is of interest because free-living V. cholerae cells are likely to have a different ecosystem behavior and infection route than particle-associated V. cholerae.
Phytoplankton blooms, particularly during the decline phase, cause large DOM input into seawater. Significantly, the rapid turnover (half-life of a few days) of labile DOM by pelagic bacterial keeps DOM concentration in check, so that generally it varies by no more than a factor of 2 (28, 42). In order to design an experiment to test the potential of V. cholerae for growth in a low-concentration but high-flux regime, we used semicontinuous-batch microcosms in which 90% of the contents were replaced every 48 h with fresh 100-kDa-filtered seawater. In addition to approximating environmental DOM renewal, we sought to reduce the container wall effect. Parallel measurements of the growth of a natural assemblage of bacteria provided a quantitative environmental context for assessing the growth performance of V. cholerae. Previous research has shown that members of the α- and γ-proteobacteria, including the groups Alteromonadaceae, Vibrionaceae, and Oceanospirillum, as well as members of the Firmicutes, Cytophagales, and Cyanobacteria, are present at the study site from which the natural assemblage was derived (see, for example, references 14, 37, and 38). We included only <100-kDa DOM, rather than the typically used DOM from <0.2- or 0.45-μm-filtered seawater fraction, in order to exclude colloids and fine particles, since the boundary between DOM and POM is indistinct and difficult to define (1, 3). We recognize the potential of colloidal organic matter to support bacterial growth, and inclusion of this material would probably have supported faster growth. Further, we sought to minimize the effect of bacteriophages on bacterial growth dynamics. Viruses may have been present at low concentrations in the marine assemblage experiments since the natural seawater 0.6-μm filtrate used to inoculate these microcosms presumably contained viruses.
V. cholerae growth performance in particle-free seawater.
V. cholerae abundance increased dramatically in bloom influenced waters (VCRT) of microcosm A (Fig. 1). Microcosm B was designed to provide a nonbloom water contrast for the growth of V. cholerae during low-enrichment periods. Artificial amendment of this nonbloom water with tryptone was used to represent the readily utilizable organic material expected to be introduced during a bloom such as during microcosm A. The goal of microcosm C was to test that the V. cholerae growth response to bloom DOM seen in microcosm A was repeatable under a bloom of a different organism. Furthermore, we sought to test whether the response could be enhanced by amendment with a variety of nutrient additions. The amendments included nitrogen, phosphorus, and carbon in formats that, once again, could mimic the introduction of readily utilizable organic material expected in the DOM fraction during a bloom. Over the entire course of these experiments abundance increases ranged from modest (1.5- to 2.5-fold) in enrichments with no additional carbon source (i.e., treatments B, F, TM, and V) to several orders of magnitude in carbon-enriched treatments, as well as VCRT. The impact of carbon amendments cannot be assigned unequivocally to the influence of improved carbon availability since the amended carbon sources included nitrogen and phosphorus sources. The only treatment in which V. cholerae abundance declined was the nonamended nonbloom waters of VCNA (Fig. 3).
As mentioned above, VCNA was the only treatment in which V. cholerae declined throughout the course of the experiment, a result probably due both to dilution effects and mortality. However, in some treatments, particularly those without amended carbon (e.g., treatments B, TM, F, and V), V. cholerae appeared to experience an initial lag in cell growth but subsequent acclimation and growth by the second or third cycle of the experiment. The protocol used to prepare V. cholerae for inoculation into the cycle 1 microcosm has previously been shown to impact cell viability (45); however, since no such lag or decline was observed in other treatments it is unlikely that inoculum preparation was the determining factor for this response. The observed lag is in contrast to the results of Singleton et al. (39), who found that cell numbers increased within the initial 2 days of inoculation into nutrient-free artificial seawater and then remained stable or declined slightly over a longer time frame. In this case cell division was proposed to be the result of completion of previously initiated multiple replication forks based on endogenous nutrient sources from the nutrient conditioned (prior to microcosm inoculation) cells in combination with decreased cell volume in a process known as reduction division (i.e., cell numbers increase at the expense of biomass). Such growth and size reduction or “reduction division” has been confirmed by Baker et al. (4). We also observed cell volume reduction of V. cholerae (Fig. 10) in some treatments. It is of interest whether all material and energy during reduction division is derived from endogenous pools as previously suggested or whether net DOM uptake makes a significant contribution. Given that cell numbers declined in VCNA but increased in treatments with bloom-influenced waters (treatments B and VCRT), it is unlikely that endogenous nutrient resources retained from the initial growth media (which was the same for all three microcosm experiments) are solely responsible for the observed growth in our experiments. Furthermore, given that in microcosm B the marine assemblage (MANA) grew, it is unlikely that the decline observed in VCNA was due to a problem such as accidental toxic contamination.
