Abstract
Functional neuroimaging studies in human subjects using positron emission tomography or functional magnetic resonance imaging (fMRI) are typically conducted by collecting data over extended time periods that contain many similar trials of a task. Here methods for acquiring fMRI data from single trials of a cognitive task are reported. In experiment one, whole brain fMRI was used to reliably detect single-trial responses in a prefrontal region within single subjects. In experiment two, higher temporal sampling of a more limited spatial field was used to measure temporal offsets between regions. Activation maps produced solely from the single-trial data were comparable to those produced from blocked runs. These findings suggest that single-trial paradigms will be able to exploit the high temporal resolution of fMRI. Such paradigms will provide experimental flexibility and time-resolved data for individual brain regions on a trial-by-trial basis.
Keywords: neuroimaging, single trial, language, prefrontal
A major limitation in conducting functional neuroimaging studies has been the use of blocked task paradigms that present many trials of the same type in immediate succession. This type of paradigm has been used because the statistical quality of individual images are relatively poor in relation to the signal changes of interest. By presenting successive trials in blocks, the data acquisition period is extended. In functional magnetic resonance imaging (fMRI), multiple images are acquired during this extended time period and relied on to obtain functional activation data with good signal-to-noise characteristics (1). In the case of positron-emission tomography (PET), the extended period is used to sample enough decay events to boost signal characteristics within the reconstructed images (2). These methods, by relying on time-blocked averaging, do not take advantage of the high temporal resolutions of the techniques, especially of fMRI. Fast echo planar MRI can acquire individual images in as little as 1/30th of a second (3) while almost all studies employing a block design have averaged data over 16- to 40-sec intervals.
Time-course data from blocked fMRI paradigms have shown that signal changes occur within seconds after a task is initiated even in prefrontal regions that demonstrate relatively small signal changes (4, 5). These findings, combined with a recent demonstration that signal changes can be observed to brief visual stimuli (18), suggest that fMRI might be able to resolve signal modulation related to single trials of a cognitive task.
We report here a procedure for signal averaging that examines activity related to single trials of a cognitive task. This procedure takes advantage of the high temporal resolution of fast fMRI and, at the same time, allows for substantial signal averaging. Such a procedure is an important complement to techniques which gather block-averaged data. For example, single-trial procedures allow for task paradigms to be developed that sequentially mix multiple trial types together or sort trial types by subject performance (e.g., whether they perform correctly on a trial or not). These design constructs have been extensively applied in studies based on event-related potentials (ERPs; e.g., see ref. 6).
Here we used a single-trial procedure to detect activation of prefrontal cortex during a cognitive task paradigm. Prefrontal activations were observed using whole brain fMRI in individual subjects, demonstrating that the procedure is highly sensitive. Visual areas were also shown to produce robust single-trial responses in the same images that showed prefrontal responses, suggesting these methods can be applied in settings that survey activity across distributed brain regions.
MATERIALS AND METHODS
Subjects.
Four right-handed subjects between the ages of 18 and 35 served in the first experiment (two males) and two subjects were in the second experiment (one male). Informed consent was obtained prior to scanning in a manner approved by the Human Studies Committee of the Massachusetts General Hospital.
General Magnetic Resonance (MR) Procedures.
Scans were acquired on a 1.5 Tesla General Electric scanner with echo planar imaging (Advanced NMR Systems, Wilmington, MA). Subjects lay in the scanner with their heads snugly surrounded by a pillow within the head coil to reduce movement. Extra cushions were used to further reduce movement. Ten to 12 functional scanning sequences ranging from 3 to 4.5 min in length were acquired for each subject in sessions lasting 2 h.
Functional scans were collected in runs of 105–256 images per slice using a T2* weighted asymmetric spin echo sequence designed to reduce contributions from large vessels (19) (TE = 70 msec, 25 msec offset). Slice thickness was set to 7 mm, with 3.125 × 3.125 mm in-plane resolution. Seventeen slices per run were acquired in experiment one and 5 slices per run were acquired in experiment two. An “image-to-image” signal-to-noise ratio was calculated for each functional run as a gross estimate of the statistical quality of the raw images. For this calculation, mean signal intensity within a rectangular region expanded to the size of the entire slice [placed near the anterior commissure–posterior commissure (AC–PC) plane] was measured for each image within the run. The mean regional signal across all images within the run was then divided by the standard deviation. T1 weighed inversion recovery echo planar images were acquired (TI = 1200 msec, 1.563 × 1.563 mm in-plane resolution) in the same orientation as the functional runs to provide detailed anatomic information aligned to the functional scans.
