Abstract
The microflora of the crop was investigated throughout the broiler production period (0 to 42 days) using PCR combined with denaturing gradient gel electrophoresis (PCR-DGGE) and selective bacteriological culture of lactobacilli followed by amplified ribosomal DNA restriction analysis (ARDRA). The birds were raised under conditions similar to those used in commercial broiler production. Lactobacilli predominated and attained populations of 108 to 109 CFU per gram of crop contents. Many of the lactobacilli present in the crop (61.9% of isolates) belonged to species of the Lactobacillus acidophilus group and could not be differentiated by PCR-DGGE. A rapid and simple ARDRA method was developed to distinguish between the members of the L. acidophilus group. HaeIII-ARDRA was used for preliminary identification of isolates in the L. acidophilus group and to identify Lactobacillus reuteri and Lactobacillus salivarius. MseI-ARDRA generated unique patterns for all species of the L. acidophilus group, identifying Lactobacillus crispatus, Lactobacillus johnsonii, and Lactobacillus gallinarum among crop isolates. The results of our study provide comprehensive knowledge of the Lactobacillus microflora in the crops of birds of different ages using nucleic acid-based methods of detection and identification based on current taxonomic criteria.
The digestive tracts of mammals and birds are home to a diverse collection of bacterial species, collectively referred to as the gut microflora (28). From gnotobiotic animal studies, the microflora is known to influence the biochemistry, immunology, physiology, and nonspecific resistance to intestinal infection of the host (9). The impact of the gut microflora on the nutritional status of farm animals is of particular interest, especially where intensive farming practices are used (4).
The crop, ileum, cecum, and colon of poultry are known to harbor bacterial populations (16, 27). Recent reports have investigated the composition of the ileal (13) and cecal (35) microflora using bacteriological culture and culture-independent methods. Lactobacilli are numerous in the ileum of broilers, whereas the cecal microflora is dominated by obligately anaerobic bacteria and bacteria yet to be cultivated. From the results of culture-based studies, it has been determined that the microflora of the crop has a simple composition and is dominated by lactobacilli (16, 27). Colonization of the surface of the stratified, squamous epithelium of the crop by lactobacilli has been reported by Fuller (6) and Morishita et al. (18). Lactobacillus salivarius, Lactobacillus fermentum or Lactobacillus reuteri, and Lactobacillus acidophilus were the species most commonly detected (16, 27). These studies were conducted prior to the reclassification of L. acidophilus, which has been divided into two DNA homology groups containing six related species (5, 11, 15). DNA homology group A consists of L. acidophilus (A1), Lactobacillus crispatus (A2), Lactobacillus amylovorus (A3), and Lactobacillus gallinarum (A4); DNA homology group B consists of Lactobacillus gasseri (B1) and Lactobacillus johnsonii (B2). Even with molecular methods, it is difficult to distinguish between members of this group. Methods that have been used successfully include the following: DNA-DNA hybridization and various biochemical properties (5, 11, 15); analysis of whole-cell protein profiles by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (3, 8, 12, 21); randomly amplified polymorphic DNA (3, 8, 12, 24); sequencing of 16S and 23S ribosomal DNA (rDNA) and elongation factor Tu (2, 14, 29, 30); oligonucleotide probes and primers for species-specific hybridizations and PCR, respectively (21, 23, 24, 32); ribotyping (25); amplified fragment length polymorphism (8); and amplified ribosomal DNA restriction analysis (ARDRA) (17, 23, 31). There is a clear need, however, for simpler methods to differentiate the members of the L. acidophilus group to ensure that large-scale microbial ecological studies are logistically possible.
A detailed analysis of the crop microflora of broiler chickens using nucleic acid-based methods has not previously been reported. Our study therefore had two main aims. First, we used PCR combined with denaturing gradient gel electrophoresis (PCR-DGGE) to compare the crop microflora of birds of different ages. Second, we cultured lactobacilli from the crops of the birds and devised an ARDRA technique by which the members of the L. acidophilus group could be rapidly identified.
MATERIALS AND METHODS
Animals, treatment, and sampling.
Ross 308 broiler chicks (Aviagen Inc., Huntsville, Ala.) were obtained from a commercial hatchery (Lilydale Hatchery, Edmonton, Alberta, Canada) and raised at the Alberta Poultry Research Centre, University of Alberta. Chicks (n = 125) were placed in eight floor pens with fresh straw as the litter material. The stocking density in each of the floor pens was 609 cm2/bird. Birds were raised under conditions similar to commercial broiler production, with feed and water provided ad libitum. The chickens were fed three different wheat-based diets with the following antimicrobial agents: (i) 0.05% (wt/wt) bacitracin methylene disalicylate (BMD) (Alpharma Canada, Mississauga, Ontario, Canada) and 0.05% (wt/wt) monensin sodium (Elanco Animal Health, Guelph, Ontario, Canada) for birds from 0 to 21 days of age, (ii) 0.05% (wt/wt) BMD and 0.05% (wt/wt) monensin sodium for birds from 22 to 35 days of age, and (iii) 0.05% (wt/wt) BMD for birds from 36 to 42 days of age. In addition to the slight differences in antimicrobial concentrations noted above, all three diets contained the same components, although the amounts of corn, wheat, canola oil, soy, and amino acids were modified in order to meet the nutritional needs of the developing birds. All nutrients were included at levels to meet or exceed the National Research Council's recommendations for broiler chickens (19). The experimental protocol was approved by the Faculty of Agriculture, Forestry, and Home Economics Animal Policy and Welfare Committee (protocol number 2002-12B).
