Abstract
Samples from diverse upland soils that oxidize atmospheric methane were characterized with regard to methane oxidation activity and the community composition of methanotrophic bacteria (MB). MB were identified on the basis of the detection and comparative sequence analysis of the pmoA gene, which encodes a subunit of particulate methane monooxygenase. MB commonly detected in soils were closely related to Methylocaldum spp., Methylosinus spp., Methylocystis spp., or the “forest sequence cluster” (USC α), which has previously been detected in upland soils and is related to pmoA sequences of type II MB (Alphaproteobacteria). As well, a novel group of sequences distantly related (<75% derived amino acid identity) to those of known type I MB (Gammaproteobacteria) was often detected. This novel “upland soil cluster γ” (USC γ) was significantly more likely to be detected in soils with pH values of greater than 6.0 than in more acidic soils. To identify active MB, four selected soils were incubated with 13CH4 at low mixing ratios (<50 ppm of volume), and extracted methylated phospholipid fatty acids (PLFAs) were analyzed by gas chromatography-online combustion isotope ratio mass spectrometry. Incorporation of 13C into PLFAs characteristic for methanotrophic Gammaproteobacteria was observed in all soils in which USC γ sequences were detected, suggesting that the bacteria possessing these sequences were active methanotrophs. A pattern of labeled PLFAs typical for methanotrophic Alphaproteobacteria was obtained for a sample in which only USC α sequences were detected. The data indicate that different MB are present and active in different soils that oxidize atmospheric methane.
Methane (CH4) is present in the atmosphere at a mixing ratio of about 1.7 ppm of volume (ppmv). An estimated 30 Tg of CH4 from the atmosphere year−1 is oxidized by aerobic methanotrophic bacteria (MB) in upland soils, accounting for about 6% of the global atmospheric CH4 sink (21, 31). Bender and Conrad (2) suggested that MB active in upland soils are specialized oligotrophs adapted to the trace level of atmospheric CH4 and possess a methane monooxygenase (MMO) with a higher substrate affinity than that of cultivated MB. It was later demonstrated that the application of single-reactant Michaelis-Menten kinetics to MMO is not always appropriate and that the apparent affinity for CH4 varies depending on the cultivation conditions (13). Nevertheless, recent studies indicate that MB in at least some soils that oxidize atmospheric CH4 are indeed taxonomically distinct from known MB (28).
The 13 recognized genera of MB are divided into two groups, type I (further divided into types I and X) and type II. These differ in phylogenetic affiliation (Gammaproteobacteria versus Alphaproteobacteria) and in diverse biochemical characteristics (21). Identification of MB in soils is often performed by the cultivation-independent detection of a fragment of pmoA, a gene encoding the active-site subunit of particulate MMO (22, 26, 30, 35, 38). This marker gene is present in all known MB, with the exception of Methylocella palustris (12) and Methylocella silvestris (14). Sequence-based pmoA phylogeny correlates well with 16S rRNA-based phylogeny, so pmoA sequences can be assigned to specific genera or even species of MB (24, 28, 35). The pmoA gene is therefore an excellent functional gene marker and has been widely used to characterize methanotrophic communities in soils, including upland soils that consume atmospheric methane (4, 18, 23, 28, 32, 42, 45). In some of these soils, a novel sequence cluster usually called the “forest sequence cluster” (here USC α) has been detected (4, 23, 28, 32). The most closely related pmoA sequence from a pure culture is that of Methylocapsa acidiphila, a type II MB isolated from acidic peat (11). Besides this novel sequence cluster, pmoA sequences related to the genera Methylocystis, Methylosinus, Methylomonas, Methylobacter, Methylomicrobium, Methylococcus, and Methylocaldum have been detected in upland soils (4, 28, 32, 42, 45).
Detection of a particular pmoA gene in a soil does not necessarily imply that the respective methanotroph is physiologically active in this soil. Thus, it remains unclear whether all of the genera of MB detected in upland soils are involved in the process of atmospheric methane oxidation. Holmes et al. (28) combined pmoA analysis of soils with 14C labeling of phospholipid fatty acids (PLFAs), which are useful biomarkers with which to distinguish different groups of MB (6, 19). Soils were incubated with 14CH4, and the resulting 14C-labeled PLFA profiles were similar but not identical to the PLFA profiles of type II MB (Alphaproteobacteria). Only pmoA sequences of USC α were detected in these soil samples. On the basis of these combined results, the existence of a novel group of methanotrophic Alphaproteobacteria involved in atmospheric methane oxidation was postulated. Later work with 14CH4 or 13CH4 labeling of PLFAs has supported this hypothesis (8, 44). Soil pH values were reported in two of these studies, and all were acidic (pH ≤5.2) (28, 44). The close relationship of the novel pmoA sequences to that of the acidophile M. acidiphila may indicate that the putative MB possessing these sequences are specialized acidophiles.
The influence of various physicochemical soil parameters (temperature, water content, pH, ammonium content, land use, fertilization, and texture) on methane oxidation activity in upland soils has been examined in several studies (36). The influence of these parameters on the composition of the methanotrophic community has only rarely been studied, e.g., the vertical distribution of MB in a soil (23) or the effects of land use (42). The aim of our study was to characterize MB communities and methane oxidation activity in diverse upland soils varying in pH, soil type, land use, and plant cover. To characterize the community of physiologically active MB, selected soil samples were incubated with 13CH4, followed by extraction and analysis of PLFAs.
MATERIALS AND METHODS
Sampling sites and soil characteristics.
Sampling sites were located near Eiterfeld, Bad Bentheim, Gerold, Marburg, Würzburg, and Göttingen (Germany), near Welschnofen and Völser Aicha (Italy), and near Denekamp (The Netherlands) (Table 1 ). Soil samples were taken in May to July 2001 and in April 2002 from the upper mineral horizon (generally 5 to 20 cm from the soil surface). The soil types were determined by reference to soil maps (1:25,000). Further characterization of the soil samples included measurements of pH in water and ammonium concentration (33). On the basis of the initial results, sites E 5FB, G 15F, G 13FH, G 44F, G 44W, and MF were selected for further analyses, and fresh samples of these soils were taken in July 2002.
TABLE 1.
Descriptions of sampling sites, soil pHs, and kinetic values of methane oxidationa
| Location or time and soil sample | Position | Soil type, German classification (corresponding FAOc soil group) | Land use or plant cover | pH | Vmax(app) | a0s | Km(app) |
|---|---|---|---|---|---|---|---|
| Eiterfeld | |||||||
| E 20 | 50°45.406′N, 9°49.721′E | Parabraunerde (Luvisol) | Farmland (wintergrain) | 7.6 | 0.00 | ||
| E 33a | 50°45.252′N, 9°48.678′E | Pseudogley Parabraunerde (Gleyic Luvisol) | Deciduous forest (Fagus sylvatica, Quercus sp.) | 6.6 | 0.94 | 0.63 | 37 |
| E 33b | 50°45.243′N, 9°48.675′E | Pseudogley Parabraunerde (Gleyic Luvisol) | Deciduous forest (Fagus sylvatica, Quercus sp.) | 5.1 | 0.08 | 0.31 | 6 |
| E L4 | 50°44.991′N, 9°48.371′E | Pararendzina (Leptosol) | Meadow, scattered with Pinus sylvestris, sampling point from meadow | 5.7 | 0.25 | 0.50 | 12 |
| E L4P | 50°44.990′N, 9°48.374′E | Pararendzina (Leptosol) | Meadow, scattered with Pinus sylvestris, sampling point under Pinus sylvestris | 5.9 | 0.13 | 0.37 | 9 |
| E L5 | 50°45.034′N, 9°48.496′E | Pseudogley Parabraunerde (Gleyic Luvisol) | Deciduous forest (Fagus sylvatica, Quercus sp., Prunus sp.) | 5.2 | 0.11 | 0.10 | 29 |
| E L6 | 50°45.002′N, 9°48.400′E | Pararendzina (Leptosol) | Meadow | 6.2 | 0.45 | 0.41 | 26 |