Visual stimuli were presented to the subject using a PowerMacintosh (Apple Computer) connected to a Sharp 2000 color LCD projector. Stimuli were projected onto a screen through a collimating lens. The screen was attached to a standard General Electric quadrature head coil and was viewed through the mirrors built into the head coil. Synchronization of the stimuli to the MR data acquisition was accomplished by manually starting the stimulus program at the same time in the MR sequence on successive runs.
Overview of Experimental Design and Analysis.
Previous PET studies have demonstrated a pathway of brain areas that are activated during overt and covert word-stem completion (7, 8). Word-stem completion involves viewing individual word beginnings (e.g., COU, GRE) and generating word completions (e.g., Couple, Green). Many activations, including those in visual, motor, and prefrontal regions, have been observed during this task as compared with a fixation control task (7). A recent study demonstrated that these activations can be reliably observed within-subject using a block-designed fMRI paradigm (20). For these reasons, word-stem completion provided a candidate cognitive task to assess the feasibility of a single-trial fMRI procedure.
Across two separate experiments, data were collected while subjects performed the word-stem completion task. For each experiment both block-trial and single-trial paradigms were studied and analyzed in multiple ways. Blocked-trial paradigms consisted of a series of closely-spaced word-stem completion trials (the “on” condition) during which stems were presented to the subject every 2.3–2.5 sec for 30–32 sec (Fig. 1A). The “on” blocks alternated with “off” blocks of a control condition of the same duration (usually consisting of visual fixation). In contrast, the single-trial paradigms consisted of a series of widely spaced word-stem completion trials during which individual stems were presented every 14–16 sec for a period of 210–256 sec (Fig. 1B). During both the block-trial and single-trial paradigms, images of the brain were obtained every 1 or 2 sec (TR = 2 sec, experiment one; TR = 1 sec, experiment two).
Figure 1.
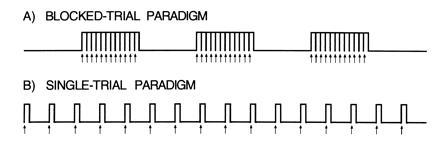
Heuristic diagrams display the difference between a blocked-trial paradigm (A) and a single-trial paradigm (B). Arrows indicate the beginnings of the individual trials. The main advantage of the single-trial paradigm is the spacing of the trials. The trials are separated in time allowing the time course of activation to be appreciated and the trials to be averaged in relation to their onset.
Data from the block-trial paradigms were analyzed with two goals in mind: first, to produce activation maps of the overall brain activity and, second, to use these activation maps to define regions of interest for analysis of the single trials.
In experiment one, data from the single-trial paradigm were analyzed to determine if signal change could be detected during individual trials of word-stem completion. For this analysis, a priori regions of interest in left prefrontal cortex, defined from the blocked-trial runs, were examined in the single-trial data. A two-step averaging procedure was utilized. First, data from the separate single-trial runs (each consisting of 15 trials) were averaged to produce an averaged single-trial run still consisting of multiple individual trials. Second, the individual trials from each single-trial run were averaged together to produce a single, representative time course for one trial of word-stem completion.
The above analyses were replicated in experiment two which collected data with a higher temporal sampling rate (TR = 1 sec) and for a larger number of trials (128). Experiment two also sought to explore how the single-trial runs could be analyzed in isolation, without reliance on blocked-trial runs to define regions of interest. Activation maps were produced solely from the single-trial runs and compared with those produced from the blocked runs. The extended single-trial runs were then explored to observe the time-course of activity over the multiple included trials.
Design and Analysis of Experiment One.
Experiment one sought to detect activations during word-stem completion within-subject using a blocked-trial paradigm and then determine the time-course of the activations using individual trials of the word-stem completion task. Whole-brain fMRI was used. Seventeen 7-mm slices (skip 1-mm between slices, TR = 2 sec) were acquired along the AC–PC plane as determined by the midsagittal section.