During the experiment, 86 birds were sampled as follows: 6 birds on day 0 (hatching day) and 10 each on 1, 3, 7, 14, 21, 28, 35, and 42 days of age. At each sampling time, one or two birds from each pen were selected randomly and in such a way that the stocking density was maintained. In a subsequent experiment, 10 birds were sampled on day 0. At each sample time, birds were euthanized and transported to the research laboratory where the crops were aseptically removed. For each bird, the crop was placed in a sterile petri plate and weighed. A section of crop tissue and contents weighing approximately 1 g or the entire crop (if it weighed less than 1 g) was transferred into a sterile 15-ml Pyrex tissue grinder and homogenized with 9 ml of 0.85% saline. For each bird, 1 ml of crop homogenate was stored at −80°C for nucleic acid-based analysis of the bacterial communities. The remaining crop homogenate was used for selective enumeration and collection of lactobacilli.
Propagation and enumeration of lactobacilli.
The crop homogenate was used to make a series of 10-fold dilutions (10−2 to 10−7) in sterile 0.85% NaCl. For each dilution, 100 μl was spread plated on Lactobacillus selective (LBS) agar (BBL) and incubated at 37°C for 48 h under anaerobic conditions (5% CO2, 10% H2, 85% N2). In the subsequent experiment using day 0 birds, 1 ml of the crop homogenate was used to make LBS pour plates. The number of CFU of presumptive lactobacilli per gram of crop for each bird was determined from the number of colonies on LBS plates. The colony morphologies on the counted LBS plates were also noted. For each crop, a total of 10 colonies, representing each colony type, were selected, restreaked on MRS agar (Difco) plates, and incubated at 37°C for 48 h under anaerobic conditions. The bacterial colonies were removed from the MRS agar plates, suspended in MRS broth containing 50% glycerol, and stored at −80°C. For routine propagation of Lactobacillus reference strains and crop isolates (Table 1), MRS broth glycerol stocks were streaked onto MRS agar and incubated at 37°C under anaerobic conditions as outlined above. Reference strains of Enterococcus and Pediococcus (Table 1) were grown on APT agar (Difco) at 30°C under aerobic conditions.
TABLE 1.
Reference strains used in this study
| Straina |
|---|
| Type strains |
| E. faecalis ATCC 19433 |
| E. faecium ATCC 19434 |
| L. acidophilus ATCC 4356 |
| L. amylovorus ATCC 33620 |
| L. aviarius subsp. aviarius ATCC 43234 |
| L. crispatus ATCC 33820 |
| L. fermentum ATCC 14931 |
| L. gallinarum ATCC 33199 |
| L. gasseri ATCC 33323 |
| L. johnsonii ATCC 33200 |
| L. reuteri ATCC 23272 |
| L. salivarius subsp. salivarius ATCC 11741 |
| Non-type strains |
| L. johnsonii ATCC 11506 |
| P. acidilactici ATCC 8042 |
| P. acidilactici PAC1.0 |
| P. pentosaceus ATCC 43200 |
ATCC, American Type Culture Collection.
DNA extraction from crop homogenates and crop isolates.
Bacterial DNA was extracted from the crop homogenate by the method of Walter et al. (33). Briefly, the frozen crop homogenate was allowed to thaw on ice and then centrifuged at 14,600 x g for 5 min at 4°C. The pellet was washed twice with 1 ml of TN150 (10 mM Tris-HCl [pH 8], 150 mM NaCl) buffer (34). After the pellet was resuspended in 1 ml of TN150 buffer, the cells were lysed by physical disruption with zirconium-silica beads (0.1-mm diameter) in a BioSpec Mini Bead-Beater-8 at 4,800 rpm for 3 min. Three phenol-chloroform-isoamyl alcohol (25:24:1) extractions were performed on each sample, and the DNA was precipitated with cold ethanol and dissolved in 30 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]). The presence of DNA was checked using a 0.7% agarose gel prior to PCR.
DNA was extracted from Lactobacillus, Enterococcus, and Pediococcus type strains and crop isolates by the method of Walter et al. (34). The DNA pellet was dissolved in 20 μl of TE buffer and diluted 20-fold for PCR.
PCR-DGGE analysis of crop DNA with universal bacterial primers and primers specific for lactic acid bacteria.
PCR was conducted using either individual DNA or pooled crop DNA as the template. The pooled samples were prepared by combining the crop DNA (1 μl of each) from all 10 crops collected at the same sampling time. The V3 region of the 16S rRNA gene from the crop DNA was amplified in a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, Calif.) using universal bacterial primers HDA1-GC and HDA2 (HDA PCR), following the protocol of Walter et al. (34) and subjected to DGGE (HDA PCR-DGGE). The DGGE analysis was performed with the Bio-Rad DCode Universal Mutation Detection System (Hercules, Calif.) by the method of Walter et al. (34). The V3 region of the 16S rDNA was amplified from the total crop DNA using group-specific bacterial primers Lac1 and Lac2-GC (Lac PCR) and subjected to DGGE (Lac PCR-DGGE) by the method of Walter et al. (33). Identification ladders for DGGE were prepared by combining the HDA or Lac PCR products prepared from DNA extracted from the type and reference Lactobacillus strains (Table 1).
DGGs were stained with ethidium bromide and viewed by UV transillumination. DGGE profiles were compared using Dice's similarity coefficient (Dsc) with the Bionumerics software package (Applied Maths, Austin, Tex.). When Dsc analysis was performed, only profiles within a gel were compared, not between gels. The average Dsc was calculated by adding the values of single profile comparisons for the age range stated and dividing by the total number of Dscs.
Identification of bacteria by sequencing DNA fragments.
DNA fragments generated by Lac primers were extracted from DGGs by the method of Knarreborg et al. (13). Following purification, the DNA was reamplified with the Lac1 and Lac2 (without GC clamp) primers using the PCR protocol described above. The resulting PCR products were purified with the QIAquick purification kit (Qiagen, Mississauga, Ontario, Canada), ligated into pGEM-T (Promega, Madison, Wis.), and used to transform Escherichia coli JM109. Transformants were plated on Luria-Bertani (LB) agar (26) containing ampicillin, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), and isopropyl-β-d-thiogalactopyranoside (IPTG) at concentrations of 100 μg/ml, 80 μg/ml, and 0.5 mM, respectively. Several colonies were subcultured, and the plasmid DNA was isolated by the alkaline lysis procedure of Sambrook et al. (26). Plasmid DNA was used as the template in a PCR with Lac1 and Lac2-GC primers, and the PCR products were analyzed by DGGE in order to compare the migration of the cloned DNA with the migration of the desired band from the original PCR-DGGE crop profile. The pGEM-T insert DNA was amplified using T7 and SP6 primers, and sequencing was conducted by the Agricultural, Food and Nutritional Science Biotech Core, University of Alberta. The sequences were compared with those in the GenBank database using the BLAST algorithm (1).
