Abstract
Background
DISC1 is considered a susceptibility gene for schizophrenia and schizoaffective disorder, but little is known regarding the potential mechanisms through which it may confer increased risk. Given that DISC1 plays a role in cerebral cortex development, polymorphisms in this gene may have relevance for neurobiological models of schizophrenia that have implicated cortical deficits in its pathophysiology.
Methods
We investigated whether the DISC1 leu607phe polymorphism was associated with prefrontal gray matter volumes using magnetic resonance imaging in a cohort of patients with schizophrenia (N=19) and healthy volunteers (N=25) and positive and negative symptoms in 200 patients with schizophrenia.
Results
Among patients and healthy volunteers, phe carriers (N=11) had significantly less gray matter in the superior frontal gyrus and anterior cingulate gyrus compared to leu/leu homozygotes (N=33). Further, among patients left superior frontal gyrus gray matter volume was significantly negatively correlated with severity of hallucinations. In addition, patients who were phe carriers (N=144) had significantly greater severity of positive symptoms (hallucinations) compared to patients who were leu/leu homozygotes (N=56).
Discussion
These findings implicate DISC1 in variation of prefrontal cortical volume and positive symptoms, thus providing a potential mechanism through which DISC1 may confer increased risk for schizophrenia or schizoaffective disorder.
Keywords: DISC1, leu607phe, MRI, frontal lobes, schizophrenia, gene, polymorphism
Introduction
There is now considerable evidence that the Disrupted in Schizophrenia 1 (DISC1) gene plays a role in schizophrenia (Porteous et al 2006). DISC1 is located at the breakpoint of a chromosomal translocation in the 1q42 region resulting in a balanced translocation that cosegregated with schizophrenia and other affective disorders (St Clair et al 1990). Subsequent studies indicated that DISC1 and Disrupted in Schizophrenia II (DISCII) were disrupted by the translocation (Millar et al 2000; Millar et al 2001; Blackwood et al 2001). Association studies provided further support for the hypothesis that DISC1 is a susceptibility gene for schizophrenia and schizoaffective disorder (e.g., Hennah et al 2003; Cannon et al. 2005). Moreover, in one of the strongest association studies to date our group reported that a missense mutation at a single nucleotide polymorphism (SNP) resulting in a phenylalanine substitution for leucine at position 607 (leu607phe) was overrepresented in patients compared to healthy individuals (Hodgkinson et al 2004).
Little is known regarding the mechanisms through which DISC1 may confer increased risk for schizophrenia. Although the functions of DISC1 have not been fully elaborated, it may be directly relevant to basic neurodevelopmental mechanisms in the central nervous system that have relevance to the pathogenesis of schizophrenia. For example, DISC1 has been implicated in brain development (Schurov et al 2004; Kamiya et al 2005), which fits well with neurodevelopmental models of schizophrenia (Bilder and Degreef, 1991; Szeszko et al 2003). Particularly relevant are data from Kamiya and colleagues (2005) who reported that suppression of DISC1 may impair migration and subsequent development of the cerebral cortex.
The identification of endophenotypes in genetics studies may help elucidate the relationship between risk genes such as DISC1 and phenomenology (Blackwood and Muir, 2004; Szeszko et al 2005). Surprisingly, however, few studies have investigated relationships among genetic variation in DISC1, gray matter structure in-vivo and symptom severity in schizophrenia. Some work suggests that genetic variation in DISC1 may be associated with delusions and hallucinations (Hennah et al 2003; DeRosse et al 2007) and that carriers of a 3 locus haplotype, which includes the leu607phe polymorphism, have prefrontal gray matter deficits compared to noncarriers (Cannon et al 2005). Better understanding how genetic variation in DISC1 is associated with cortical measures and symptoms in schizophrenia as well as the potential relationship between gray matter structure and symptoms could provide important clues to the neurobiology of schizophrenia.