For each subject, six to eight runs of blocked-trial word-stem completion were acquired. Each run consisted of four blocks of an “off” condition, usually simple fixation, alternating with three blocks of a word-stem completion “on” condition. Each block was 30 sec long with 12 trials of word-stem completion presented during each “on” block (2.5 sec between trial onsets, 1.5-sec stimulus duration). Subjects were instructed to covertly generate a word completion for each word beginning presented. For subject 4, the “off” period was passively viewing false-font strings. This difference causes the visual areas to be attenuated in the comparison but has little effect on the frontal and motor activations.
Runs of blocked-trial word-stem completion were followed by four runs in which individual trials of word-stem completion were presented. Fifteen trials were presented per single-trial run. Trials occurred at regular intervals throughout the 210-sec run (14 sec between trial onsets). This procedure allowed a total of 60 individual trials to be acquired per subject (4 runs each with 15 trials).
Analysis was conducted on individual subjects by first averaging all of the blocked-trial runs and separately averaging all of the single-trial runs. This resulted in two separate averaged runs per subject: the blocked-trial run included 3 blocked trials of word-stem completion and the single-trial run included 15 individual trials.
Activation images were constructed for the blocked-trial runs using the nonparametric Kolmogorov–Smirnov (K-S) statistic (10, 11). K-S maps were created that showed activations above a P < 0.001 threshold within each subject. K-S maps were constructed after an in-plane Hanning spatial filter was applied that smoothed adjacent voxels. Within these maps, an expected activation within left inferior prefrontal cortex, near frontal-opercular cortex, was identified (see Fig. 2). For each subject, a region was manually traced to include the activated pixels within this region. Data from the single-trial runs were not used for region definition in experiment one. The time course for the mean signal intensity within these regions was obtained for each subject. A linear slope was subtracted from the overall time series to remove drift for this and all subsequent time-course data.
Figure 2.
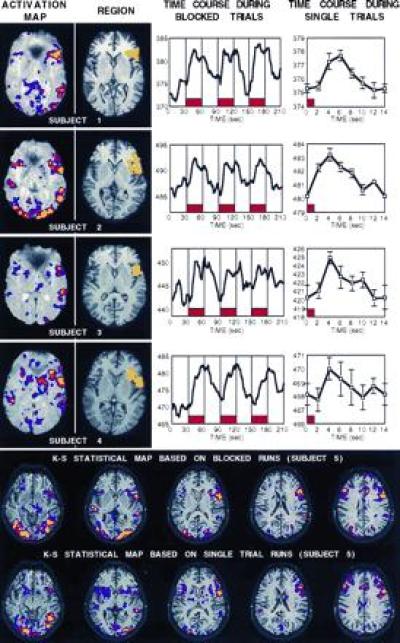
(Upper) The top figure shows experiment one data for each of the four subjects. For each subject, four kinds of data are shown left to right including: an activation map (threshold = P < 0.01, red = P < 10−5, yellow = P < 10−8) plotted superimposed on top of a raw T2* weighted functional image; the region constructed to include the left inferior prefrontal activation (yellow) on top of a T1-weighted echo planar image showing more detailed anatomy; the region’s mean MR signal intensity during the runs with blocked trials of word-stem completion (placement of blocks shown in red, time course smoothed to three time points); and the region’s mean MR signal intensity during the single-trial runs (placement of trial shown in red, time point at 0 sec duplicated at time point 14 sec to show continuation of trial, no temporal smoothing, plus standard error of the mean). (Lower) K-S activation maps are shown for all slices collected in subject 5, plotted on top of the T1-weighted images (threshold lowered to P < 0.05 to allow more complete comparison, red = P < 10−4). Top activation map shows data derived from the blocked runs while the bottom map shows data derived solely from the single-trial runs. The two maps overlap considerably.
Time-course data for mean activity in the left inferior prefrontal regions were then examined in the single-trial runs (averaged over the previously defined regions of interest). The time course for a single averaged trial was constructed by averaging the time-course data in reference to the onset of the word-stem cues, much like ERP data are averaged (6). The procedure resulted in a single 14-sec averaged time course that was the composite of 60 trials of data.
Design and Analysis of Experiment Two.
The previous experiment used whole brain MR scanning to detect activation during averaged single trials. However, the temporal sampling was relatively sparse with one image (time point) collected every 2 sec. The second experiment sought to replicate the first experiment in a paradigm that sampled activity with a higher temporal frequency (1/sec). To accomplish this goal, we sampled only five slices along the AC–PC plane at a level likely to detect the left inferior prefrontal activation and the visual activations (five 7-mm slices, skip 1 mm between slices, TR = 1 sec).