Identification of Lactobacillus isolates with ARDRA.
The total DNA extracted from each reference strain or crop isolate was used as the template for PCR amplification of either the 16S rRNA gene (16S rDNA) or the 16S rRNA gene plus the entire 16S-23S rRNA intergenic region (16-23S rDNA). The total 16S rRNA gene (1.5 kb) was amplified using SacI-POmod (5′-CCGAGCTCAACAGAGTTTGATCCTGGCTCAG-3′) and SalI-T7-PC5 (5′-GGTCGACCGTTAATACGACTCACTATAGGGATACCTTGTTACGACTT-3′) primers (22). The following primers were used to amplify the 16-23S rDNA (2 kb): (i) Lb16a (5′-GTGCCTAATACATGCAAGTCG-3′), which corresponds to nucleotides (nt) 17 to 36 of the 16S rDNA of L. crispatus ATCC 33820 (GenBank accession no. AF257097) (this study), and (ii) 23-1B (5′-GGGTTCCCCCATTCGGA-3′), which corresponds to nt 123 to 113 of Lactobacillus 23S rDNA and which was developed by Tannock et al. (29). PCR was performed as follows: (i) 5 min at 94°C; (ii) 25 cycles, with 1 cycle consisting of 45 s at 94°C, 30 s at 53°C, and 1.5 min at 72°C; and (iii) a final extension step of 7 min at 72°C. The PCR products were digested with HaeIII (16S rDNA and 16-23S rDNA) or MseI (16-23S rDNA) following the manufacturer's directions (Invitrogen [Burlington, Ontario, Canada] and New England Biolabs [Pickering, Ontario, Canada]). The resulting banding patterns were analyzed on a 2% agarose gel.
To confirm the species designation of the crop isolates, the V2-V3 region of the 16S rDNA was sequenced using primers Lb16a, HDA2, Lac1, and Lac2 (without GC clamp). Sequencing and analysis were conducted as outlined above.
RESULTS
Comparison of the migrations of 16S rDNA fragments generated from members of the L. acidophilus group in DGGs.
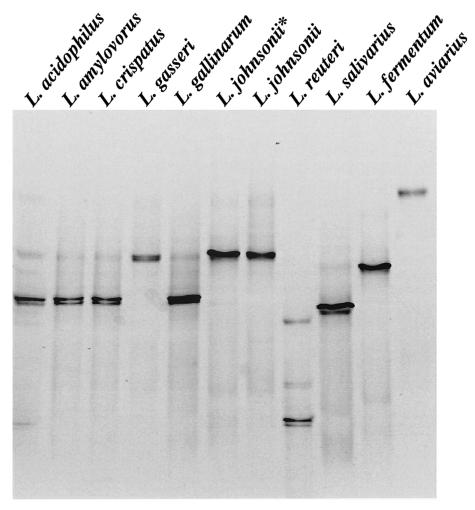
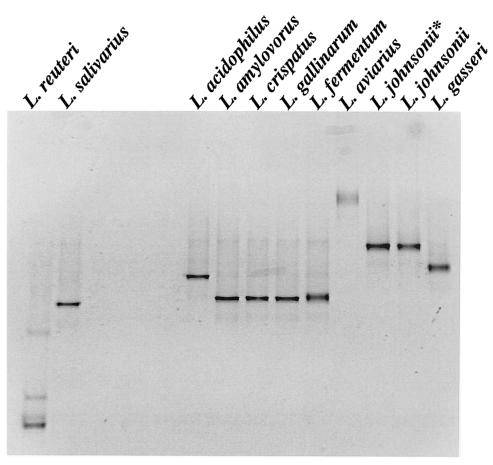
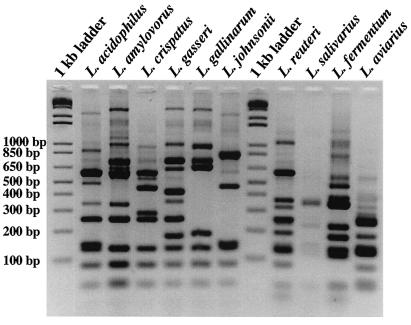
Comparison of the migrations of the HDA PCR-DGGE fragments of Lactobacillus reference strains showed that L. acidophilus, L. amylovorus, L. crispatus, and L. gallinarum fragments had almost identical migration positions (Fig. 1A) and could not be easily differentiated. HDA PCR-DGGE, however, could distinguish between DNA homology group A and B L. acidophillus strains, as well as other less closely related Lactobacillus species (Fig. 1A). Fragments generated from L. crispatus, L. gallinarum, L. amylovorus, and L. fermentum DNA in Lac PCR-DGGE migrated to the same position (Fig. 1B). Lac PCR-DGGE did, however, distinguish L. acidophilus from other group A species and could also distinguish L. johnsonii from L. gasseri. Therefore, both HDA and Lac PCR-DGGE could identify L. reuteri, L. salivarius, and L. aviarius (Fig. 1), whereas Lac PCR-DGGE could identify L. johnsonii, L. gasseri, and L. acidophilus (Fig. 1B). L. crispatus, L. gallinarum, and L. amylovorus could not be distinguished from each other.
FIG. 1.