| E W7 | 50°43.654′N, 9°43.654′E | Rendzina (Leptosol) | Meadow | 7.2 | 0.01 | ||
| E 5FB | 50°43.461′N, 9°47.118′E | Rendzina (Leptosol) | Mixed forest (Fagus sylvatica, Quercus sp., scattered Pinus sylvestris) | 7.5 | 3.11 | 1.36 | 56 |
| E 5FL | 50°43.463′N, 9°47.081′E | Rendzina (Leptosol) | Mixed forest (Pinus sylvestris, Larix decidua, scattered Fagus sylvatica) | 6.2 | 0.48 | 0.10 | 114 |
| E 26F | 50°43.023′N, 9°48.501′E | Parabraunerde (Luvisol) | Deciduous forest (Fagus sylvatica, Quercus sp.) | 4.9 | 0.07 | 0.08 | 21 |
| E 26W | 50°43.010′N, 9°48.507′E | Parabraunerde (Luvisol) | Pasture | 5.9 | 0.24 | 0.08 | 78 |
| E 26A | 50°43.008′N, 9°48.504′E | Parabraunerde (Luvisol) | Farmland (wintergrain) | 6.6 | 0.04 | 0.06 | 17 |
| E 56A | 50°42.971′N, 9°45.506′E | Pseudogley (Stagnic Gleysol) | Farmland (rape) | 6.9 | 0.01 | 0.03 | 12 |
| E 56W | 50°42.971′N, 9°45.506′E | Pseudogley (Stagnic Gleysol) | Pasture | 5.6 | 0.11 | 0.32 | 8 |
| E 56F | 50°42.960′N, 9°45.423′E | Pseudogley (Stagnic Gleysol) | Mixed forest (Pinus sylvestris, Larix decidua, Fagus sylvatica, Quercus rubra, Populus tremula) | 4.6 | 0.16 | 0.45 | 9 |
| Gerold | |||||||
| GE BWN | 47°28.950′N, 11°11.967′E | Not determined | Meadow | 5.8 | 0.51 | 0.67 | 18 |
| GE BF | 47°28.950′N, 11°11.967′E | Not determined | Mixed forest (Fagus sylvatica, Picea abies) | 6.8 | 0.80 | 0.73 | 27 |
| Welschnofen/ Völser Aicha | |||||||
| WO DFH | 46°25.342′N, 11°36.030′E | Not determined | Coniferous forest (Picea abies, Pinus cembra, Larix decidua) | 5.6 | 1.55 | 1.74 | 22 |
| VA DFT | 46°29.204′N, 11°29.324′E | Not determined | Mixed forest (Fagus sylvatica, Picea abies) | 4.4 | 1.11 | 6.41 | 4 |
| VA DWT | 6°29.294′N, 11°29.318′E | Not determined | Meadow | 6.8 | 0.77 | 0.64 | 30 |
| Marburg, MF | 51°00.000′N, 9°50.625′E | Braunerde (Cambisol) | Mixed forest (Fagus sylvatica, Quercus robur) | 4.0 | 1.26 | 5.08 | 6 |
| Bad Bentheim/ Denekamp | |||||||
| BB NH | 51°21.505′N, 7°04.285′E | Podsole (Podzol) | Heathland (Calluna vulgaris) | 4.6 | 0.08 | 0.24 | 8 |
| BB NNW | 51°21.383′N, 7°04.267′E | Podsole (Podzol) | Coniferous forest (Pinus sylvestris) | 4.3 | 0.06 | 0.32 | 4 |
| BB WNWb | 51°21.104′N, 7°06.169′E | Podsole (Podzol) | Coniferous forest (Pinus sylvestris) | 3.9 | 0.01 | 0.16 | 2 |
| BB WAS | 51°21.004′N, 7°06.190′E | Podsole (Podzol) | Farmland (closed for 0.5 yr) | 5.6 | 0.25 | 0.11 | 58 |
| Göttingen | |||||||
| G 15F | 51°33.974′N, 10°0.657′E | Pseudogley Parabraunerde (Gleyic Luvisol) | Deciduous forest (Fagus sylvatica, scattered Tilia platiphyllos, Acer pseudoplatanus) | 7.7 | 0.95 | 0.39 | 60 |
| G 15W | 51°33.971′N, 10°0.645′E | Pseudogley Parabraunerde (Gleyic Luvisol) | Meadow | 7.3 | 0.39 | 0.05 | 196 |
| G 15Ab | 51°33.964′N, 10°0.655′E | Pseudogley Parabraunerde (Gleyic, Luvisol) | Farmland (wintergrain) | 7.8 | 0.05 | 0.03 | 38 |
| G 13FH | 51°31.096′N, 10°2.171′E | Rendzina (Leptosol) | Deciduous forest (Fagus sylvatica, Fraxinus excelsior, Acer platanoides) | 7.1 | 0.93 | 0.23 | 100 |
| G 44W | 51°30.411′N, 10°1.154′E | Rendzina (Leptosol) | Meadow | 7.8 | 1.22 | 1.51 | 20 |
| G 44F | 51°30.423′N, 10°1.167′E | Rendzina (Leptosol) | Deciduous forest (Fagus sylvatica, Fraxinus excelsior) | 8.0 | 2.39 | 1.00 | 58 |
Methane oxidation.
Ten-gram amounts of sieved (<3-mm mesh) soil were incubated at 25°C in 120-ml serum vials closed with butyl rubber septa. Triplicates of each sample were incubated under atmospheric CH4 or under elevated CH4 mixing ratios (100 to 400 ppmv). The decrease in CH4 in the headspace was measured with an SRI 8610C gas chromatograph (SRI Instruments, Torrance, Calif.) equipped with a flame ionization detector (GC-FID) (detector temperature, 140°C; Porapak Q column, 6 feet long, 1/8 in. in diameter, 80/100 mesh [Supelco, Taufkirchen, Germany]; oven temperature, 100°C). A linear decrease in CH4 versus time was always observed in the elevated-CH4 vials and was used to estimate maximum apparent CH4 oxidation rates [Vmax(app)]. Incubation under atmospheric CH4 mixing ratios resulted in an exponential decrease in CH4, from which the specific affinity a0s (first-order uptake rate constant) was calculated by using the least-squares iterative fitting procedure of Origin 6.1 (Microcal Software, Inc., Northampton, Maine). Incubation times varied depending on the activity of the sample and included 3 to 12 measurement points (elevated CH4) or 4 to 10 measurement points (atmospheric CH4).
Methane oxidation kinetics were determined more intensely in the fresh samples collected for the 13CH4-labeling experiment. Vials (35 ml) were filled with 5 ml of soil slurry (2 volumes of distilled water per g of soil), injected with CH4 mixing ratios of 2 to 150 ppmv (duplicates at each level), and incubated on a rotary shaker at 180 rpm and 25°C. CH4 oxidation rates were estimated by linear regression (maximum decline in CH4 of 33%, maximum incubation time of 34 h) and plotted against the CH4 concentration at the time midpoint. Vmax(app) and the apparent half-saturation constant [Km(app)] were estimated by least-squares fitting to a Michaelis-Menten hyperbolic model.
DNA extraction and PCR amplification.
DNA was extracted from 0.5 g of soil (stored at −20°C immediately after sampling) with a Fast DNA SPIN Kit (Bio 101, La Jolla, Calif.). To increase DNA recovery, the final elution of the DNA was performed twice with 100 μl of DNase-free water. Additional purification was achieved with polyvinylpolypyrrolidone as described by Henckel et al. (23), again with twofold elution of the DNA with TE buffer (10 mM Tris base, 1 mM EDTA, pH 8). The DNA was finally purified and concentrated with a QIAquick PCR Purification Kit (Qiagen, Hilden, Germany).
A partial fragment of pmoA was amplified with primers A189f and A682b (38) and primers A189f and mb661 (10). For denaturing gradient gel electrophoresis (DGGE), a GC clamp was attached to the 5′ end of the A189f primer (22). A touchdown PCR program (22) with annealing temperatures decreasing from 62 to 55°C was used with 30 cycles when primers A189f and A682b were used and 35 cycles when primers A189f and mb661 were used. A fragment of the mmoX gene encoding the active-site subunit of soluble MMO was amplified with primers described by Auman et al. (1). The PCR program consisted of an initial denaturation step of 5 min at 94°C, followed by 35 cycles of 94°C for 1 min, 55°C for 1.5 min, and 72°C for 1 min, with a final extension step of 72°C for 7 min. Amplification of a gene fragment of the 16S rRNA was performed with primers UNI 533f and UNI 907r (47) as described previously (22). All PCR mixtures contained each primer at 0.5 μM, 1× Premix F (Epicentre Technologies, Madison, Wis.), 1 U of Taq DNA polymerase (Qbiogene, Heidelberg, Germany), and 1 μl of template DNA and were run on either a GeneAmp PCR System 9700 (Perkin-Elmer Applied Biosystems, Weiterstadt, Germany) or a Primus 96 Cycler (MWG Biotech, Ebersberg, Germany).
DGGE.
For each sample, separation of the mixed pmoA products by DGGE was performed on PCR products of both primers A189f and A682b and primers A189f and mb661. DGGE was performed as described previously (22), in a gradient of 35 to 80% denaturant at 61°C and 180 V for 5 h. Visible bands were excised and reamplified. PCR products of bands that migrated closely in DGGE gels were checked for purity in a second DGGE gel. Although reamplification of bands usually gave a single pmoA product to be sequenced, occasionally nonspecific products were also evident. Three procedures were used to remove these nonspecific products: (i) primer mb661 or A650 (4), instead of A682b, was used for reamplification of excised bands; (ii) the complete PCR product was loaded onto an agarose gel, and the band with the expected size of pmoA was excised and purified with a QIAquick Gel Extraction Kit (Qiagen); or (iii) PCR products were cloned with a TOPO TA cloning kit (Invitrogen, Karlsruhe, Germany). The pmoA products obtained from positive clones were verified by comparing their migration in DGGE to that of the original excised pmoA gene fragment.