A procedure similar to that of experiment one was used with two exceptions. First, four runs of the blocked-trial word-stem completion were collected with four “off” blocks and four “on” blocks. Each block was 32 sec with 14 trials per block (2.3 sec between trial onsets, 1.5-sec stimulus duration) for a total run length of 256 sec. Second, 8 single-trial runs were collected with 16 trials per run spaced 16 sec apart. These two modifications resulted in a larger portion of the session being devoted to acquisition of single-trial data with more data collected during each trial (8 runs each with 16 trials yielding a total of 128 trials compared with 60 trials in experiment one). The reason for this design modification was that overall signal-to-noise ratio was anticipated to drop due to the shortened TR.
Analysis was similar to that in experiment one. Blocked-trial runs were averaged, and a left inferior prefrontal region was traced. This region was then applied to the independently acquired single-trial runs to determine the time course of activation. For this analysis, the 16 individual trials from the composite of the 8 single-trial runs were averaged to determine the mean time course for a single trial of word-stem completion.
Three additional analyses were performed to further explore the characteristics of the single-trial data. First, visual regions were traced in extrastriate cortex to compare with the left inferior prefrontal regions. This analysis was conducted to determine whether the time course of activation across distributed brain regions could be appreciated. Second, the time course data from the extended single-trial runs (after averaging across the eight runs) were examined. These runs contained separate data for each of 16 trials and allowed the data to be inspected to determine whether responses to the separate trials could be observed, and whether the magnitude of signal was constant during the entire length of the run.
Finally, activation maps were constructed from the single-trial runs and compared with maps constructed from the blocked runs. Two reasons exist for conducting this analysis. Most importantly, the procedure serves as an independent comparison between the blocked-trial and single-trial runs to determine if similar regions are activated across the two procedures. In addition, the analysis suggests a way of analyzing data to obtain activation maps without the use of blocked-trial runs. This was accomplished by assigning individual time points in the single-trial runs as part of the “on” or “off” condition. The first 4 time points per trial were considered “off” and time points 6–12 were considered “on.” These time points were selected based on the time course of the single trials from experiment one.
RESULTS
Experiment One.
The “image-to-image” signal-to-noise ratio was between 79:1 to 89:1 for the averaged blocked-trial runs and 61:1 to 70:1 for the averaged single-trial runs. Fig. 2 shows the main results from experiment one. In each subject, a robust activation could be detected in left inferior prefrontal cortex in the averaged blocked-trial runs. Voxels within this area, which included the anterior portion of the operculum and extended laterally, could be detected at the P < 0.0001 level in each subject. Regions were defined on these activations and time courses across the blocked-trial runs revealed robust signal modulation in relation to the blocked trials of word-stems.
Of central importance to this paper was the finding that a consistent, but small (<1%), single-trial response could be detected in the left prefrontal region in each subject. Post-hoc statistical tests revealed that magnitude of signal was significantly increased above the baseline magnitude 4–6 sec after the stimulus onset in each subject (P < 0.05 Wilcoxen signed rank test; for subject 4, P = 0.08). The signal change peaked after 4–8 sec in each subject and lasted on the order of 8–10 sec. The magnitude of signal change in the prefrontal regions was considerably less in the single-trial paradigm (about 0.7%) than in the blocked-trial paradigm (about 1.9%). However, because there was not an extended baseline period, it was not possible to determine if the signal change had reached its absolute baseline level in the 14-sec time period during the single-trial runs.
Experiment Two.
The image-to-image signal-to-noise ratio was between 49:1 to 55:1 for the averaged blocked-trial runs and 69:1 to 77:1 for the averaged single-trial runs. Examination of the time courses for the entire unspliced (but averaged) single-trial runs revealed that the signal change could be observed in many of the individual trials (Fig. 3). The signal change was considerably more robust for the visual extrastriate region as compared with the left inferior prefrontal region. Additionally, the signal appeared to diminish slightly as the run progressed. Subject 5 showed a much more stable time course throughout the run than for subject 6, consistent with the well behaved single-trial time courses discussed below.
Figure 3.