PCR-DGGE profiles generated from Lactobacillus type strains used in this study. (A) HDA PCR-DGGE profiles on a 22 to 55% DGG. (B) Lac PCR-DGGE profiles on a 30 to 45% DGG. The species are indicated above the lanes. L. johnsonii* is the non-type strain L. johnsonii ATCC 11506 listed in Table 1.
DGGE profiles of the crop microflora.
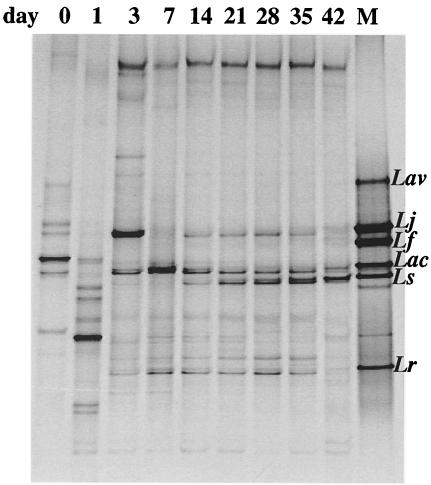
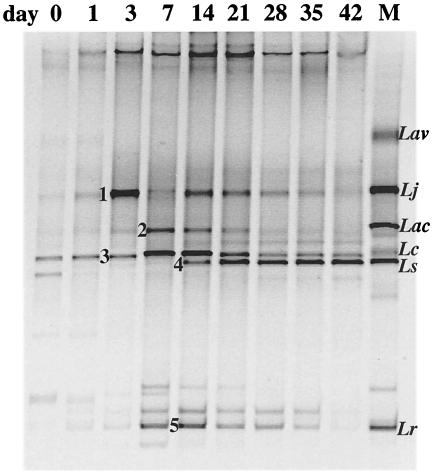
Consensus profiles generated by HDA primers from pooled DNA extracted from individual crop samples were compared using Dscs which indicated that the composition of the microflora changed markedly between days 0 and 7 (average Dsc of 27.9%) and then remained relatively stable from days 14 to 42 (average Dsc of 79.1%). The HDA PCR-DGGE profiles of the pooled samples from days 0 and 1 contained several fragments, indicating that a variety of bacterial species was present (Fig. 2). Analysis of the lactic acid bacteria (LAB) in the crop over time revealed trends similar to those obtained with HDA PCR-DGGE (Fig. 2 and 3). The Lac PCR-DGGE profiles showed that the composition of the LAB populations remained relatively stable from days 14 to 42 (average Dsc of 80%). Whereas HDA PCR-DGGE profiles from days 0 to 7 were very different from those of older birds, Lac PCR-DGGE profiles showed less disparity (average Dsc of 52.6%). The migration positions of DNA fragments in the Lac PCR-DGGE profiles matched those of the Lactobacillus identification ladder from day 3 onwards, which was also evident from the HDA PCR-DGGE (Fig. 2 and 3), but this was not the case with profiles from day 0 and day 1 chicks except for a band representing L. crispatus, L. gallinarum, and/or L. amylovorus (Fig. 3).
FIG. 2.
PCR-DGGE profiles generated from pooled crop DNA, using primer pair HDA1-GC and HDA2 (22 to 55% DGG). The sample time is indicated above the lanes. Lane M contains the identification ladder composed of PCR products from the following reference strains of Lactobacillus: L. aviarius (Lav) ATCC 43234, L. johnsonii (Lj) ATCC 33200, L. fermentum (Lf) ATCC 14931, L. acidophilus (Lac) ATCC 4356, L. salivarius (Ls) ATCC 11741, and L. reuteri (Lr) ATCC 23272.
FIG. 3.
PCR-DGGE profiles generated from pooled crop DNA, using primer pair Lac1 and Lac2-GC (30 to 45% DGG). The sample time is indicated above the lanes. Lane M contains the identification ladder composed of PCR products from the following reference strains of Lactobacillus: L. aviarius (Lav) ATCC 43234, L. johnsonii (Lj) ATCC 33200, L. acidophilus (Lac) ATCC 4356, L. crispatus (Lc) ATCC 33820, L. salivarius (Ls) ATCC 11741, and L. reuteri (Lr) ATCC 23272. Numbered fragments were extracted and sequenced as outlined in the text.
To confirm the species identification for the major (most intensely stained) fragments, DNA fragments generated by Lac PCR-DGGE were extracted, cloned, and sequenced. The sequences obtained for fragments 1, 2, 3, 4, and 5 (Fig. 3) confirmed the presence of L. johnsonii (100% identity, AJ002515), L. acidophilus (99%, M59902), L. crispatus (99.4%, AF257097) or L. gallinarum (99.4%, AJ417737), L. salivarius (99.4%, AF335475), and L. reuteri (98.7%, AF257097), respectively. Overall, L. salivarius was present from days 14 to 42 (Fig. 2 and 3); L. acidophilus was present from days 7 to 21 (Fig. 3); L. johnsonii was present from days 3 to 35 (Fig. 2 and 3); L. reuteri was present at all ages, as was one or more of the group A L. acidophilus species L. gallinarum, L. crispatus, and L. amylovorus (Fig. 2 and 3). These data were supported by examination of Lac PCR-DGGE profiles generated for individual birds from day 3 onwards: L. crispatus, L. gallinarum, and/or L. amylovorus were present in 85.7% (60 of 70) of the birds, and L. johnsonii was present in 84.3% (59 of 70) of the birds. Only 4 of 70 crops contained fragments representing L. fermentum (data not shown).
Enumeration of lactobacilli in chicken crops.