Sequencing and phylogenetic analysis.
PCR products from excised DGGE bands were purified with a QIAquick PCR Purification Kit (Qiagen) and sequenced on an ABI 377 DNA sequencer with BigDye terminator chemistry as specified by the manufacturer (Perkin-Elmer Applied Biosystems). Phylogenetic tree reconstructions based on deduced amino acid sequences of partial pmoA and amoA sequences were performed with the ARB software package (46) and the PHYLIP software package, version 3.6a2.1 (17). Original tree construction included sequences of all DGGE bands, all available pmoA sequences in the GenBank database (April 2003), and selected public-domain amoA sequences. A selection was then made of 28 representative sequences from this study plus 38 public-domain sequences.
Labeling experiments with [13C]methane.
Incubation of 25-g amounts of sieved soil under 50 ppmv of labeled 13CH4 (99%; Cambridge Isotope Laboratories, Mass.) or unlabeled CH4 in air was performed in 1,000-ml screw-cap flasks sealed gas tight with silicone septa. Incubation was performed at 25°C for 4 weeks. Methane was measured at 1- to 5-day intervals (depending on the activity of the sample) by GC-FID and added again each time the mixing ratio fell below 10 ppmv. Control labeling experiments were also done with the MB strains Methylosinus trichosporium KS24b (24) and Methylocaldum sp. strain E10a. The latter was isolated from site E 20 by methods described previously (24). Its 16S rRNA sequence is 97.8% identical to that of Methylocaldum tepidum LK6. Cultures were grown in liquid mineral salts medium A under an atmosphere containing 20% (vol/vol) CH4 (25).
Care was taken to control for secondary labeling effects of 13CO2 in soil incubations. Tubes containing 5 ml of 5 M NaOH solution were inserted into flasks to fix emerging 13CO2. After 2 weeks of incubation, the 13C/12C isotope ratio of CO2 in the headspace of the flasks was measured with a Thermo Finnigan MAT Delta Plus isotope mass spectrometer (Thermoquest, Bremen, Germany) coupled to a Hewlett-Packard (Waldbronn, Germany) 6890 gas chromatograph (detector temperature, 150°C; Pora Plot Q column, 27.5 m by 0.32 mm [diameter] [Chrompack, Frankfurt, Germany]; oven temperature, 25°C) (GC-IRMS). This measurement detected a slight increase in labeled 13CO2 in the headspace of samples incubated with 13CH4 (increase in label in CO2, <1 atom%). Therefore, the flasks were flushed well with air and the NaOH solution was replaced at this time. As a further control of secondary labeling effects, soil samples MF and G 44F were incubated with 13CO2 instead of 13CH4. 13CO2 was released from a solution of NaH13CO3 (99.9%; Cambridge Isotope Laboratories) and added via syringe to the incubation flasks. The final mixing ratio of 225 ppmv of 13CO2 was verified by measurement with a GC-8A GC-FID (Shimadzu, Kyoto, Japan) (detector temperature, 350°C; 50/100 mesh Porapak QS column, 2 m long by 1/8 in. in diameter [Alltech, Unterhaching, Germany]; oven temperature, 40°C) equipped with a methanizer (NiCr-Ni catalyst column, 20 cm long by 1/8 in. in diameter [Chrompack]). The 13CO2 added was equal to the total amount of 13CH4 oxidized in the soil sample with the highest methane oxidation activity.
PLFA analysis.
Lipids were extracted from 10 g of soil by a modified Bligh and Dyer method and fractionated on silica columns (CUSIL15Z; ICT, Bad Homburg, Germany). PLFAs were subjected to mild alkaline methanolysis as described previously (34). Cells from pure cultures were harvested by centrifugation (10,000 × g, 20 min) and washed twice with 0.9% NaCl solution before PLFA extraction.
Separation, identification, and quantification of fatty acid methyl esters (FAMEs) were performed by gas chromatography mass spectrometry (GC-MS) as described previously (37). The positions of double bonds and cyclopropyl groups were determined by analysis of dimethyl disulfide adducts (39). Carbon isotope ratios of the individual FAMEs were determined with the GC-IRMS system described above. The GC-IRMS apparatus was equipped with the same nonpolar column as was the GC-MS apparatus (5% phenyl methyl silicone capillary column, 30 m by 0.25 mm [diameter] [Hewlett-Packard]), and the same temperature program was used to separate FAMEs (0.5 min at 120°C, increase of 5°C/min to 240°C and finally 240°C for 2 min) in order to obtain similar PLFA profiles by both analyses. The injector temperature of the GC-MS was set to 250°C, and a split ratio of 29.2 was used, while the injector temperature of the GC-IRMS system was set to 300°C and a splitless injection mode was used. To calculate isotope ratios (δ13C) for the PLFAs, δ13C values of the FAMEs were corrected with a mass balance for the carbon atom of the methyl group that was added during methanolysis (43). For each PLFA, the incorporation of 13C (I, expressed as micrograms of 13C per gram of total PLFAs) was calculated as follows: I = (Fl − Fu) × (Ax), where Ax is the peak area of PLFAx divided by the sum of the peak areas of all of the PLFAs. F is the fraction of 13C in PLFAx of samples incubated with 13C (Fl) or samples incubated with 12C (Fu) and was calculated as follows: F = 13C/(13C + 12C) = R/(R + 1). The carbon isotope ratio (R) was derived from the measured δ13C values as follows: R = (δ13C/1,000 + 1) × RVPDB, with RVPDB = 0.0112372.
Nucleotide sequence accession numbers.
Representative pmoA nucleotide sequences obtained during this study have been deposited in the GenBank, EMBL, and DDBJ nucleotide sequence databases under accession numbers AJ579657 to AJ579676.
RESULTS
Characterization of sampling sites.
Soil samples were taken from seven regions in Central Europe incorporating different soil types, land uses, and plant covers (Table 1). Care was taken to select soil types of various pHs to test the hypothesis that USC α represents a specialized acidophile. Soil pH values ranged from 3.9 to 8.0.
No soil showed net CH4 production, although a few had no measurable CH4 uptake. The Vmax(app) values for methane oxidation at 25°C ranged from <0.01 to 3.11 nmol of CH4 g of dry weight−1 h−1. Specific affinity (a0s) reached values greater than 1 ml g of dry weight−1 h−1 in four forest soils and meadow soil sample G 44W. Km(app) values calculated from the data in Table 1 ranged from a CH4 mixing ratio in air of 2 ppmv (BB WNW) to 196 ppmv (G 15W) (or 3 to 271 nM dissolved CH4). A correlation between Vmax(app) and a0s was not observed, nor was there any significant correlation of these kinetic values with soil pH or ammonium or ammonia concentrations (data not shown).
Characterization of the MB community.
The community of MB was characterized in 35 samples by cultivation-independent retrieval of partial pmoA genes, followed by comparative sequence analysis of derived amino acid sequences (PmoA). PCR amplification of pmoA from all samples was successful, with the exception of GEBWN, GEBF, and BB NH. Some of the sequences retrieved with primers A189f and A682b were closely related to the amoA gene (encoding a subunit of ammonia monooxygenase) of ammonia-oxidizing Betaproteobacteria (Fig. 1 and Table 2). This was expected, as primers A189f and A682b amplify both pmoA and amoA gene fragments, while A189f and mb661 are specific for pmoA (10, 27).
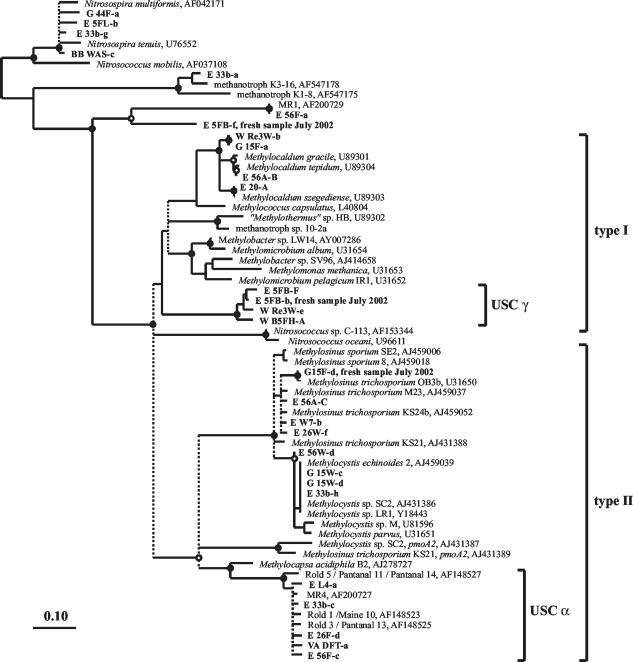
FIG. 1.