FIG. 10.
DAPI-stained V. cholerae N16961 images of VCRT T0 (A) and VCRT T192 (B), as well as nonbloom influenced VCNA T0 (C) and VCNA T192 (D). All images were exposed for 100 ms and are shown at 1,000× magnification.
In natural assemblages of pelagic marine bacteria, the average cell size is similar to that of V. cholerae cells that have undergone reduction division. Likewise, the diminutive pelagic bacteria tend to become larger upon attachment to marine particles or during growth in rich medium (2). Although reduction division in the free-living state may be a mechanism to increase population size at the expense of biomass accumulated during transient growth in enriched microenvironments, diminution also greatly increases cell surface area/volume ratio. Thus, it may constitute an adaptive response to enhance DOM uptake from seawater. Therefore, we might conclude that V. cholerae growth performance and strategies are not novel; rather, they may reflect a common adaptive ability of pelagic marine bacteria not just to survive via dormancy but rather to acclimate in order to achieve growth. This hypothesis is supported by the positive growth rates seen in the third and fourth cycles of treatments in which V. cholerae growth had initially lagged (Fig. 7), indicating that after an acclimation period it was able to utilize ambient nutrient sources to achieve net growth.
One factor that has shaped the view that copepods play a major role in the proliferation of V. cholerae is the presence of chitinase in this organism (22). This enzyme is capable of degrading chitin, a structural polysaccharide in many marine invertebrates including copepods. Genes encoding chitinase have been found in many marine bacteria (see, for example, references 5 and 36), although no study has examined whether this implies specific niche adaptation to carapaces or copepods. Our results showing successful V. cholerae growth solely on the dissolved fraction of natural seawater, even in comparison to the natural bacterial assemblage (Fig. 2 and 4), provide another key insight into the ecology of this organism. This apparent plasticity is not unexpected given the results of previous studies. For example V. cholerae has been shown to tolerate a wide range of pH, salinity, and temperature (see, for example, references 32, 39, and 40). In addition, it has been found to attach not only to copepods (20, 25) but also to a variety of algal cells (21, 26). Furthermore, Tamplin et al. (43) found that V. cholerae O1 had a significantly higher degree of binding to whole phytoplankton cells and detritus than to whole copepods in Bangladesh fresh waters. These results do not refute the idea that copepods play a role in V. cholerae proliferation but rather highlight the ecophysiological plasticity of this organism and its ability to succeed under various environmental scenarios.
Relationship between CFU and V. cholerae abundance and growth in seawater.
We examined the dependence of CFU on direct count cell abundance and growth rate in microcosm C by using regression analysis. There was a significant positive relationship between CFU and direct counts in carbon amended treatments, but in other treatments this generally was not the case (Table 2). Similarly, Singleton et al. (39) found no significant difference between mean CFU and mean total cell counts in seawater enriched with 1 mg of tryptone per liter or more. However, in lower DOC concentrations these authors found CFU gave considerably lower abundance estimates than direct counts. These results highlight the difficulty associated with interpreting experimental results based on CFU, especially if treatments differ in DOM concentrations.
In 1982 the ability of V. cholerae to enter a viable but nonculturable (VBNC) state was noted (45) and led to the development of direct count methods (see, for example, references 19 and 46). The “biological meaning” of the VBNC state has been debated for some time (see, for example, references 8, 31, and 44). Some reviews of the V. cholerae literature (see, for example, references 22 and 27) have described VBNC as a state of dormancy (see also reference 44). Kaper et al. (27) described VBNC as “… a dormant state in which it is still viable but not culturable in conventional laboratory media… cells are reduced in size and become ovoid.” In our study, CFU and growth rate were highly related (r2 = 0.40, P < 0.001) within carbon-amended treatments but not in other treatments (Fig. 9). Interestingly, after an initial growth lag (and cell death) during the first two cycles of treatments TM, F, and V, the growth rate was stimulated above that of treatment B (Fig. 7), which was itself experiencing positive growth. Thus, even when a low CFU count might be interpreted to indicate that the V. cholerae is dormant, nongrowing, or dying, it may actually be growing rapidly. Likewise, although a low portion (typically 0.1%) of the natural marine assemblage forms colonies on traditional media, microautoradiography after [3H]thymidine or 3H-labeled amino acid incorporation has shown that a large fraction (typically >50%) is growing (see, for example, references 15 and 34). Our results indicate that V. cholerae behaves similarly to the natural bacterial assemblages in this respect as well: certain environmental conditions may render growing cells unable to form colonies on nutrient agar. Taken together, these results indicate that the absence of culturable V. cholerae in the environment may at times accompany the presence of V. cholerae enjoying robust growth. In our study, V. cholerae appeared capable of growth rates comparable to the coastal bacterial assemblages (Fig. 2, 4). The success of V. cholerae in these conditions and the mimicry of high grazing mortality (via dilutions at the end of each cycle) suggest that V. cholerae may be able to compete effectively in the natural environment.