For each sampling time, the number of CFU of presumptive lactobacilli per gram of crop for each bird was determined and used to calculate the mean log10 CFU per gram and standard deviation. For day 1 birds, lactobacilli were below the detection range in five birds, and a large range in log10 CFU/gram was observed for the other five birds (5.84 ± 1.23 log10 CFU/g). From days 1 to 7, the number of lactobacilli increased 1,000-fold, reaching a maximum average population of 9.00 ± 0.41 log10 CFU/g on day 7. From day 14 onwards, the average number of lactobacilli stabilized between 8.14 and 8.51 log10 CFU/g, corresponding to the period of compositional stability revealed by the PCR-DGGE results. In the initial sampling, the numbers of Lactobacillus in the day 0 crops were below the detection limit of spread plating. A subsequent experiment was conducted using an additional 10 day 0 crops using pour plating instead of spread plating. Colonies were observed for bacteria from two crops. From the bacteria from one crop, two colonies of different morphology grew; from the bacteria from the other crop, 36 colonies with the same morphology grew. Two and four colonies, respectively, were selected for further investigation.
Identification of lactobacilli and differentiation between the L. acidophilus group isolates using ARDRA.
The Lac PCR-DGGE analysis indicated that most crops contained L. reuteri, L. salivarius, and representatives of different species of the L. acidophilus group. In order to rapidly and accurately identify the Lactobacillus isolates obtained from each bird, an ARDRA technique was developed. Initially, only the 16S rRNA gene was amplified from the type strains of the L. acidophilus group and other Lactobacillus reference strains representing different species (Table 1). The 1.5-kb PCR products were digested with various restriction enzymes. HaeIII digestion generated species-specific banding patterns for L. reuteri, L. salivarius, L. fermentum, and L. aviarius as well as group-specific banding patterns for L. acidophilus group A and B species (data not shown).
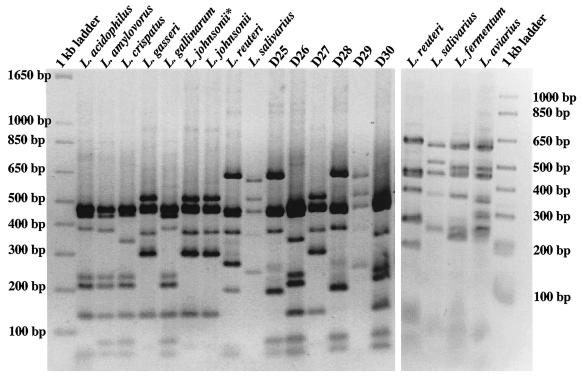
Tannock et al. (29) used the sequence of the 16S-23S rRNA intergenic region to identify various members of the L. acidophilus group to the species level, and in silico restriction analysis of the intergenic region supported inclusion of this region in order to generate species-specific patterns. The 16-23S rDNA (2 kb) was amplified with primers Lb16a and 23-1B and PCR products generated from type strains were digested with HaeIII. Identical banding patterns were observed for L. amylovorus ATCC 33620 and L. gallinarum ATCC 33199. The banding pattern for L. acidophilus ATCC 4356 was similar to that obtained for ATCC 33620 and ATCC 33199 but differed slightly in the number and size of fragments below 100 bp (Fig. 4A). The banding pattern for L. crispatus ATCC 33820 differed from that of ATCC 33620, ATCC 33199, and ATCC 4356 in that the second largest fragment was ∼350 bp instead of ∼400 bp (Fig. 4A), and this difference was used to discriminate L. crispatus from the other group A species. The HaeIII banding pattern for L. johnsonii ATCC 33200 and ATCC 11506 and L. gasseri ATCC 33323 were the same but were different from those for L. crispatus and the other group A species. Unique fragmentation patterns were also evident for L. reuteri ATCC 43272, L. salivarius subsp. salivarius ATCC 11741, L. fermentum ATCC 14931, and L. aviarius ATCC 43234 (Fig. 4A). Subsequently, the HaeIII-ARDRA of the 16-23S rDNA was used to make an initial identification of the crop isolates (Fig. 4A, lanes D25 to D30). Slight variations in the HaeIII-ARDRA patterns were noted for L. reuteri (Fig. 4A, compare banding patterns of the type strain, D25, and D28) and L. crispatus (data not shown) and were characterized by the absence and/or different intensities of bands between 250 to 300 bp for L. reuteri and between 300 bp to 400 bp for L. crispatus. The identity of the crop isolates with these L. reuteri-like and L. crispatus-like patterns was confirmed by sequencing (discussed below) (Table 2). The variability observed with these patterns is likely due to strain-specific differences in the 16-23S spacer regions (10, 20).
FIG. 4.
ARDRA profiles of type and reference strains and crop isolates. (A) HaeIII fragmentation patterns of the 16-23S rRNA gene amplified from the type and reference strains and crop isolates as indicated. L. johnsonii* is the non-type strain L. johnsonii ATCC 11506 listed in Table 1. (B) MseI fragmentation patterns of the 16-23S rRNA gene amplified from type strains of the L. acidophilus group as indicated. In panels A and B, the 1-kb ladder (Invitrogen) was used as the molecular weight marker, and the corresponding fragment sizes are indicated at the sides of the gels.
TABLE 2.
Identification of chicken crop isolates by HaeIII- and MseI-ARDRA and 16S rRNA gene sequence analysis
| No. of isolates | Characterization by:
|
Sequence analysis of the V2-V3 region of 16S rDNA in representative strain
|
||||
|---|---|---|---|---|---|---|
| HaeIII-ARDRA | MseI-ARDRAa | Strain (bp sequence obtained) | Species | % Identity | Genbank accession no. | |
| 24 | L. amylovorus | L. gallinarum | D64 (573) | L. gallinarum | 99.5 | AJ417737 |
| L. gallinarum | ||||||
| 48 | L. crispatus | NT | D139 (573) | L. crispatus ATCC 33820 | 99.5 | AF257097 |
| 7 | L. crispatus-like | NT | D68 (294) | L. crispatus ATCC 33820 | 98.9 | AF257097 |
| 25 | L. johnsonii | L. johnsonii | D33 (581) | L. johnsonii | 99.8 | M99704 |
| L. gasseri | 14-1 (388) | L. johnsonii | 99.3 | AJ002515 | ||
| 22 | L. reuteri | NT | D15 (486) | L. reuteri DSM 20016T | 99.1 | X76328 |
| Lactobacillus spp. | 98.6 | AY005048 | ||||
| 24 | L. reuteri-like | NT | D3 (464) | Lactobacillus spp. | 99.3 | AY005048 |
| L. reuteri DSM 20016T | 98.6 | X76328 | ||||
| 16 | L. salivarius | NT | D29 (611) | L. salivarius subsp. salivarius | 99.2 | AF335475 |
| 16 | P. acidilactici | NT | 11-1 (427) | P. acidilactici | 99.7 | AF515229 |
| 16-1 (519) | P. acidilactici | 99.4 | AJ305322 | |||
| 4 | E. faecium | NT | 96-1 (375) | E. faecium | 99.6 | AY172570 |
NT, not tested.