Consensus tree showing the relationship of the amino acid sequences derived from partial pmoA sequences retrieved from soils to public-domain PmoA and AmoA sequences. The base tree was constructed on the basis of 141 amino acid residues with a neighbor-joining algorithm with a Kimura correction. Multifurcations indicate branch points where the topologies of distance matrix-based neighbor-joining trees with Kimura or PAM correction, of a maximum-likelihood tree, and of a parsimony tree differed. These are indicated by dashed lines. Bootstrap values were calculated with the PHYLIP software package on the basis of 1,000 data resamplings. Bootstrap values of ≥90% are indicated by black circles, and values of ≥80% are indicated by white circles. The cluster of AmoA sequences (top) was set as an outgroup. The bar represents 0.10 change per position.
TABLE 2.
Methanotrophic community of different soil samples, based on detection of the pmoA genea
| Sample | pH | PmoA
|
PmoA or AmoA (unknown)b | AmoA (ammonia oxidizer) | ||||
|---|---|---|---|---|---|---|---|---|
| Methylocaldum | Methylosinus | Methylocystis | USC α | USC γ | ||||
| E 20 | 7.6 | + | + | + | ||||
| E 33a | 6.6 | + | + | + | + | |||
| E 33b | 5.1 | + | + | + | A | + | ||
| E L4 | 5.7 | + | + | |||||
| E L4P | 5.9 | + | + | + | ||||
| E L5 | 5.2 | + | + | A | ||||
| E L6 | 6.2 | + | + | + | ||||
| E W7 | 7.2 | + | + | + | + | + | ||
| E 5FB | 7.5 | + | + | |||||
| E 5FL | 6.2 | + | + | + | ||||
| E 26F | 4.9 | + | + | |||||
| E 26W | 5.9 | + | + | + | + | |||
| E 26A | 6.6 | + | A | + | ||||
| E 56A | 6.9 | + | + | A | + | |||
| E 56W | 5.6 | + | + | + | ||||
| E 56F | 4.6 | + | + | + | B | |||
| GE BWN | 5.8 | |||||||
| GE BF | 6.8 | |||||||
| WO DFH | 5.6 | + | ||||||
| VA DFT | 4.4 | + | ||||||
| VA DWT | 6.8 | + | + | |||||
| MF | 4.0 | + | ||||||
| BB NH | 4.6 | |||||||
| BB NNW | 4.3 | + | ||||||
| BB WAS | 5.6 | + | ||||||
| G 15F | 7.7 | + | + | + | ||||
| G 15W | 7.3 | + | ||||||
| G 13FH | 7.1 | + | + | |||||
| G 44W | 7.8 | + | ||||||
| G 44F | 8.0 | + | + | |||||
| W B5FH | 6.3 | + | + | |||||
| W B5FP | 6.9 | + | + | |||||
| W Re4F | 6.1 | + | + | + | ||||
| W Re1F | 7.6 | + | + | + | ||||
| W Re3W | 7.8 | + | + | |||||
| Samples from July 2002 | ||||||||
| E 5FB | 6.5 | + | + | C | ||||
| MF | 4.3 | + | ||||||
| G 15F | 7.9 | + | + | + | ||||
| G 13FH | 6.8 | + | + | B, C | + | |||
| G 44F | 7.9 | + | + | + | + | |||
| G 44W | 8.0 | + | + | + | ||||
See Table 1 for site descriptions.
A, B, and C indicate the affiliations of the detected sequences with different unknown clusters.
PmoA sequences closely related to those of the genera Methylocaldum, Methylosinus, and Methylocystis were detected in 70% of the soil samples analyzed (Table 2). Methylocaldum-like sequences were in three main groups: identical to those of Methylocaldum szegediense, closely related to Methylocaldum tepidum (>99% identity), and belonging to a novel branch with 94 to 95% identity to known Methylocaldum strains. The last are represented by sequences G 15F-a and W Re3W-b (Fig. 1). All Methylosinus-like PmoA sequences fell clearly within the M. trichosporium cluster. Methylocystis-like sequences clustered into a group represented by many sequences in the GenBank database, including Methylocystis sp. strain SC2 and Methylocystis sp. strain LR1 (Fig. 1).
Many soil samples also contained sequences less closely related to those of cultivated MB species. USC α (Table 2 and Fig. 1), which has previously been detected in upland soils that consume atmospheric methane, was very common. Another cluster of sequences (USC γ) was distantly related to PmoA sequences of methanotrophic Gammaproteobacteria (Fig. 1). The highest identity values of USC γ amino acid sequences to those of cultivated MB were only 72 to 75%, to Methylocaldum strains, Methylococcus capsulatus, and Methylobacter sp. strain LW14. In addition, soil samples E 33b, E L5, E 56A, and E 26A yielded PmoA sequences closely related to those of methanotrophic isolates K3-16 and K1-8, which were recently described by Pacheco-Oliver et al. (41). Two further sequence types may represent either AmoA or PmoA: (i) sequences from E 56F and G 13FH (collected in 2002) that were related to MR1 (23) and (ii) sequences represented by E 5FB-f, which were detected in soil samples E 5FB and G 13FH, which were collected in 2002 (Table 2).
A fragment of the mmoX gene was not detected in any sample. Either MB possessing soluble MMO are uncommon in upland soils, or the PCR systems used to detect mmoX are not as sensitive as those used for pmoA. Thus, no marker genes for methanotrophs were detected in the GEBWN, GEBF, and BB NH soil samples, although these soil samples exhibited methane oxidation activity (Table 1). Severe inhibition of the PCR assays of these three samples by coextracted inhibitory substances in the DNA extracts was unlikely, since PCR assays for the amplification of a partial 16S rRNA gene fragment were not inhibited (data not shown).
Relationship between soil pH and MB community composition.
To gain insight into the distribution of MB in upland soils in relation to soil characteristics, the MB community composition of the different soil samples was analyzed in comparison to methane oxidation activity, land use, ammonium and ammonia concentrations, and pH values by correspondence analyses with SYSTAT version 10.2 (SPSS Inc., Richmond, Calif.). Only pH showed a clear and significant influence on MB distribution, and only analyses for this factor are presented below.
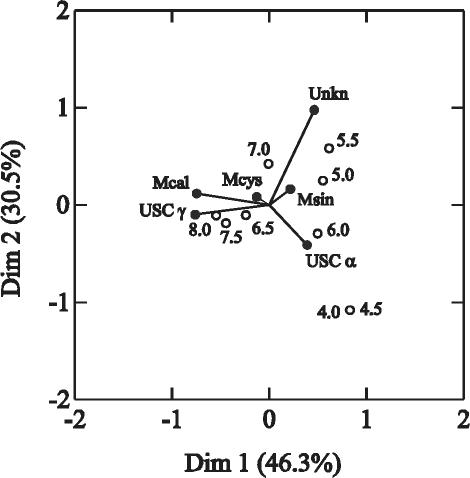
The 36 soil samples from which pmoA products were retrieved (samples GE BWN, GE BF, BB NH, BB WNW, and G 15A excluded) and atmospheric methane uptake was detectable (samples E 20 and E W7 excluded) were grouped to the closest 0.5 pH unit (i.e., 4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5, and 8) in order to improve the degrees of freedom in the correspondence analyses, and the correspondence of pH levels to the species groups in Table 2 was calculated (Fig. 2). The occurrence of Methylocaldum spp. and USC γ strongly influenced the separation of samples with different pH values, such that the higher-pH soils corresponded well to the presence of these clusters. The significance of this trend in USC γ was confirmed with a contingency analysis. The null hypothesis that the USC γ sequences were equally distributed in high-pH versus low-pH soils (with the median soil pH of 6 chosen as the separation point between high and low) was rejected (Fisher's exact test, P ≤ 0.001), indicating that these sequences were significantly more likely to be detected in soils with pHs of >6. Methylocystis spp. occurred in soils with a broad pH range and had little value in sample differentiation. The presence of sequences of USC α and Methylosinus spp. was typical for acidic soils (Fig. 2). A contingency analysis on USC α as described above demonstrated that they were more likely to be detected in soils with pHs of <6 than in soils with pHs of >6 (Fisher's exact test, P = 0.025), although they were also detected in some higher-pH soils (Table 2).
FIG. 2.
Correspondence plot showing the relationship between soil pH values and the detection of the different pmoA sequences in 36 soil samples. The first two dimensions explain 76.8% of the variance among samples. The pH values were classified into nine groups and are represented by the empty circles in the plot. The different taxa are represented by filled circles and are indicated as follows: Mcal, Methylocaldum spp.; Mcys, Methylocystis spp.; Msin, Methylosinus spp.; Unkn, sequences of the unknown clusters that may represent either PmoA or (Dim1 and Dim2) AmoA.
Statistical analysis with regard to land use or plant cover was not possible because of insufficient data. Nevertheless, it is remarkable that sequences of USC α and USC γ were not detected in any of the four farmland soils analyzed but occurred in 30 of 34 grassland and forest soils. Sequences related to those of Methylocaldum, Methylosinus, and Methylocystis were detected in different soils, independent of land use and plant cover.