To our knowledge there are no published reports of free-living V. cholerae growth rates in natural seawater. We predict that free-living V. cholerae in coastal and estuarine waters grow rapidly under DOM-enriched conditions existing during phytoplankton blooms. This should be testable, in a culture-independent mode, by the concurrent application of species-specific probes (e.g., monoclonal antibodies or nucleic acid probes) and [3H]thymidine microautoradiography (34). Nonculturable V. cholerae O1 has been shown to cause disease symptoms (see reference 8 and references therein), suggesting that nonculturable free-living V. cholerae can be infectious. One factor that may provide insight into predicting infectivity routes is understanding whether infectivity is growth rate dependent.
The potential of V. cholerae to rapidly develop and sustain large and dynamic free-living populations, as seen here, has implications for its infection route and for strategies to minimize the inadvertent ingestion of this pathogen. Colwell et al. (11) developed a simple and effective method found to reduce the incidence of cholera in Bangladeshi villages by 48%. The method is based on reducing the particle-associated V. cholerae load of drinking water, obtained from wells or rivers, by filtration through Sari cloth (which retained >20-μm particles). Free-living V. cholerae, passing into the filtered water and harboring the infectious dose (minimum of 104 cells; see Lipp et al. [29] and references therein) in 1 to 100 ml of water, could have contributed to the remaining 52% of the infections encountered in that study. Our study underscores the importance of understanding the ecophysiology of free-living V. cholerae and their potential role in cholera transmission.
Acknowledgments
We appreciate many helpful discussions with project coinvestigator D. Bartlett and his comments on the manuscript. K. Hamasaki, R. Long, F. Malfatti, H. Yoshihara, and E. Zamora assisted with various parts of the experiments. We also thank Anne Sugiura for comments on the manuscript. We are grateful for the helpful comments and suggestions made by two anonymous reviewers.
This work was funded by NIH grant AI46600-03 to F.A.
REFERENCES
- 1.Alldredge, A. L., U. Passow, and B. E. Logan. 1993. The abundance of a class of large, transparent organic particles in the ocean. Deep Sea Res. 40:1131-1140. [Google Scholar]
- 2.Ammerman, J. W., J. A. Fuhrman, A. Hagström, and F. Azam. 1984. Bacterioplankton growth in seawater. I. Growth kinetics and cellular characteristics in seawater cultures. Mar. Ecol. Prog. Ser. 18:31-39. [Google Scholar]
- 3.Azam, F., D. C. Smith, G. F. Steward, and A. Hagström. 1993. Bacteria-organic matter coupling and its significance for oceanic carbon cycling. Microb. Ecol. 28:167-179. [DOI] [PubMed] [Google Scholar]
- 4.Baker, R. M., F. L. Singleton, and M. A. Hood. 1983. Effects of nutrient deprivation on Vibrio cholerae. Appl. Environ. Microbiol. 46:930-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassler, B. L., C. Yu, Y. C. Lee, and S. Roseman. 1991. Chitin utilization by marine bacteria, degradation and catabolism of chitin oligosaccharides by Vibrio furnissi. J. Biol. Chem. 266:24276-24286. [PubMed] [Google Scholar]
- 6.Blake, P. A. 1994. Historical perspectives on pandemic cholerae, p. 293-295. In I. K. Wachsmuth, P. A. Blake, and O. Olsvik (ed.), Vibrio cholerae and cholera: molecular to global perspectives. ASM Press, Washington, D.C.
- 7.Colwell, R. R. 1996. Global climate and infectious disease: the cholera paradigm. Science 274:2025-2031. [DOI] [PubMed] [Google Scholar]
- 8.Colwell, R. R. 2000. Viable but nonculturable bacteria: a survival strategy. J. Infect. Chemother. 6:121-125. [DOI] [PubMed] [Google Scholar]
- 9.Colwell, R. R., and A. Huq. 2001. Marine ecosystems and cholera. Hydrobiologia 460:141-145. [Google Scholar]
- 10.Colwell, R. R., and A. Huq. 1994. Vibrios in the environment: viable but non-culturable Vibrio cholerae, p. 117-133. In I. K. Wachsmuth, P. A. Blake, and Ø. Olsvik (ed.), Vibrio cholerae and cholera: molecular to global perspectives. ASM Press, Washington, D.C.