We tested the hypothesis that the DISC1 leu607phe polymorphism would be associated with prefrontal gray matter volume and symptoms in patients. We focused on prefrontal regions given that twin studies of schizophrenia suggest that the genetic underpinnings of brain structural deficits may lie in these regions (Cannon et al 2002). Moreover, both positive (Lennox et al 2000; Erkwoh et al 1997) and negative symptoms (Sanfilipo et al 2000) have been linked with prefrontal cortical abnormalities in schizophrenia. Based on prior work (Hennah et al 2003; Cannon et al 2005; Hodgkinson et al 2004) we hypothesized that phe carriers would have less prefrontal cortical gray matter and greater severity of symptoms compared to leu/leu homozygotes. We focused on the DISC1 leu607phe SNP because it: (a) was most strongly associated with disease in our prior study (Hodgkinson et al 2004); and (b) was part of a 3 locus haplotype within DISC1 that was overtransmitted to patients (Hennah et al 2003; Cannon et al 2005), which was associated with lower prefrontal gray matter density (Cannon et al 2005).
Method
Subjects
Twenty-five (11 males, 14 females; mean age = 27.1, SD = 6.8) healthy volunteers with no history of Axis I psychiatric illness as determined from the SCID-NP (Spitzer and Williams, 1988) and 19 (14 males, 5 females; mean age = 26.3, SD = 5.9) patients participated in this study. All patients were derived from a larger cohort of patients participating in clinical treatment trials of antipsychotic medications at Zucker Hillside Hospital who had both magnetic resonance (MR) imaging and genotype data available for analysis. Genotype distributions were as follows (patient, healthy volunteer): phe/phe (2,0), leu/phe (3, 6) and leu/leu (14,19). Diagnoses of patients were based on the Structured Clinical Interview for Axis I DSM-IV Disorders (First et al 1994) and included schizophrenia (n=17), schizoaffective (N=1) or schizophreniform (N=1) disorder. All patients with MR imaging data were experiencing a first-episode of illness with a median of 0 weeks exposure to antipsychotic treatment (range = 0 to 23 weeks). Eleven patients were antipsychotic drug-naïve at the time of the MR imaging exam. Mean age at onset of illness was 22.7 (SD = 5.1) years.
Classification of handedness for individuals with MR imaging data was based on a modified version of the Edinburgh Inventory consisting of twenty items (Oldfield, 1971) using the following formula: (total right – total left) / (total right + total left). Subjects with a laterality quotient greater than .70 were classified as dextral and the rest were classified as nondextral (Schachter et al 1987). Mean (SD) laterality quotient for patients was .76 (SD = .58) and for controls was .72 (SD = .53). Handedness for 1 patient and 2 healthy volunteers was based on preference for handwriting alone.
Two hundred patients with a diagnosis of schizophrenia (N=156) or schizoaffective disorder (N=44) based on the Structured Clinical Interview for Axis I DSM-IV Disorders (First et al 1994) had genotype data available and complete item information available from the psychosis module for analysis. Among the 200 patients 11 were phe/phe homozygotes, 45 were leu/phe heterozygotes and 144 were leu/leu homozygotes.
Exclusion criteria for patients and healthy volunteers included any known genetic, neurologic or seizure disorder or meeting DSM-IV criteria for mental retardation. Additional exclusion criteria for healthy volunteers included an Axis I diagnosis. The sample was limited to Caucasians to control for potential population effects. All procedures were approved by the North Shore - Long Island Jewish Health System IRB and written informed consent was obtained from all participants.
Symptom Measures
We assessed positive and negative symptoms using the psychosis module of the Structured Clinical Interview for Axis I DSM-IV Disorders (First et al 1994). A negative symptom score was formed by averaging the avolition, alogia and affective flattening scores. The positive symptom score was formed by averaging the delusion and hallucination items. The delusion score was computed as the average of referential delusions, paranoid delusions, grandiose delusions, somatic delusions, other delusions, delusions of being controlled, thought broadcasting, and bizarre delusions. The hallucination score was computed as the average of auditory hallucinations, visual hallucinations, tactile hallucinations and other hallucinations.
Magnetic Resonance (MR) Imaging Methods
MR imaging exams were conducted at the Long Island Jewish Medical Center in the coronal plane using a 3D Fast SPGR with IR Prep (TR = 12.7 or 14.7, TE=4.5 or 5.5ms, FOV=22cm) on a 1.5 Tesla whole body superconducting imaging system (General Electric, Milwaukee, WI). This sequence produced 124 contiguous images (slice thickness = 1.5 mm) through the whole head with nominal in-plane resolution of .86mm × .86mm in a 256 × 256 matrix. All measurements were completed using the MEDx software program (Medx, 1998). MR images were aligned along the anterior and posterior commissures for standardization across subjects and flipped randomly in the right-left axis. Scans were mixed together randomly and no identifying information was available to the operator from the scan. All measurements were thus completed by an operator who was blind to group membership, genotype status and hemisphere. All scans were reviewed by a member of the research team during acquisition and any scan with significant artifacts was repeated.