Labeling experiments with [13C]methane.
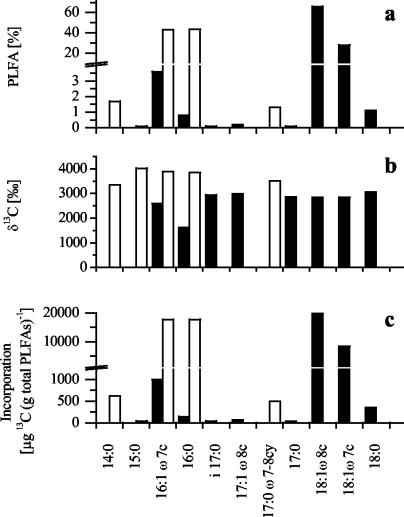
Growth in the presence of 1% (vol/vol) labeled 13CH4 plus 19% unlabeled CH4 in the gas headspace led to strong labeling (1,600 to 4,000‰) of all of the PLFAs in M. trichosporium KS24b and Methylocaldum sp. strain E10a (Fig. 3). Since the separation efficiency was better in GC-MS analysis than in GC-IRMS analysis, separate detection of 18:1ω8c and 18:1ω7c was possible, but the quantification of separate isotope ratios for these two PLFAs in M. trichosporium KS24b was problematic because the peaks were not baseline separated in GC-IRMS analysis. Because of the similar labeling intensities of all of the PLFAs, the incorporation of label into the different PLFAs closely reflected the PLFA profiles of the two strains (Fig. 3). 16:1ω7c and 16:0 are the dominant PLFAs in Methylocaldum sp. strain E10a, while 18:1ω8c and 18:1ω7c are the main PLFAs of M. trichosporium KS24b.
FIG. 3.
PLFA profile and 13C-labeling pattern of the PLFAs of M. trichosporium KS24b (filled bars) and Methylocaldum sp. strain E10a (open bars). The relative amount (a) and δ13C value (b) of each PLFA were used to calculate 13C incorporation into each PLFA relative to the total amount of PLFAs (c).
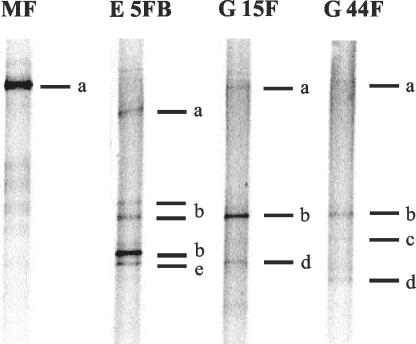
Fresh soil samples were taken in July 2002 from sampling sites that had shown high methane oxidation activity and contained sequences of USC γ or USC α or only pmoA sequences closely related to pmoA of cultivated MB. The MB communities in these samples were similar, but not identical, to those of the earlier samples (Table 2 and Fig. 4). Either the USC γ or the USC α sequences were detected in all of the fresh soil samples. In soil sample MF, only pmoA sequences of USC α were detected. Soil samples E 5FB and G 15F showed intense DGGE bands for USC γ in the DGGE gel, compared to the bands of the other detected taxa within the respective samples. Four samples were selected for the 13CH4-labeling experiment and a more extensive analysis of methane oxidation kinetics.
FIG. 4.
DGGE banding patterns of pmoA PCR products amplified with primers A189f and A682b from soil samples (July 2002) used in the 13CH4-labeling experiment. The bands were identified as follows: a, USC α; b, USC γ; c, Methylocystis sp.; d, Methylosinus sp.; e, unknown (PmoA and AmoA).
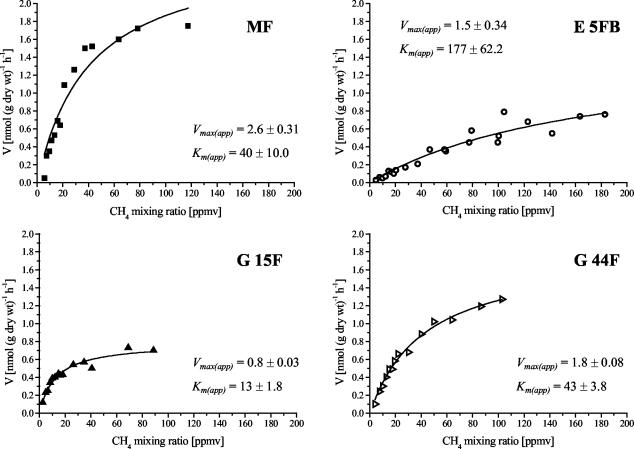
As shown in Fig. 5, all four samples showed a typical Michaelis-Menten kinetic. The Km(app) values between 13 and 177 ppmv of CH4 (17 to 244 nM dissolved CH4) were typical for the “high-affinity” uptake described by Bender and Conrad (2), and no evidence of additional “low-affinity” activity was observed. The four soil samples were incubated under 13CH4 (or unlabeled CH4) mixing ratios that varied during the incubations from about 10 to 50 ppmv. This value was chosen to be near the estimated Km(app), or within the first-order range of methane uptake. Rate measurements excluded the possibility that shifts occurred within the active community of MB during the incubation. A shift could potentially be caused by induction of methane-oxidizing activity in resting cells or growth of MB. Methane oxidation rates did not increase during the incubation period of 4 weeks (data not shown), indicating that only the already active atmospheric methane oxidizers were labeled.
FIG. 5.
Methane oxidation kinetics of the soil samples used in the 13C-labeling experiment. Data were fitted to the Michaelis-Menten hyperbolic curve to estimate Vmax(app) (nanomoles of CH4 per gram of dry weight per hour ± 1 standard error of fitting) and Km(app) (ppmv of CH4 ± 1 standard error of fitting). Each data point represents the mean of duplicate samples.
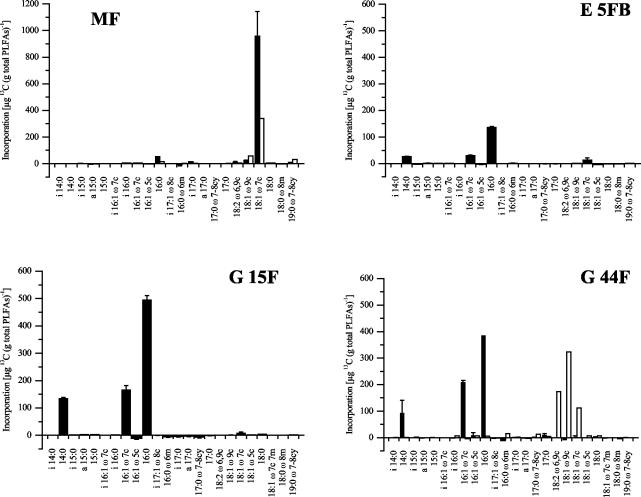
The total PLFA profiles were very similar in all of the soil samples. The profile of soil sample G 44F is shown as an example in Fig. 6. Saturated and onefold-unsaturated PLFAs with 16 and 18 carbon atoms dominated the profiles. In soil sample MF, incubation with 13CH4 led to incorporation of 13C into PLFAs 16:0, i17:0, and 18:1ω7c (Fig. 7). The incorporation of label into 16:0 and i17:0 was much weaker than that into 18:1ω7c. PLFA 18:1ω8c, typical of Methylosinus and Methylocystis spp., was below the detection limit of the GC-MS method used. The dimethyl disulfide derivatization method used to confirm the positions of double bounds also failed to reveal detectable amounts of 18:1ω8c. The labeled profile in soil MF is most similar to that of type II MB of the genera Methylocella and Methylocapsa.
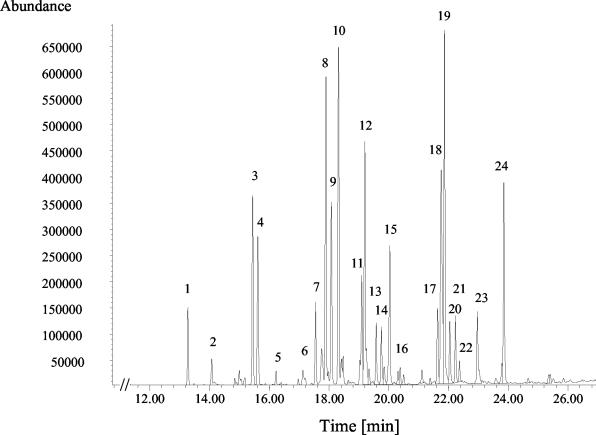
FIG. 6.