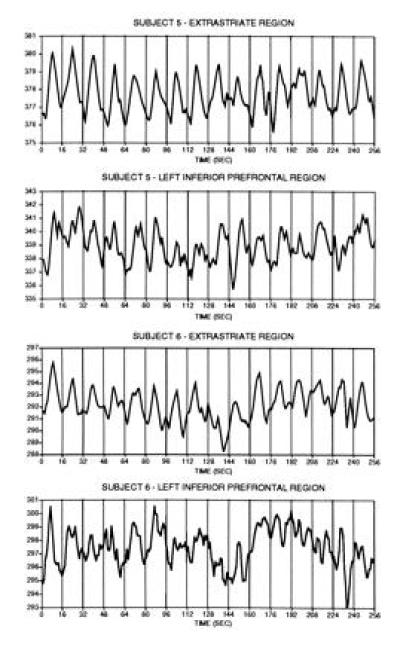
Time-course data from the extended single-trial runs in experiment two are shown for each subject (eight runs averaged per subject, see text). Data from each run comprise the mean MR signal magnitude within the individual regions indicated (separate data are shown for extrastriate and left inferior prefrontal regions). Each vertical line represents the start of a trial, each of which was spaced 16 sec apart. Clear signal changes in relation to the 16 temporally separate trials can be observed and most clearly for the extrastriate regions. Single-trial responses were more robust in the earlier trials as compared with the later trials. These signal changes were averaged to produce the time-course data presented in Fig. 4.
Fig. 4 shows the main result from experiment two. A robust single-trial signal increase could be detected in the left inferior prefrontal cortex and also within extrastriate cortex for both subjects. The time course of the prefrontal response was similar to that observed in experiment one, showing a peak around 8 sec and lasting about 10 sec. Subject 6 showed a rather fast onset of response similar to subject 2 of experiment one.
Figure 4.
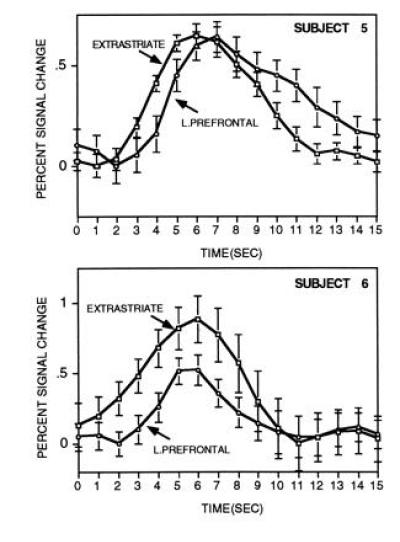
The time course for averaged single-trial responses are shown for separate brain regions in experiment two (plus standard error of the mean). Both regions come from the same slice and were selected based on data from the blocked runs. Each time course comprises the average of 128 individual trials and is plotted in relation to percent signal change to make the comparison across brain regions. Time-course data are smoothed across adjacent time points. As can be seen, robust signal increases occur starting at about two sec after the onset of the stimulus (0 sec) and continue for about 10 sec. The prefrontal response was delayed in relation to the extrastriate response in both subjects.
For both subjects, the time course of the visual response was temporally shifted in relation to the prefrontal response, starting about one sec before the frontal response. This was most evident for subject 5, whose sampled magnitude of activation was equal across the two regions, which allows the time courses to be compared without contributions from differences in magnitude of response. For this subject, comparison of the individual time points between the two regions showed they were significantly different during the rise (seconds 3–4, 4–5, 5–6, and 6–7; all P < 0.05 Wilcoxen signed rank test; see Fig. 4) and during the fall of the response (seconds 11–12 and 12–13; both P < 0.05). For subject 6, the frontal response occurred after the extrastriate response but the data were sufficiently noisy and the magnitude of the responses were different enough to make the comparison less informative (see Fig. 3).
Finally, comparisons of the activation maps constructed based on the single-trial runs were qualitatively similar to those observed from the blocked-trial runs (Fig. 2). Activation was significant (P < 0.001) in both maps in left inferior prefrontal cortex, visual areas, motor areas, and cingulate. For subject 5, the correspondence between activation in these areas was high. This correspondence extended to regions which decreased in activation, with both maps (single trial and blocked trial) in both subjects showing a robust decrease (P < 0.001) in medial parietal cortex similar to that observed in previous PET studies (7, 21).
DISCUSSION
Examination of the time course of fMRI activation to averaged single trials of word-stem completion showed highly reliable signal changes (Fig. 2). These changes were detected within-subject using whole brain fMRI. Moreover, signal changes of less than 1% in magnitude were present in prefrontal regions presumably activated by cognitive demands of the task (for a discussion of these demands see ref. 9). In this regard, the present study complements two recent reports (abstracts) using single-trial paradigms. Savoy et al. (18) reported that signal increases to passive visual stimuli as brief as 34 msec could be detected within-subject using fMRI and surface coils. More subtle cognitive responses were detected in averaged subject groups in an odd-ball paradigm by McCarthy et al. (12).