- 11.Colwell, R. R., A. Huq, M. Sirajul Islam, K. M. A. Aziz, M. Yunus, N. Huda Khan, A. Mahmud, R. Sack, G. B. Nair, J. Chakraborty, D. A. Sack, and E. Russek-Cohen. 2003. Reduction of cholera in Bangladeshi villages by simple filtration. Proc. Natl. Acad. Sci. USA 100:1051-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colwell, R. R., and J. A. Patz. 1997. Climate, infectious disease, and health: an interdisciplinary perspective. The American Academy of Microbiology, Washington, D.C. [PubMed]
- 13.Epstein, P. R. 1995. Emerging diseases and ecosystems instability: new threats to public health. Am. J. Public Health 85:168-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fandino, L. B., L. Riemann, G. F. Steward, R. A. Long, and F. Azam. 2001. Variations in bacterial community structure during a dinoflagellate bloom analyzed by DGGE and 16S rDNA sequencing. Aquat. Microb. Ecol. 23:119-130. [Google Scholar]
- 15.Fuhrman, J. A., and F. Azam. 1982. Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: evaluation and field results. Mar. Biol. 66:109-120. [Google Scholar]
- 16.Guillard, R. R. L. 1975. Culture of phytoplankton for feeding marine invertebrates, p. 29-60. In W. L. Smith and M. H. Chanley (ed.), Culture of marine invertebrate animals. Plenum Press, Inc., New York, N.Y.
- 17.Hagström, A., J. W. Ammerman, S. Henrichs, and F. Azam. 1984. Bacterioplankton growth in seawater. II. Organic matter utilization during steady-state growth in seawater cultures. Mar. Ecol. Prog. Ser. 18:41-48. [Google Scholar]
- 18.Harvell, C. D., K. Kim, J. M. Burkholder, R. R. Colwell, P. R. Epstein, D. J. Grimes, E. E. Hofmann, E. K. Lipp, A. D. M. E. Osterhaus, R. M. Overstreet, J. W. Porter, G. W. Smith, and G. R. Vasta. 1999. Emerging diseases: climate links and anthropogenic factors. Science 285:1505-1510. [DOI] [PubMed] [Google Scholar]
- 19.Hasan, J. A. K., A. Huq, G. B. Nair, S. Garg, A. K. Mukhopadhyay, L. Loomis, D. Bernstein, and R. R. Colwell. 1995. Development and testing of monoclonal antibody-based rapid immunodiagnostic test kits for direct detection of Vibrio cholerae O139 synonym Bengal. J. Clin. Microbiol. 33:2935-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidelberg, J. F., K. B. Heidelberg, and R. R. Colwell. 2002. Bacteria of the g-subclass Proteobacteria associated with zooplankton in Chesapeake Bay. Appl. Environ. Microbiol. 68:5498-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hood, M. A., and P. A. Wointer. 1997. Attachment of Vibrio cholerae under various environmental conditions and to selected substrates. FEMS Microbiol. Ecol. 22:215-223. [Google Scholar]
- 22.Huq, A., and R. R. Colwell. 1996. Vibrios in the marine and estuarine environment: tracking Vibrio cholerae. Ecosystem Health 2:198-214. [Google Scholar]
- 23.Huq, A., and R. R. Colwell. 1995. Vibrios in the marine and estuarine environments. J. Mar. Biotechnol. 3:60-63. [Google Scholar]
- 24.Huq, A., E. B. Small, P. A. West, M. I. Huq, R. Rahman, and R. R. Colwell. 1983. Ecological reolationships between Vibrio cholerae and planktonic crustacean copepods. Appl. Environ. Microbiol. 45:275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huq, A., P. A. West, E. B. Small, M. I. Huq, and R. R. Colwell. 1984. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar O1 associated with live copepods in laboratory microcosms. Appl. Environ. Microbiol. 48:420-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Islam, S. M., B. S. Drasar, and D. J. Bradley. 1990. Long-term persistence of toxigenic Vibrio cholerae O1 in the muscilaginous sheath of a blue-green alga. J. Trop. Med. Hyg. 93:113-139. [PubMed] [Google Scholar]
- 27.Kaper, J. B., J. G. Morris, and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirchman, D. L., Y. Suzuki, C. Garside, and H. W. Ducklow. 1991. High turnover rates of dissolved organic carbon during a spring phytoplankton bloom. Nature 352:612-614. [Google Scholar]