Partial gas chromatogram of methylated PLFAs detected by GC-MS of soil sample G 44F. Peak numbers indicate PLFAs as follows: 1, i14:0; 2, 14:0; 3, i15:0; 4, a15:0; 5, 15:0; 6, i16:1ω7c; 7, i16:0; 8, 16:1ω7c; 9, 16:1ω5c; 10, 16:0; 11, i17:1ω8c; 12, 16:0ω6m; 13, i17:0; 14, a17:0; 15, 17:0ω7-8cy; 16, 17:0; 17, 18:2ω6,9c; 18, 18:1ω9c; 19, 18:1ω7c; 20, 18:1ω5c; 21, 18:0; 22, 18:1ω7c 7m; 23, 18:0ω8m; 24, 19:0ω7-8cy. Branched fatty acids are indicated by i (iso), a (anteiso), m (methyl), and cy (cyclo).
FIG. 7.
13C-labeling patterns of the PLFAs of soil samples incubated under 10 to 50 ppmv of 13CH4 (filled bars) or under 225 ppmv of 13CO2 (open bars). Incubation with 13CO2 was only performed with samples MF and G 44F. For samples incubated with 13CH4, data are the mean of triplicates ± 1 standard error of the mean.
A 13C-labeling pattern more typical of type I MB was evident in soil samples E 5FB, G 44F, and G 15F. These profiles were dominated by PLFAs 14:0, 16:1ω7c, and 16:0 (Fig. 7). The labeling intensity was stronger in PLFAs of soil samples G 44F and G 15F than in PLFAs of soil sample E 5FB, which is reasonable considering the relative rates of methane oxidation in these soil samples (Fig. 5).
An additional experiment was performed to exclude the possibility that secondary labeling effects were caused by 13CO2 originating through 13CH4 oxidation by methanotrophs. Soil samples G 44F and MF were incubated with an amount of 13CO2 equal to the total amount of 13CH4 consumed in methane incubations. This led to labeling of 18:1 PLFAs in both samples, also of PLFA 19:0ω7-8cy in soil sample MF, and of 18:2ω6,9c in soil sample 44F (Fig. 7). Thus, soil sample G 44F showed different labeling patterns after 13CH4 incubation than after 13CO2 incubation. In soil sample MF, 18:1ω7c was labeled after incubation with both substrates. However, since the labeling was significantly lower after incubation with 13CO2, the labeling of this PLFA after incubation with 13CH4 was probably direct labeling of MB and not the result of secondary effects.
DISCUSSION
Upland soils are characterized by high-affinity methane oxidation, with Km(app) values for CH4 in the nanomolar range (2). The Km(app) values measured in the present work were all similar to values published previously for upland soils (7 to 200 ppmv of CH4) (2, 3, 9, 15, 20), as were the Vmax(app) values (2). Although there was considerable variability, all of the soils tested therefore showed kinetic properties typical of atmospheric methane oxidation. Atmospheric methane oxidation activity in upland soils is dependent on plant cover and land use, such that rates in woodland > rates in grassland > rates in farmland (48). Although not statistically tested, our data confirm this trend insofar as the highest a0s values (which represent the initial slope of the hyperbolic curve at low CH4 mixing ratios) were observed in forest soils. Where several adjacent sampling sites with different land uses were analyzed, a0s decreased with increasing anthropogenic disturbance from woodland to pasture to cropland: specifically, in samples E 26F, E 26W, and E 26A; E 56F, E 56W, and E 56A; or G 15F, G 15W, and G 15A (Table 1).
PmoA sequence analysis revealed that the soils contained MB closely related to cultivated species and also some only distantly related to known MB. We did not detect pmoA sequences related to those of the genera Methylomonas, Methylomicrobium, Methylobacter, and Methylococcus, which have been detected in forest soils from Denmark and the United Kingdom (4, 42), but we did commonly find pmoA sequences closely related to Methylocaldum, Methylocystis, and Methylosinus. Sequences of USC α were very common in our soils, as expected from previous studies (4, 23, 28, 32). This cluster has been postulated to represent as yet uncultivated MB active at consuming atmospheric methane (28). We also frequently detected pmoA sequences in a cluster (USC γ) that grouped most closely with sequences of type I MB but had only low identity values (<75%) with PmoA of cultivated species. A few other sequence types were detected, but only infrequently.
Sequences of USC α or USC γ have never been detected in wetland soils. The occurrence of these sequences seems to be restricted to upland soils that consume atmospheric methane. A correlation between the occurrence of these groups and the soil pH value was also evident from our data. The USC γ sequences were significantly more likely to be detected in soils with pH values of >6.0 than in more acidic soils, whereas the opposite trend was evident for USC α. Different MB are known to have different pH optima (11, 21), but pH can only be considered an indicator rather than a cause in this study. The neutral or alkaline soils studied were primarily Leptosols derived from a calcareous substrate, and some chemical factor other than pH may be decisive in determining whether a particular MB group is present. Alternatively, secondary pH effects, for example, on micronutrient availability, may be critical.
The 13CH4-labeling experiment was performed to identify the active MB in soils in which pmoA sequences of USC γ, of USC α, or of cultivated MB were detected. A CH4 mixing ratio (10 to 50 ppmv) near the Km(app) for methane oxidation of the soils was used for incubations. Control measurements ensured that only the already active high-affinity population was labeled and that secondary labeling effects of 13CO2 were minimal. In soil sample MF, labeling of PLFAs 16:0, i17:0, and 18:1ω7c was obtained after 13CH4 incubation. PLFA 18:1ω7c is the main fatty acid of M. acidiphila and Methylocella spp. (11, 14). PFLAs typical of type I MB (e.g., 14:0 and 16:1ω7c) or of other type II MB (e.g., 18:1ω8c) were not detectably labeled. A labeling pattern similar to that of soil sample MF was previously found in another upland soil (8), although a different branched 17:0 PLFA was labeled instead of i17:0. Fatty acid i17:0 has been found only in small amounts (<0.6%) in a few cultivated MB (7, 11), and the labeling of branched 17:0 PLFAs was previously interpreted to mean that active MB in soil are unusual compared to known species (8). This may also be the case in soil sample MF; however, such a conclusion is dangerous because i17:0 accounted for only a tiny percentage of the 13C incorporated. PLFA-labeling profiles similar to that of soil sample MF were also obtained by incubation of six acidic upland soil samples under 14C-labeled methane, followed by scintillation counting of fractionated FAMEs (28, 44). Although this fractionation procedure resulted in limited separation efficiency, one labeled PLFA in soil sample MF would fall within each of the labeled fractions described in these previous studies (28, 44). The use of labeled PLFAs as chemotaxonomic marker molecules correlates well with the pmoA recovery in soil sample MF. The only pmoA sequences detected in soil sample MF belonged to USC α, a unique cluster of which the type II MB M. acidiphila is the closest relative. Taken together, these results strongly support the hypothesis of Holmes et al. (28) that as yet uncultivated methanotrophic Alphaproteobacteria possessing pmoA sequences of USC α are responsible for atmospheric methane oxidation in some upland soils.
On the other hand, the more neutral soil samples E 5FB, G 44F, and G 15F showed PLFA labeling patterns clearly distinct from that of soil sample MF. The combination of labeled 14:0 and 16:1ω7c suggests that MB more closely related to cultivated type I MB were active in these soil samples. PLFA 16:1ω7c contributes 8 to 57% of the total amount of PLFAs in all characterized type I MB but much less in type II MB (<9%) (7, 40). Fatty acid 14:0 is abundant only in type I MB. It contributes up to 25% of the total PLFA content of Methylomonas spp. and smaller amounts (1 to 10%) to that of other type I MB (5, 7). Only traces (<0.1%) have been found in Methylosinus spp. and Methylocystis spp. (7, 40), and it is undetectable in Methylocella spp. and M. acidiphila (11, 14).
At the very least, the labeling results indicate that different MB are active in different soils and include strains related to either type I (Gammaproteobacteria) or type II (alphaproteobacteria) MB. The most likely explanation for the combined pmoA and PLFA data for soil samples E 5FB, G 44F, and G 15F is that unknown methanotrophic Gammaproteobacteria possessing pmoA sequences of USC γ are the most active MB. This pmoA sequence type was detected in all three soils and was clearly the dominant pmoA product obtained from two. It was the only pmoA sequence detected that was closely related to pmoA sequences of type I MB, and it is therefore likely that the organisms containing these sequences were the same MB labeled with 13CH4. However, it cannot be excluded that another type I MB that we did not detect was present and active. We have observed, for example, that the pmoA genes of several Methylobacter strains are poorly amplified with the standard primer sets used here (data not shown). Sequences from USC α and sequences related to those of Methylocystis and Methylosinus spp. were also detected in one or all of the E 5FB, G 44F, and G 15F soil samples, but labeled PLFAs 18:1ω8c and 18:1ω7c indicative of Methylocystis and Methylosinus spp. (7, 40), and presumably also of USC α, were not found. This finding demonstrates that not all of the methanotrophs detectable in an upland soil sample are equally involved in the process of methane oxidation. USC γ was only detected in soils with pHs of >6 and may therefore represent a neutrophilic or alkalophilic MB. This would explain why earlier experiments with acidic soils failed to show a PFLA-labeling pattern like that observed in the more neutral soil samples E 5FB, G 44F, and G 15F.