Total intracranial contents
Measurement of total intracranial contents was completed in MEDx by computing the volume of the total cerebrum, cerebrospinal fluid, cerebellum and brainstem. Inter-rater reliability between two raters as assessed by intra-class correlations [ICCs]) in 9 cases was .99.
Frontal Lobe Subregions
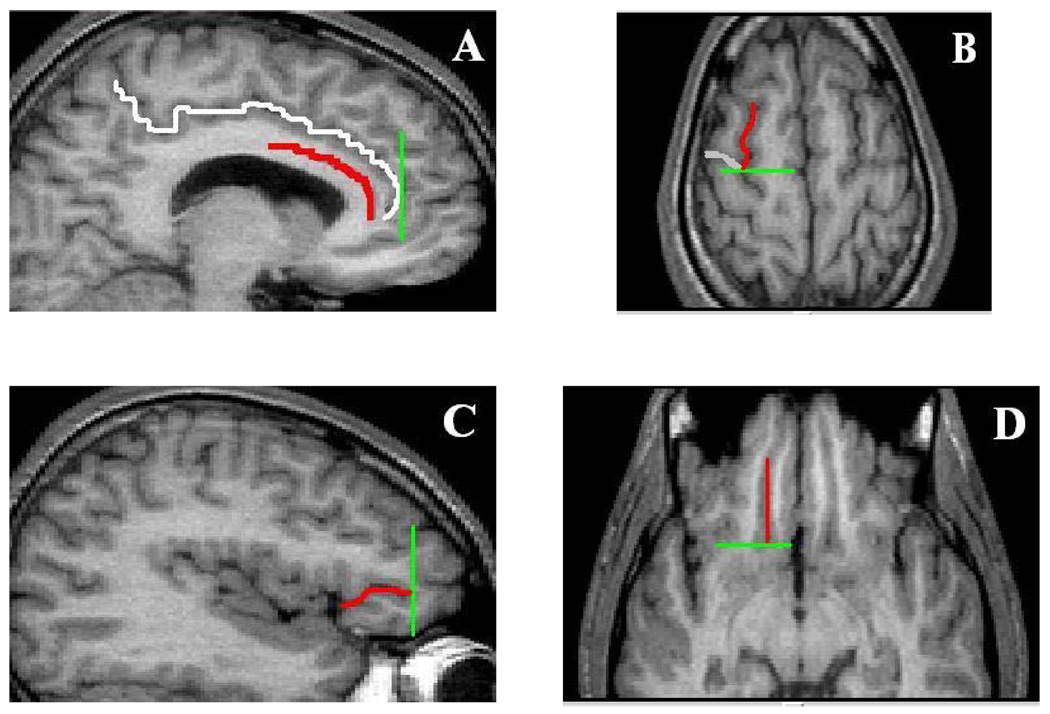
The frontal lobe subregions were measured in the coronal plane using methods described previously (Szeszko et al 1999; Szeszko et al 2004) that were adapted from Rademacher et al (1992). An illustration of these regions at the level of the genu of the corpus callosum is provided in Figure 1 and the limiting boundaries for these regions are illustrated in Figure 2. This method utilizes the cerebral sulci in combination with a set of coronal planes that "close" the borders of selected regions of interest. Although the most anterior portion of these frontal regions may be excluded from measurement, the major strength of an approach based on the sulcal anatomy is to allow consistency of measurement of cytoarchitectonic regions across subjects given the fundamental problem associated with several prior schemes based on the use of invariant landmarks. The boundaries of the superior frontal gyrus were (anterior, posterior, lateral, medial): tip of the cingulate sulcus, connection of the superior and precentral sulci, superior frontal sulcus and cingulate sulcus. The boundaries of the anterior cingulate gyrus were (anterior, posterior, ventral, dorsal): tip of the cingulate sulcus, connection of the superior and precentral sulci, callosal sulcus and cingulate sulcus. Based on our empirical (Szeszko et al 1999; Szeszko et al 2000) and theoretical (Bilder and Szeszko, 1996; Bilder and Degreef, 1991; Christensen and Bilder, 2000) work the superior frontal gyrus and anterior cingulate gyrus volumes were summed together to form a single measure of “dorsal” brain volume to minimize potential Type I error in the analysis of the brain structure volumes. The boundaries of the orbital frontal region, which served as the “ventral” brain volume were (anterior, posterior, lateral and medial): last appearance of the anterior horizontal ramus, last appearance of the olfactory sulcus, anterior horizontal ramus/circular sulcus of insula and the olfactory sulcus. Because one of the limiting sulci required for measurement of the orbital frontal region (i.e., the anterior horizontal ramus) was not present in every hemisphere (Szeszko et al 1999; Ono et al 1990), orbital frontal volumes could not be computed in the right hemisphere for 2 patients and 5 healthy volunteers and in the left hemisphere for 1 patient and 4 healthy volunteers.