- 29.Lipp, E. K., A. Huq, and R. R. Colwell. 2002. Effects of global climate on infectious disease: the cholera model. Clin. Microbiol. Rev. 15:757-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lobitz, B., L. Beck, A. Huq, B. Wood, G. Fuchs, A. S. G. Faruque, and R. R. Colwell. 2000. Climate and infectious disease: use of remote sensing for detection of Vibrio cholerae by indirect measurements. Proc. Natl. Acad. Sci. USA 97:1438-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDougald, D., S. A. Rice, D. Weichart, and S. Kjelleberg. 1998. Nonculturability: adaptation or debilitation? FEMS Microbiol. Ecol. 25:1-9. [Google Scholar]
- 32.Miller, C. J., B. S. Drasar, and R. G. Feachem. 1984. Response of toxigenic Vibrio cholerae O1 to physio-chemical stresses in the aquatic environment. J. Hyg. 93:475-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munro, P. M., and R. R. Colwell. 1996. Fate of Vibrio cholerae O1 in seawater microcosms. Water Res. 30:47-50. [Google Scholar]
- 34.Ouverney, C. C., and J. A. Fuhrman. 1999. Combined microautoradiography-16S rRNA probe technique for determination of radioisotope uptake by specific microbial cell types in situ. Appl. Environ. Microbiol. 65:1746-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pascual, M., M. J. Bouma, and A. P. Dobson. 2002. Cholera and climate: revisiting the quantitative evidence. Microbes Infect. 4:237-245. [DOI] [PubMed] [Google Scholar]
- 36.Ramaiah, N., R. T. Hill, J. Chun, J. Ravel, M. H. Matte, W. L. Straube, and R. R. Colwell. 2000. Use of a chiA probe for detection of chitinase genes in bacteria from the Chesapeake Bay. FEMS Microbiol. Ecol. 34:63-71. [DOI] [PubMed] [Google Scholar]
- 37.Riemann, L., and F. Azam. 2002. Widespread N-acetyl-d-glucosamine uptake among pelagic marine bacteria and its ecological implications. Appl. Environ. Microbiol. 68:5554-5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riemann, L., G. F. Steward, and F. Azam. 2000. Dynamics of bacterial community composition and activity during a mesocosm diatom bloom. Appl. Environ. Microbiol. 66:578-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singleton, F. L., R. Attwell, S. Jangi, and R. R. Colwell. 1982. Effects of temperature and salinity on Vibrio cholerae growth. Appl. Environ. Microbiol. 44:1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singleton, F. L., R. W. Attwell, M. S. Jangi, and R. R. Colwell. 1982. Influence of salinity and organic nutrient concentration on survival and growth of Vibrio cholerae in aquatic microcosms. Appl. Environ. Microbiol. 43:1080-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, D. C., M. Simon, A. L. Alldredge, and F. Azam. 1992. Intense hydrolytic enzyme activity on marine aggregates and implications for rapid particle dissolution. Nature 359:139-142. [Google Scholar]
- 42.Smith, D. C., G. F. Steward, R. A. Long, and F. Azam. 1995. Bacterial mediation of carbon fluxes during a diatom bloom in a mesocosm. Deep Sea Res. 42:75-97. [Google Scholar]
- 43.Tamplin, M. L., A. L. Gauzens, A. Huq, D. A. Sack, and R. R. Colwell. 1990. Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplnkton of Bangladesh waters. Appl. Environ. Microbiol. 56:1977-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wai, S. N., Y. Mizunoe, and S. Yoshida. 1999. How Vibrio cholerae survive during starvation. FEMS Microbiol. Lett. 180:123-131. [DOI] [PubMed] [Google Scholar]
- 45.Xu, H. S., N. Roberts, F. L. Singleton, R. W. Attwell, D. J. Grimes, and R. R. Colwell. 1982. Survival and viability of non-culturable Escherichia coli and Vibrio cholerae in th estuarine and marine environment. Microb. Ecol. 8:313-323. [DOI] [PubMed] [Google Scholar]
- 46.Xu, H. S., N. C. Roberts, L. B. Adams, P. A. West, R. J. Siebling, A. Huq, M. I. Huq, R. Rahman, and R. R. Colwell. 1984. An indirect fluorescent antibody staining procedure for detection of Vibrio cholerae serovar O1 cells in aquatic environmental samples. J. Microbiol. Methods 2:221-231. [Google Scholar]
- 47.Yoon, W. B., and R. A. Rosson. 1990. Improved method of enumeration of attached bacteria for study of fluctuation in the abundance of attached and free-living bacteria in response to diel variation in seawater turbidity. Appl. Environ. Microbiol. 56:595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]