The comparison of the pmoA data and the PLFA patterns in this and earlier studies is based on the usefulness of both biomolecules as phylogenetic markers. Although pmoA is, for the most part, an excellent phylogenetic marker, two studies have demonstrated that some caution is necessary in the interpretation of pmoA sequences. The recently described methanotrophic isolates K3-16 and K1-8 are related to Methylosinus and Methylocystis on the basis of 16S rRNA sequence comparison but only distantly related on the basis of pmoA sequence comparison (41). As well, a second pmoA-like gene (pmoA2) has recently been found in some strains of Methylosinus and Methylocystis. These sequences have <80% identity to the previously known pmoA gene (pmoA1) sequences of the respective organisms (16). Together, these two studies indicate that a pmoA-based phylogeny does not perfectly reflect the 16S rRNA-based phylogenetic affiliation of MB and that novel clusters of pmoA sequences do not necessarily indicate that novel groups of uncultivated MB exist.
Nevertheless, we feel that the most likely explanation for our data is that pmoA sequences of USC α and USC γ belong to unknown groups of MB active in upland soils. No other pmoA gene sequences were detected together with those of USC α in soil samples MF, WO DFT, VA DFH, BB NNW, and G 13FH. The USC γ sequences were detected together with sequences of other genera; however, in the samples used for 13C labeling, none of the other MB detected would be expected to give a labeling profile typical of type I MB. Taken together, the data presented here indicate that different groups of MB are responsible for atmospheric methane uptake in different soils and that the MB in neutral-to-alkaline Leptosols are most closely related to type I MB of the Gammaproteobacteria. Indirect evidence from other studies has suggested that MB closely related to cultivated species may also be active in certain upland soils (15, 29), so in all, many diverse species of MB may be involved in the process of atmospheric methane oxidation.
Table 1a.
| Würzburg | |||||||
| W B5FH | 49°49.041′N, 9°54.955′E | Podsolige Braunerde (Cambisol) | Mixed forest (Fagus sylvatica, Carpinus betulus, Pinus sylvestris, Larix decidua) | 6.3 | 0.44 | 0.33 | 33 |
| W B5FP | 49°48.960′N, 9°54.804′E | Podsolige Braunerde (Cambisol) | Mixed forest (Acer pseudoplatanus, Fagus sylvatica, Quercus robur, Pinus sylvestris) | 6.9 | 0.49 | 0.20 | 60 |
| W Re4F | 49°51.540′N, 9°54.521′E | Protorendzina (Leptosol) | Deciduous forest (Carpinus betulus, Prunus sp., Quercus robur, Acer campestris) | 6.1 | 0.29 | 0.15 | 46 |
| W Re1F | 49°52.553′N, 9°52.355′E | Protorendzina (Leptosol) | Mixed forest (Pinus sylvestris, Quercus petraea, Fagus sylvatica, Acer campestre) | 7.6 | 0.37 | 0.15 | 58 |
| W Re3W | 49°52.234′N, 9°51.684′E | Mullartige Rendzina (Leptosol) | Meadow | 7.8 | 0.74 | 0.34 | 53 |
| July 2002 | |||||||
| E 5FB | 50°43.461′N, 9°47.118′E | Rendzina (Leptosol) | Mixed forest (Fagus sylvatica, Quercus sp., scattered Pinus sylvestris) | 6.5 | 1.52 | 0.21 | 177 |
| MF | 51°00.000′N, 9°50.625′E | Braunerde (Cambisol) | Mixed forest (Fagus sylvatica, Quercus robur) | 4.3 | 2.62 | 1.58 | 40 |
| G 15F | 51°33.974′N, 10°0.657′E | Pseudogley Parabraunerde (Gleyic Luvisol) | Deciduous forest (Fagus sylvatica, scattered Tilia platiphyllas, Acer pseudoplatanus) | 7.9 | 0.78 | 1.53 | 13 |
| G 13FH | 51°31.096′N, 10°2.171′E | Rendzina (Leptosol) | Deciduous forest (Fagus sylvatica, Fraxinus excelsior, Acer platanoides) | 6.8 | 0.80 | ||
| G 44F | 51°30.423′N, 10°1.167′E | Rendzina (Leptosol) | Deciduous forest (Fagus sylvatica, Fraxinus excelsior) | 7.9 | 1.80 | 1.02 | 43 |
| G 44W | 51°30.411′N, 10°1.154′E | Rendzina (Leptosol) | Meadow | 8.0 | 1.38 |
Kinetic values are the maximum zero-order methane uptake rate (Vmax(app) [nanomoles of CH4 per gram of dry weight per hour]), the apparent Michaelis-Menten kinetic constant (Km(app) [ppmv of CH4]), which was calculated from Vmax(app)/a0s, and the specific affinity (a0s [milliliters of gas per gram of dry weight per hour]). a0s is a first-order rate constant. The unit milliliters refers not to milliliters of CH4 but rather to standardization to a 1-ml total gas volume. To calculate rates of atmospheric methane uptake at 25°C multiply a0s by the mixing ratio of methane in the atmosphere (1.7 ppmv of CH4 = 0.071 nmol of CH4 ml−1).
Sample was not analyzed by molecular methods.
FAO, Food and Agriculture Organization of the United Nations.
Acknowledgments
This work was financed by grant DU 377/1-1 from the Deutsche Forschungsgemeinschaft to P.D.
We thank Jürgen Heyer for help with the collection of soil samples and botanical characterization of the sampling sites and Peter Claus for GC-IRMS analyses.
REFERENCES
- 1.Auman, A. J., S. Stolyar, A. M. Costello, and M. E. Lidstrom. 2000. Molecular characterization of methanotrophic isolates from freshwater lake sediment. Appl. Environ. Microbiol. 66:5259-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender, M., and R. Conrad. 1992. Kinetics of CH4 oxidation in oxic soils exposed to ambient air or high CH4 mixing ratios. FEMS Microbiol. Ecol. 101:261-270. [Google Scholar]
- 3.Benstead, J., and G. M. King. 1997. Response of methanotrophic activity in forest soil to methane availability. FEMS Microbiol. Ecol. 23:333-340. [Google Scholar]
- 4.Bourne, D. G., I. R. McDonald, and J. C. Murrell. 2001. Comparison of pmoA PCR primer sets as tools for investigating methanotroph diversity in three Danish soils. Appl. Environ. Microbiol. 67:3802-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman, J. P., S. A. McCammon, and J. H. Skerratt. 1997. Methylosphaera hansonii gen. nov., sp. nov., a psychrophilic, group I methanotroph from Antarctic marine-salinity, meromictic lakes. Microbiology 143:1451-1459. [DOI] [PubMed] [Google Scholar]
- 6.Bowman, J. P., J. H. Skerratt, P. D. Nichols, and L. I. Sly. 1991. Phospholipid fatty acid and liposaccharide fatty acid signature lipids in methane utilizing bacteria. FEMS Microbiol. Ecol. 85:15-22. [Google Scholar]
- 7.Bowman, J. P., L. I. Sly, P. D. Nichols, and A. C. Hayward. 1993. Revised taxonomy of the methanotrophs: description of Methylobacter gen. nov., emendation of Methylococcus, validation of Methylosinus and Methylocystis species, and a proposal that the family Methylococcaceae includes only the group I methanotrophs. Int. J. Syst. Bacteriol. 43:735-753. [Google Scholar]
- 8.Bull, I. D., N. R. Parekh, G. H. Hall, P. Ineson, and R. P. Evershed. 2000. Detection and classification of atmospheric methane oxidizing bacteria in soil. Nature 405:175-178. [DOI] [PubMed] [Google Scholar]
- 9.Conrad, R. 1996. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol. Rev. 60:609-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costello, A. M., and M. E. Lidstrom. 1999. Molecular characterization of functional and phylogenetic genes from natural populations of methanotrophs in lake sediments. Appl. Environ. Microbiol. 65:5066-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dedysh, S. N., V. N. Khmelenina, N. E. Suzina, Y. A. Trotsenko, J. D. Semrau, W. Liesack, and J. M. Tiedje. 2002. Methylocapsa acidiphila gen. nov., sp. nov., a novel methane-oxidizing and dinitrogen-fixing acidophilic bacterium from Sphagnum bog. Int. J. Syst. Evol. Microbiol. 52:251-261. [DOI] [PubMed]
- 12.Dedysh, S. N., W. Liesack, V. N. Khmelenina, N. E. Suzina, Y. A. Trotsenko, J. D. Semrau, A. M. Bares, N. S. Panikov, and J. M. Tiedje. 2000. Methylocella palustris gen. nov., sp. nov., a new methane-oxidizing acidophilic bacterium from peat bogs, representing a novel subtype of serine-pathway methanotrophs. Int. J. Syst. Evol. Microbiol. 50:955-969. [DOI] [PubMed] [Google Scholar]
- 13.Dunfield, P. F., and R. Conrad. 2000. Starvation alters the apparent half-saturation constant for methane in the type II methanotroph Methylocystis strain LR1. Appl. Environ. Microbiol. 66:4136-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunfield, P. F., V. N. Khmelenina, N. E. Suzina, Y. A. Trotsenko, and S. N. Dedysh. 2003. Methylocella silvestris sp. nov., a novel methanotrophic bacterium isolated from an acidic forest cambisol. Int. J. Syst. Evol. Microbiol. 53:1231-1239. [DOI] [PubMed]
- 15.Dunfield, P. F., W. Liesack, T. Henckel, R. Knowles, and R. Conrad. 1999. High-affinity methane oxidation by a soil enrichment culture containing a type II methanotroph. Appl. Environ. Microbiol. 65:1009-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunfield, P. F., M. T. Yimga, S. N. Dedysh, U. Berger, W. Liesack, and J. Heyer. 2002. Isolation of a Methylocystis strain containing a novel pmoA-like gene. FEMS Microbiol. Ecol. 41:17-26. [DOI] [PubMed] [Google Scholar]
- 17.Felsenstein, J. 1993. PHYLIP: phylogeny inference package. University of Washington, Seattle.