In silico restriction mapping analysis indicated that MseI may generate species-specific patterns for the L. acidophilus group. Digestion of the 16-23S rDNA PCR products from the reference strains of the L. acidophilus group produced unique restriction patterns, with considerable variation between 200 to 850 bp (Fig. 4B). The MseI-ARDRA was therefore used to identify the crop isolates that had been previously placed in the L. johnsonii-L. gasseri and L. amylovorus-L. gallinarum-L. acidophilus HaeIII-ARDRA groups. All the crop isolates from the L. acidophilus group were identified as L. johnsonii, L. crispatus, or L. gallinarum (Table 2). As shown in Fig. 4B, MseI also generated unique patterns for L. reuteri ATCC 43272, L. salivarius subsp. salivarius ATCC 11741, L. fermentum ATCC 14931, and L. aviarius ATCC 43234. Although MseI could be used to identify these strains, we chose to use HaeIII because the banding pattern was simpler.
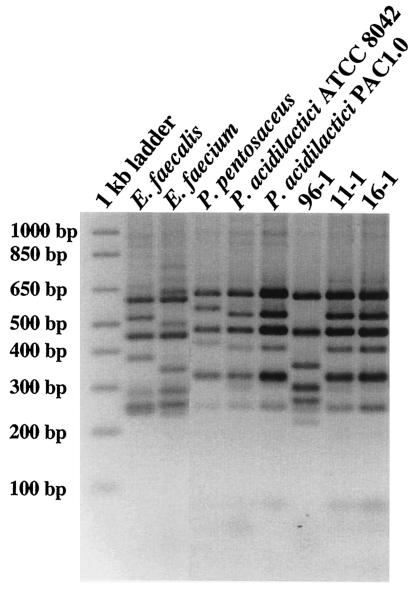
Six isolates from day 0 and 14 isolates from day 1 generated HaeIII- and MseI-ARDRA banding patterns that did not match those of the Lactobacillus reference strains. In an attempt to identify these isolates, other species of LAB were analyzed. HaeIII-ARDRA generated species-specific patterns for Enterococcus faecalis, Enterococcus faecium, Pediococcus acidilactici, and Pediococcus pentosaceus (Fig. 5). Unique patterns were also observed for Lactococcus lactis, Leuconostoc gelidum, and Carnobacterium piscicola (data not shown), suggesting this technique may be applicable for identifying species within these genera as well. Sixteen of the 20 isolates generated HaeIII banding patterns that corresponded to that of P. acidilactici (Fig. 5, strains 11-1 and 16-1); the remaining four isolates generated HaeIII banding patterns that corresponded to E. faecium (Fig. 5, strain 96-1) (Table 2).
FIG. 5.
HaeIII-ARDRA profiles of 16-23S rRNA gene amplified from type strains, other LAB reference strains, and crop isolates. The 1-kb ladder (Invitrogen) was used as the molecular weight marker, and the corresponding fragment sizes are indicated to the left of the gel.
A total of 166 Lactobacillus crop isolates were identified using the HaeIII- and MseI-ARDRA methods, and the results are summarized in Table 2. The 16S rDNA sequences that were obtained from these isolates confirmed the species designation obtained by ARDRA (Table 2). Although the homology obtained for the L. reuteri-like and L. crispatus-like representatives was lower than the representatives of the L. reuteri and L. crispatus patterns, the sequence of the V2-V3 region was highly homologous to the corresponding regions in the type strains of each species. Of the 166 Lactobacillus isolates, 55 (33%) were L. crispatus, 46 (28%) were L. reuteri, 25 (15%) were L. johnsonii, 24 (14%) were L. gallinarum, and 16 (9.5%) were L. salivarius. Twelve isolates generated HaeIII-ARDRA patterns that were different from those of the reference lactobacilli and LAB species tested and have not yet been identified.
DISCUSSION
Earlier studies found that L. salivarius, L. reuteri, and L. acidophilus (old classification) inhabited the crop and that these species were present throughout the chicken digestive tract (16, 27). To our knowledge, ours is the first study utilizing nucleic acid-based techniques to investigate the composition of the Lactobacillus population in the crop throughout the development of broilers raised under commercial production conditions. The results showed that the crop microflora varied in composition during the life of the bird with some species, such as L. acidophilus and L. salivarius, appearing in a developmental succession, while other species (i.e., L. reuteri and L. johnsonii plus one or more of the species L. crispatus, L. gallinarum, and L. amylovorus) were consistently detected. Sequence analysis of DGGE fragments demonstrated, for the first time, the presence of L. johnsonii and L. crispatus and/or L. gallinarum in the crop of broilers.