Figure 1.
Illustration of the Frontal Lobe Regions-of-Interest
Notes. Superior frontal gyrus = yellow; Anterior cingulate gyrus = blue; Orbital frontal lobe = Green. Outlined regions were automatically segmented into gray and white matter using a thresholding method generated from gray-level histograms (see text and Otsu, 1979 for details)
Figure 2.
Illustration of the Limiting Boundaries for the Frontal Lobe Regions
Notes. Panel A. Cingulate sulcus (white), callosal sulcus (red) and tip of the cingulate sulcus (green); Panel B. Superior frontal sulcus (SFS; red), precentral sulcus (PRC; white) and connection of the SFS and PRC (green); Panel C. anterior horizontal ramus (ahr; red) and anterior tip of the ahr (green); Panel D. Olfactory sulcus (OLS, red) and posterior tip of the OLS (green)
All regions were outlined manually in the coronal plane on a slice by slice basis and included both gray and white matter (see Figure 1). After outlining the frontal region of interest an operator segmented it into gray and white matter using a thresholding method generated from gray-level histograms (Otsu, 1979) as described previously (Szeszko et al 2004; Lim et al 1992). Given our apriori hypothesis only gray matter volumes were included in analyses. Intra-class correlations between 2 or 3 operators (number of cases ranged from 8–10) for the prefrontal gray matter volumes were (right hemisphere, left hemisphere): anterior cingulate gyrus gray matter (.90, .94), superior frontal gyrus gray matter (.92, .97), orbital frontal lobe gray matter (.92, .99).
Genotyping
Genotyping methods have been described in detail previously (Hodgkinson et al 2004). Briefly, DISC1 leu607phe genotypes were determined using a 5’ – exonuclease allelic discrimination (Taqman) assay using Reference SNP ID: rs6675281 on an Applied Biosystems 7900 Analyzer (Foster City, CA). Genotyping accuracy as determined by regenotyping one in six samples, randomly selected, produced an overall accuracy rate > 99%.
Statistical Procedures
All statistical tests were conducted using SAS version 9.1 (SAS, 2002–2003). The mixed models approach for repeated measures analysis of covariance was used to investigate the relationship between leu607phe genotype and the frontal gray matter volumes in patients and healthy volunteers. When appropriate the 95% confidence intervals are presented for the difference in group means (lower to upper bound). To control Type-I error we first investigated all higher order interactions in the original model and retained only those that remained statistically significant in the final model, along with all main effects. In addition, to increase statistical power in the analyses we combined the small number of phe/phe homozygotes with the leu/phe carriers to form a single group (phe carriers). The statistical model thus included group (patient versus healthy volunteer) and genotype status (phe carriers versus leu/leu homozygotes) as between subjects factors and gray matter region (dorsal versus ventral) and hemisphere (right verus left) as within subjects factors. Statistical covariates included age, sex and total intracranial contents. Age was included as a covariate because it correlated with the brain structure volumes. Intracranial volume was included as a covariate to control for nonspecific differences in brain size among individuals. Sex was included as a covariate due to the imbalance in sex distribution between the groups.