- 18.Fjellbirkeland, A., V. Torsvik, and L. Øvreås. 2001. Methanotrophic diversity in an agricultural soil as evaluated by denaturing gradient gel electrophoresis profiles of pmoA, mxaF and 16S rDNA sequences. Antonie Leeuwenhoek 79:209-217. [DOI] [PubMed] [Google Scholar]
- 19.Guckert, J. B., D. B. Ringelberg, D. C. White, R. S. Hanson, and B. J. Bratina. 1991. Membrane fatty acids as phenotypic markers in the polyphasic taxonomy of methylotrophs within the Proteobacteria. J. Gen. Microbiol. 137:2631-2641. [DOI] [PubMed] [Google Scholar]
- 20.Gulledge, J., and J. P. Schimel. 1998. Low-concentration kinetics of atmospheric CH4 oxidation in soil and mechanism of NH4+ inhibition. Appl. Environ. Microbiol. 64:4291-4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanson, R. S., and T. E. Hanson. 1996. Methanotrophic bacteria. Microbiol. Rev. 60:439-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henckel, T., M. Friedrich, and R. Conrad. 1999. Molecular analyses of the methane-oxidizing microbial community in rice field soil by targeting the genes of the 16S rRNA, particulate methane monooxygenase, and methanol dehydrogenase. Appl. Environ. Microbiol. 65:1980-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henckel, T., U. Jäckel, S. Schnell, and R. Conrad. 2000. Molecular analyses of novel methanotrophic communities in forest soil that oxidize atmospheric methane. Appl. Environ. Microbiol. 66:1801-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heyer, J., V. F. Galchenko, and P. F. Dunfield. 2002. Molecular phylogeny of type II methane-oxidizing bacteria isolated from various environments. Microbiology 148:2831-2846. [DOI] [PubMed]
- 25.Heyer, J., Y. Malashenko, U. Berger, and E. Budkova. 1984. Verbreitung methanotropher Bakterien. Z. Allg. Mikrobiol. 24:725-744. [Google Scholar]
- 26.Hoffmann, T., H. P. Horz, D. Kemnitz, and R. Conrad. 2002. Diversity of the particulate methane monooxygenase gene in methanotrophic samples from different rice field soils in China and The Philippines. Syst. Appl. Microbiol. 25:267-274. [DOI] [PubMed] [Google Scholar]
- 27.Holmes, A. J., A. Costello, M. E. Lidstrom, and J. C. Murrell. 1995. Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol. Lett. 132:203-208. [DOI] [PubMed] [Google Scholar]
- 28.Holmes, A. J., P. Roslev, I. R. McDonald, N. Iversen, K. Henriksen, and J. C. Murrell. 1999. Characterization of methanotrophic bacterial populations in soils showing atmospheric methane uptake. Appl. Environ. Microbiol. 65:3312-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horz, H.-P., A. S. Raghubanshi, J. Heyer, C. Kammann, R. Conrad, and P. F. Dunfield. 2002. Activity and community structure of methane-oxidising bacteria in a wet meadow soil. FEMS Microbiol. Ecol. 41:247-257. [DOI] [PubMed] [Google Scholar]
- 30.Horz, H.-P., M. T. Yimga, and W. Liesack. 2001. Detection of methanotroph diversity on roots of submerged rice plants by molecular retrieval of pmoA, mmoX, mxaF, and 16S rRNA and ribosomal DNA, including pmoA-based terminal restriction fragment length polymorphism profiling. Appl. Environ. Microbiol. 67:4177-4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Intergovernmental Panel on Climate Change. 2001. The scientific basis—contribution of working group I to the third assessment report of the Intergovernmental Panel on Climate Change (IPCC). In J. T. Houghton, Y. Ding, D. J. Griggs, M. Noguer, P. J. van der Linden, and D. Xiaosu (ed.), Climate change 2001. Cambridge University Press, Cambridge, United Kingdom.
- 32.Jensen, S., A. J. Holmes, R. A. Olsen, and J. C. Murrell. 2000. Detection of methane oxidizing bacteria in forest soil by monooxygenase PCR amplification. Microb. Ecol. 39:282-289. [PubMed] [Google Scholar]
- 33.Kandeler, E., and H. Gerber. 1988. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 6:68-72. [Google Scholar]
- 34.Knief, C., K. Altendorf, and A. Lipski. Linking autotrophic activity in environmental samples with specific bacterial taxa by detection of 13C-labeled fatty acids. Environ. Microbiol., in press. [DOI] [PubMed]
- 35.Kolb, S., C. Knief, S. Stubner, and R. Conrad. 2003. Quantitative detection of methanotrophs in soil by novel pmoA-targeted real-time PCR assays. Appl. Environ. Microbiol. 69:2423-2429. [DOI] [PMC free article] [PubMed]
- 36.Le Mer, J., and P. Roger. 2001. Production, oxidation, emission and consumption of methane by soils: a review. Eur. J. Soil Biol. 37:25-50. [Google Scholar]
- 37.Lipski, A., and K. Altendorf. 1997. Identification of heterotrophic bacteria isolated from ammonia-supplied experimental biofilters. Syst. Appl. Microbiol. 20:448-457. [Google Scholar]
- 38.McDonald, I. R., and J. C. Murrell. 1997. The particulate methane monooxygenase gene pmoA and its use as a functional gene probe for methanotrophs. FEMS Microbiol. Lett. 156:205-210. [DOI] [PubMed] [Google Scholar]
- 39.Nichols, P. D., J. B. Guckert, and D. C. White. 1986. Determination of monounsaturated fatty acid double-bond position and geometry for microbial monocultures and complex consortia by capillary GC-MS of their dimethyl disulfide adducts. J. Microbiol. Methods 5:49-55. [Google Scholar]
- 40.Nichols, P. D., G. A. Smith, C. P. Antworth, R. S. Hanson, and D. C. White. 1985. Phospholipid and lipopolysaccharide normal and hydroxy fatty acids as potential signatures for methane-oxidizing bacteria. FEMS Microbiol. Ecol. 31:327-335. [Google Scholar]
- 41.Pacheco-Oliver, M., I. R. McDonald, D. Groleau, J. C. Murrell, and C. B. Miguez. 2002. Detection of methanotrophs with highly divergent pmoA genes from Arctic soils. FEMS Microbiol. Lett. 209:313-319. [DOI] [PubMed] [Google Scholar]
- 42.Reay, D. S., S. Radajewski, J. C. Murrell, N. McNamara, and D. B. Nedwell. 2001. Effects of land use on the activity and diversity of methane oxidizing bacteria in forest soils. Soil Biol. Biochem. 33:1613-1623. [Google Scholar]
- 43.Rieley, G. 1994. Derivatization of organic compounds prior to gas chromatographic-combustion-isotope ratio mass spectrometric analysis: identification of isotope fractionation processes. Analyst 119:915-919. [Google Scholar]
- 44.Roslev, P., and N. Iversen. 1999. Radioactive fingerprinting of microorganisms that oxidize atmospheric methane in different soils. Appl. Environ. Microbiol. 65:4064-4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinkamp, R., W. Zimmer, and H. Papen. 2001. Improved method for detection of methanotrophic bacteria in forest soils by PCR. Curr. Microbiol. 42:316-322. [DOI] [PubMed] [Google Scholar]
- 46.Strunk, O., O. Gross, B. Reichel, M. May, S. Hermann, N. Stuckman, B. Nonhoff, M. Lenke, A. Ginhart, A. Vilbig, T. Ludwig, A. Bode, and K. H. Schleifer. 1998. ARB: a software environment for sequence data. Technische Universität München, Munich, Germany.
- 47.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willison, T. W., C. P. Webster, K. W. T. Goulding, and D. S. Powlson. 1995. Methane oxidation in temperate soils: effects of land use and the chemical form of nitrogen fertilizer. Chemosphere 30:539-546. [Google Scholar]