A rapid and simple ARDRA method was developed to distinguish between the members of the L. acidophilus group. MseI-ARDRA generated unique patterns for all species of the L. acidophilus group, identifying L. crispatus, L. johnsonii, and L. gallinarum among crop isolates. Ventura et al. (31) also described an ARDRA technique in which only the 16S rDNA was amplified, which was then digested with three enzymes, Sau3AI, HinfI, and DraI. Sau3AI digestion yielded group A- and group B-specific patterns and differentiated among species of lactobacilli that were less closely related, such as L. paracasei, L. salivarius, L. reuteri, and L. fermentum. Following group designations provided by Sau3AI digestion, HinfI produced a unique banding pattern for L. acidophilus and L. gallinarum, and DraI could distinguish L. crispatus and L. amylovorus species and L. johnsonii and L. gallinarum species. All three enzymes, however, were used to distinguish between the group A species, and two enzymes were required to identify the group B species. Roy et al. (23) proposed a combined group- or species-specific PCR followed by ARDRA. Group-specific PCR was used to differentiate group A from group B lactobacilli. A second PCR was used to amplify the 16S rDNA, and L. acidophilus and L. amylovorus were identified using HinfI. Using an isoschizomer of MseI, Tru9I, Roy et al. were able to distinguish between L. crispatus and L. gallinarum species and L. gasseri and L. johnsonii species. Thus, at least two PCRs and two restriction digestions were required to identify the L. acidophilus group species. The ARDRA used in our study simplified identification in that a single MseI restriction digestion of one PCR product easily distinguished both closely related and less related lactobacilli. HaeIII-ARDRA distinguished group A L. acidophilus from group B, differentiated L. crispatus from other group A L. acidophilus, and produced unique patterns for the non-Lactobacillus LAB studied.
Our study has provided detailed knowledge of the acquisition of the Lactobacillus microflora in the broiler crop. Of particular importance was the observation of the dynamics of the crop microflora during the life of the birds, demonstrating both rapid changes during days 1 to 7 and the establishment of a stable microflora after day 14. Further, since it has been proposed that the crop microflora acts as a bacterial inoculum for the remainder of the gut (7), knowledge of the composition of this bacterial collection is critical in understanding the contribution of the microflora members to the well-being of the avian host and for selection of species for probiotics. Given the crop microflora dynamics observed in our study, it is doubtful that efficacious and scientifically valid probiotics can be derived without the use of this information because it impinges on the types of bacteria that will inoculate the digesta in the crop, suppress the multiplication of contaminating bacteria, and influence the biochemistry of the broiler gut (4, 7). The baseline information generated by this study will be essential in planning husbandry methods that utilize feed supplements other than antimicrobial drugs for the efficient production of broilers.
Acknowledgments
This research was supported by the Natural Science and Engineering Research Council (NSERC) of Canada and the Canadian Foundation for Innovation (CFI). G. E. Allison is a recipient of a Tier II Canada Research Chair. K. E. Hagen is a recipient of an NSERC Postgraduate Scholarship.
We thank Todd Klaenhammer (North Carolina State University) and Lynn McMullen (University of Alberta) for providing reference cultures. We also thank Matt Rawluk and Dorthe Nielsen for assistance with sampling and plating. The assistance of the animal research technicians at the Alberta Poultry Research Centre, University of Alberta, was greatly appreciated.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Meyers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Chavagnat, F., M. Haueter, J. Jimeno, and M. G. Casey. 2002. Comparison of partial tuf gene sequences for the identification of lactobacilli. FEMS Microbiol. Lett. 217:177-183. [DOI] [PubMed] [Google Scholar]
- 3.Du Plessis, E. M., and L. M. Dicks. 1995. Evaluation of random amplified polymorphic DNA (RAPD)-PCR as a method to differentiate Lactobacillus acidophilus, Lactobacillus crispatus, Lactobacillus amylovorus, Lactobacillus gallinarum, Lactobacillus gasseri, and Lactobacillus johnsonii. Curr. Microbiol. 31:114-118. [DOI] [PubMed] [Google Scholar]
- 4.Feighner, S. D., and M. P. Dashkevicz. 1987. Subtherapeutic levels of antibiotics in poultry feeds and their effects on weight gain, feed efficiency, and bacterial cholyltaurine hydrolase activity. Appl. Environ. Microbiol. 53:331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujisawa, T., Y. Benno, T. Yaeshima, and T. Mitsuoka. 1992. Taxonomic study of the Lactobacillus acidophilus group, with recognition of Lactobacillus gallinarum sp. nov. and Lactobacillus johnsonii sp. nov. and synonymy of Lactobacillus acidophilus group A3 (Johnson et al. 1980) with the type strain of Lactobacillus amylovorus (Nakamura 1981). Int. J. Syst. Bacteriol. 42:487-491. [DOI] [PubMed] [Google Scholar]
- 6.Fuller, R. 1973. Ecological studies on the Lactobacillus flora associated with the crop epithelium of the fowl. J. Appl. Bacteriol. 36:131-139. [Google Scholar]
- 7.Fuller, R., and B. E. Brooker. 1974. Lactobacilli which attach to the crop epithelium of the fowl. Am. J. Clin. Nutr. 27:1305-1312. [DOI] [PubMed] [Google Scholar]
- 8.Gancheva, A., B. Pot, K. Vanhonacker, B. Hoste, and K. Kersters. 1999. A polyphasic approach towards the identification of strains belonging to Lactobacillus acidophilus and related species. Syst. Appl. Microbiol. 22:573-585. [DOI] [PubMed] [Google Scholar]
- 9.Gordon, H. A., and L. Pesti. 1971. The gnotobiotic animal as a tool in the study of host-microbial relationships. Bacteriol. Rev. 35:390-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen, M. A., J. A. Webster, and N. Straus. 1993. Rapid identification of bacteria on the basis of polymerase chain reaction-amplified ribosomal DNA spacer polymorphisms. Appl. Environ. Microbiol. 59:945-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson, J. L., C. F. Phelps, C. S. Cummins, J. London, and F. Gasser. 1980. Taxonomy of the Lactobacillus acidophilus group. Int. J. Syst. Bacteriol. 30:53-68. [Google Scholar]
- 12.Klein, G., A. Pack, C. Bonaparte, and G. Reuter. 1998. Taxonomy and physiology of probiotic lactic acid bacteria. Int. J. Food Microbiol. 41:103-125. [DOI] [PubMed] [Google Scholar]
- 13.Knarreborg, A., M. A. Simon, R. M. Engberg, B. B. Jensen, and G. W. Tannock. 2002. Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl. Environ. Microbiol. 68:5918-5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kullen, M. J., R. B. Sanozky-Dawes, D. C. Crowell, and T. R. Klaenhammer. 2000. Use of the DNA sequence of variable regions of the 16S rRNA gene for rapid and accurate identification of bacteria in the Lactobacillus acidophilus complex. J. Appl. Microbiol. 89:511-516. [DOI] [PubMed] [Google Scholar]
- 15.Lauer, E., C. Helming, and O. Kandler. 1980. Heterogeneity of the species Lactobacillus acidophilus (Moro) Hansen and Moquot as revealed by biochemical characteristics and DNA-DNA hybridization. Zentbl. Bakteriol. Mikrobiol. Hyg. 1 Abt. Orig. C 1:150-168. [Google Scholar]
- 16.Mead, G. C. 1997. Bacteria in the gastrointestinal tract of birds, p. 216-240. In R. I. Mackie, B. A. White, and R. E. Isaacson (ed.), Gastrointestinal microbiology, vol. 2. Gastrointestinal microbes and host interactions. Chapman and Hall, New York, N.Y.