These findings have broad implications for the design and analysis of fMRI experiments. By taking advantage of the high temporal resolution of fMRI, neuroimaging studies are no longer restricted to the use of blocked-trial paradigms which require many trials of the same type in succession. Single trials of word-stem completion produce hemodynamic responses of sufficient magnitude to be detected using fast fMRI techniques at a 1.5 Tesla field strength. Inspection of the averaged single-trial runs reveal that responses to the 16 individual trials can be observed in the averaged runs even before the complete set of trials are averaged together (Fig. 3).
Activation maps of spatially distributed activations were constructed using only the data from the single-trial runs. These maps were similar to those collected during blocked-trial runs (Fig. 2), suggesting that studies exclusively containing runs of single trials will be sufficient to construct activation maps of overall brain activity. Thus, the use of single-trial paradigms may provide flexibility in task design by allowing different trial types to be mixed, while still allowing survey activation maps to be created.
Similar to studies of visual impulse responses (18), the hemodynamic response was delayed in onset and temporally extended over many seconds (Figs. 2 and 4). Examination of the time course of two distributed brain regions in experiment two revealed that visual and prefrontal regions showed temporally offset signal changes. In subject 5, the visual response preceded the prefrontal response by about 1 sec and decayed at a considerably faster rate than the prefrontal response. Subject 6 showed a trend for an earlier visual response similar to subject 5 (Fig. 4).
One interpretation of this result is that the shift in time course reveals the temporal separation of the activity within these two regions, such as has been proposed for a similar finding in a blocked-designed fMRI paradigm (22). Consistent with this possibility, overt word generation during the word-stem completion task takes about 1.2 sec (7). However, ERP data for similar word generation tasks have suggested prefrontal responses may initiate in as a little as a few hundred milliseconds (13), suggesting the temporal separation may be due to other factors as well. An alternative explanation for the staggered time courses is that different regions of the brain have different apparent hemodynamic time courses. Such a possibility may result because different regions of interest, as sampled at the resolution of fMRI, contain systematic differences in the distribution of blood vessel diameters. As variance in vessel diameter has been demonstrated to produce hemodynamic response functions with different time courses (14), it is possible that such an effect could also underlie the shift in time course. Nonetheless, the observed temporal offsets were in line with expectations about when the regions are likely to become activated in the temporal sequence of the task and are thus intriguing.
Many unresolved issues exist concerning how studies using single-trial paradigms should be conducted. It will be important to determine more optimal analyses for exploring single-trial data both when comparing different trial types and when constructing activation maps based on the single-trial runs. The present analyses utilized conservative nonparametric statistics and provided some promising results. It seems quite possible that analyses which correlate the single-trial runs to idealized single-trial response functions, or empirically derived response functions, might be considerably more powerful (15, 16) and provide information about temporal onsets (23). Analyses based on the Fourier frequency spectrum might also prove useful (15). A further issue needing systematic exploration is how far apart trials should be separated. Methods applied in the ERP field have been able to deconvolve the separate contributions of overlapping trials and these methods may also apply to fMRI data (17).
In summary, reliable fMRI signal changes were detected in response to averaged single trials of a cognitive task. The demonstration that fMRI techniques are sensitive to signal from individual trials allows a new spectrum of task designs. Such designs might include (i) mixing distinct trial types together in single runs, (ii) correlating behavioral performance on individual trials with the fMRI activation signal, and (iii) examining the fMRI time course of activation on a trial-by-trial basis.
Acknowledgments
We thank Terrance Campbell and Mary Foley for technical assistance, and Robert Weisskoff and Mike Posner for thoughtful comments on an earlier version of this manuscript. This work was supported by National Institutes of Health Grants P1DA09467 and AG-08377, and grants from the Charles A. Dana Foundation and the McDonnell Center for Higher Brain Function.
Footnotes
Abbreviations: PET, positron-emission tomography; fMRI, functional magnetic resonance imaging; K-S, Kolmogorov–Smirnov; ERP, event-related potential; MR, magnetic resonance.