- 17.Miteva, V., I. Boudakov, G. Ivanova-Stoyancheva, B. Marinova, V. Mitev, and J. Mengaud. 2001. Differentiation of Lactobacillus delbrueckii subspecies by ribotyping and amplified ribosomal DNA restriction analysis (ARDRA). J. Appl. Microbiol. 90:909-918. [DOI] [PubMed] [Google Scholar]
- 18.Morishita, Y., T. Mitsuoka, C. Kaneuchi, S. Yamamoto, and M. Ogata. 1971. Specific establishment of lactobacilli in the digestive tract of germ-free chickens. Jpn. J. Microbiol. 15:531-538. [DOI] [PubMed] [Google Scholar]
- 19.National Research Council. 1994. Nutrient requirements of poultry, 9th ed. National Academy Press, Washington, D.C.
- 20.Nour, M. 1998. 16S-23S and 23S-5S intergenic spacer regions of lactobacilli: nucleotide sequence, secondary structure and comparative analysis. Res. Microbiol. 149:433-448. [DOI] [PubMed] [Google Scholar]
- 21.Pot, B., C. Hertel, W. Ludwig, P. Descheemaeker, K. Kersters, and K. H. Schleifer. 1993. Identification and classification of Lactobacillus acidophilus, L. gasseri and L. johnsonii strains by SDS-PAGE and rRNA-targeted oligonucleotide probe hybridization. J. Gen. Microbiol. 139:513-517. [DOI] [PubMed] [Google Scholar]
- 22.Rodtong, S., and G. W. Tannock. 1993. Differentiation of Lactobacillus strains by ribotyping. Appl. Environ. Microbiol. 59:3480-3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy, D., S. Sirois, and D. Vincent. 2001. Molecular discrimination of lactobacilli used as starter and probiotic cultures by amplified ribosomal DNA restriction analysis. Curr. Microbiol. 42:282-289. [DOI] [PubMed] [Google Scholar]
- 24.Roy, D., P. Ward, D. Vincent, and F. Mondou. 2000. Molecular identification of potentially probiotic lactobacilli. Curr. Microbiol. 40:40-46. [DOI] [PubMed] [Google Scholar]
- 25.Ryu, C. S., J. W. Czajka, M. Sakamoto, and Y. Benno. 2001. Characterization of the Lactobacillus casei group and the Lactobacillus acidophilus group by automated ribotyping. Microbiol. Immunol. 45:271-275. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, New York, N.Y.
- 27.Sarra, P. G., L. Morelli, and V. Bottazzi. 1992. The lactic microflora of fowl, p. 3-19. In B. J. B. Wood (ed.), The lactic acid bacteria in health and disease. Elsevier Applied Science, London, United Kingdom.
- 28.Savage, D. C. 1977. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 31:107-133. [DOI] [PubMed] [Google Scholar]
- 29.Tannock, G. W., A. Tilsala-Timisjarvi, S. Rodtong, J. Ng, K. Munro, and T. Alatossava. 1999. Identification of Lactobacillus isolates from the gastrointestinal tract, silage, and yoghurt by 16S-23S rRNA gene intergenic spacer region sequence comparisons. Appl. Environ. Microbiol. 65:4264-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarnberg, M., T. Jakobsson, J. Jonasson, and U. Forsum. 2002. Identification of randomly selected colonies of lactobacilli from normal vaginal fluid by pyrosequencing of the 16S rDNA variable V1 and V3 regions. APMIS 110:802-810. [DOI] [PubMed] [Google Scholar]
- 31.Ventura, M., I. A. Casas, L. Morelli, and M. L. Callegari. 2000. Rapid amplified ribosomal DNA restriction analysis (ARDRA) identification of Lactobacillus spp. isolated from fecal and vaginal samples. Syst. Appl. Microbiol. 23:504-509. [DOI] [PubMed] [Google Scholar]
- 32.Ventura, M., and R. Zink. 2002. Specific identification and molecular typing analysis of Lactobacillus johnsonii by using PCR-based methods and pulsed-field gel electrophoresis. FEMS Microbiol. Lett. 217:141-154. [DOI] [PubMed] [Google Scholar]
- 33.Walter, J., C. Hertel, G. W. Tannock, C. M. Lis, K. Munro, and W. P. Hammes. 2001. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67:2578-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter, J., G. W. Tannock, A. Tilsala-Timisjarvi, S. Rodtong, D. M. Loach, K. Munro, and T. Alatossava. 2000. Detection and identification of gastrointestinal Lactobacillus species by using denaturing gradient gel electrophoresis and species-specific PCR primers. Appl. Environ. Microbiol. 66:297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu, X. Y., T. Zhong, Y. Pandya, and R. D. Joerger. 2002. 16S rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl. Environ. Microbiol. 68:124-137. [DOI] [PMC free article] [PubMed] [Google Scholar]