References
- 1.Deyoe E A, Bandettini P, Neitz J, Miller D, Winans P. Neurosci Methods. 1994;15:171–187. doi: 10.1016/0165-0270(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 2.Raichle M E. In: The Handbook of Physiology. Plum F, Mountcastle V, editors. Vol. 5. Bethesda, MD: Am. Physiol. Assoc.; 1987. pp. 643–674. [Google Scholar]
- 3.Cohen M S, Weisskoff R M. Magn Reson Imaging. 1991;9:1–37. doi: 10.1016/0730-725x(91)90094-3. [DOI] [PubMed] [Google Scholar]
- 4.Hinke R M, Hu X, Stillman A E, Kim S-G, Merkle H, Salmi R, Ugurbil K. NeuroReport. 1993;14:675–678. doi: 10.1097/00001756-199306000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Binder J R, Rao S M, Hammeke T A, Frost J A, Bandettini P A, Jesmanowicz A, Hyde J S. Arch Neurol. 1995;52:593–601. doi: 10.1001/archneur.1995.00540300067015. [DOI] [PubMed] [Google Scholar]
- 6.Picton T W, Lins O G, Scherg M. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Vol. 10. Amsterdam: Elsevier; 1995. pp. 3–74. [Google Scholar]
- 7.Buckner R L, Petersen S E, Ojemann J G, Miezin F M, Squire L R, Raichle M E. J Neurosci. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckner R L, Raichle M E, Petersen S E. J Neurophysiol. 1995;74:2163–2173. doi: 10.1152/jn.1995.74.5.2163. [DOI] [PubMed] [Google Scholar]
- 9.Buckner R L, Petersen S E. Semin Neurosci. 1996;8:47–55. [Google Scholar]
- 10.Press W H, Flanner B P, Teukolsky S A, Veterling W T. Numerical Recipes in C: The Art of Scientific Computing. New York: Cambridge Univ. Press; 1988. [Google Scholar]
- 11.Stuart A, Ord J K. Kendall’s Advanced Theory of Statistics. New York: Oxford Univ. Press; 1991. [Google Scholar]
- 12.McCarthy G, Luby M, Gore J C, Goldman-Rakic P. NeuroImage. 1996;3:S548. (abstr.). [Google Scholar]
- 13.Abdullaev Y G, Posner M I, Srinivasan R, Tucker E M. NeuroImage. 1996;3:S425. (abstr.). [Google Scholar]
- 14.Lee A T, Glover G H, Meyer C H. Magn Reson Med. 1995;33:745–754. doi: 10.1002/mrm.1910330602. [DOI] [PubMed] [Google Scholar]
- 15.Bandettini P A, Jesmanowicz A, Wong E C, Hyde J S. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- 16.Boynton G M, Engel S A, Glover G H, Heeger D J. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woldorff M. Psychophysiology. 1993;30:98–119. doi: 10.1111/j.1469-8986.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]
- 18.Savoy, R. L., Bandettini, P. A., O’Craven, K. M., Kwong, K. K., Davis, T. L., Baker, J. R., Weisshoff, R. M. & Rosen, B. R. (1995) 15th Annu. Proc. Soc. Magn. Reson. Med. 450 (abstr.).
- 19.Baker, J. R., Hoppel, B. E., Stern, E. E., Kwong, K. K., Weisskoff, R. M. & Rosen, B. R. (1993) 12th Annu. Proc. Soc. Magn. Reson. Med. 1400 (abstr.).
- 20.Buckner R L, Kale A M, Tootell R B H, Petersen S E, Raichle M E, Rosen B R. Soc Neurosci Abstr. 1996;22:7. [Google Scholar]
- 21.Shulman G L, Buckner R L, Corbetta M, Miezin F, Raichle M, Petersen S E. NeuroImage. 1996;3:S197. (abstr.). [Google Scholar]
- 22.Binder, J. R., Rao, S. M., Hammeke, T. A., Bandettini, P. A., Jesmanowicz, A., Frost, J. A., Wong, E. C., Haughton, V. M. & Hyde, J. S. (1993) 12th Annu. Proc. Soc. Magn. Reson. Med. 5 (abstr.).
- 23.Binder, J. R., Jesmanowicz, A., Rao, S. M., Bandettini, P. A., Hammeke, T. A. & Hyde, J. S. (1993) 12th Annu. Proc. Soc. Magn. Reson. Med. 1382 (abstr.).