Repeated measures analysis of variance was used to investigate the relationship between leu607phe genotype and positive and negative symptoms in patients. Given the small number of phe/phe homozygotes and the fact that they did not differ significantly from leu/phe heterozygotes in symptoms we combined these two groups into a single group (phe carriers) for analysis. Thus, of the 200 patients 56 were classified as phe carriers and 144 were classified as leu/leu homozygotes. Sample characteristics for the two genotype groups are illustrated in Table 1. In each analysis genotype group (phe carriers versus leu/leu homozygotes) was the between subjects factor. In the negative symptom analysis the within subjects factor was negative symptom score (i.e., avolition, alogia and affective flattening). In the positive symptom analysis the within subjects factor was positive symptom score (i.e., delusions and hallucinations).
Table 1.
Sample Characteristics for Symptom Data Analysis
| Phe Carriers N=56 | leu/leu Homozygotes N=144 | Test Statistic | df | p value | |
|---|---|---|---|---|---|
| Sex (M, F) | 41, 15 | 99, 45 | χ2 = .38 | 1 | 0.54 |
| Age (years) | 40.7(9.3) | 38.7(10.9) | t = 1.3 | 198 | 0.21 |
| Age at onset of psychotic symptoms (years) 1 | 21.4(5.3) | 21.1(6.2) | t = .33 | 190 | 0.74 |
| Global assessment of functioning 1 | 38.3(15.6) | 38.4(15.2) | t = −.03 | 191 | 0.99 |
| Hallucination Severity | 1.8(0.5) | 1.6(0.4) | t = 2.6 | 198 | 0.01 |
| Delusion Severity | 2.1(0.5) | 2.0(0.5) | t = 1.3 | 198 | 0.20 |
| Avolition | 1.8(0.9) | 1.8(0.9) | t = .03 | 198 | 0.98 |
| Alogia | 1.5(0.8) | 1.6(0.9) | t = −1.3 | 198 | 0.19 |
| Affective Flattening | 1.7(0.8) | 1.8(0.8) | t = −.80 | 198 | 0.43 |
Notes: Data are presented as mean ± SD in parentheses, unless otherwise indicated.
There were missing data for the following variables: age at onset of psychotic symptoms (3 risk carriers, 5 non-risk carriers), and global assessment of functioning (1 risk carrier, 6 non-risk carriers).
Group differences for continuous demographic variables were examined using independent groups t tests. Chi-square tests were used to examine differences in categorical variables. Pearson Product Moment correlations were used for investigating brain structure in relationship to symptoms. Alpha was set to .05 for all analyses. Genotype frequencies were investigated using chi-square analysis to test for Hardy-Weinberg equilibrium.
Results
Magnetic Resonance (MR) Imaging Data
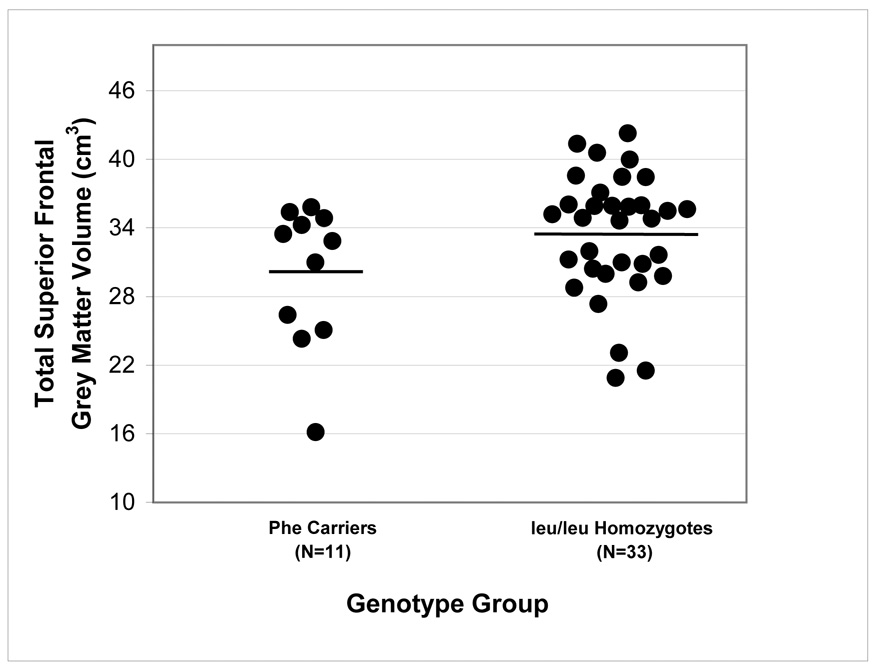
Mixed models repeated measures analysis of covariance did not reveal any significant main effects of group, hemisphere, or sex. There was, however, a significant genotype-by-brain region interaction for the prefrontal cortical volumes (F = 4.48, df = 1,38, p = .04), which remained significant after removal of nondextral subjects from the analysis (F = 7.01, df = 1,31, p = .01). Overall, carriers of the phe allele (N=11) had less gray matter in the dorsal brain region compared to leu/leu homozygotes (N=33) (t = −2.24, df = 1,38, p = .03; 95% CI = −261 to −3609). The genotype-by-brain region-by-hemisphere interaction was not statistically significant indicating that the observed findings were not specific to either the right or left hemisphere. Followup analyses revealed that this effect was statistically significant for both the superior frontal gyrus and anterior cingulate gyrus. Specifically, carriers of the phe allele had less gray matter in the superior frontal gyrus (t = −2.19, df = 1,38 p = .035; 95% CI = −6,700 to −370) and anterior cingulate gyrus (t = −2.34, df = 1,38 p = .025; 95% CI = −2,213 to −196) compared to leu/leu homozygotes. Individual brain volumes are illustrated by genotype in Figure 3 for the anterior cingulate gyrus and Figure 4 for the superior frontal gyrus. There were no significant differences between the two genotype groups in orbital frontal volumes. There was a significant region-by-hemisphere interaction (F = 6.54, df = 1,35, p = .02) such that overall, subjects had more gray matter in the left compared to the right orbital frontal cortex (t = 2.81, df=1,35, p=.008; 95% CI = 178 to 1,002). Allelic distribution for the genotype groups in the MRI analysis did not differ significantly from Hardy-Weinberg equilibrium.
Figure 3.
Total Anterior Cingulate Gray Matter Volume by leu607phe Genotype
Notes: Horizontal lines represent mean values.
Figure 4.
Total Superior Frontal Gray Matter Volume by leu607phe Genotype
Notes: Horizontal lines represent mean values.
Given the main effect of DISC1 leu607phe genotype we examined possible differences in sample characteristics between phe carriers and leu/leu homozygotes that may have influenced the observed findings. There were no significant differences between the two genotype groups in distributions of sex, age, education, handedness, clinical diagnosis of schizophrenia or total intracranial contents (see Table 2). Nevertheless, we investigated the genetic hypothesis separately in patients and healthy volunteers in a post-hoc analysis. Genotype accounted for a larger percentage of the variance in superior frontal gyrus volume among patients (F=7.59, df=1,14, p=.015, 95% CI = −11,142 to −1,388; effect size=.35) compared to healthy volunteers (F = 3.50, df = 1,20, p = .076, 95% CI = −7288 to 2110; effect size = .149). Similarly, genotype accounted for a larger percentage of the variance in anterior cingulate gyrus volume among patients (F=5.74, df=1,14, p=.031, 95% CI = −3,082 to −170; effect size = .29) compared to healthy volunteers (F=1.32, df = 1,20, p = .26, 95% CI = −2891 to 157; effect size = .062).
Table 2.
Sample Characteristics for Subjects with Brain Volume Data by Genotype
| Phe Carriers (N=11) | Leu/leu Homozygotes (N=33) | Statistic | df | p | |
|---|---|---|---|---|---|
| Sample Characteristics | |||||
| Group (Patients, Healthy Volunteers) | 5/6 | 14/19 | χ2 = .03 | 1 | .86 |
| Sex (Male, Female) | 8/3 | 17/16 | χ2 = 1.51 | 1 | .22 |
| Handedness (Right, Left) | 9/2 | 29/4 | χ2 = .26 | 1 | .61 |
| Age (years) | 26.0 (6.8) | 27.0 (6.3) | t = −.48 | 42 | .64 |
| Education (years) | 14.9 (2.4) | 14.4 (1.9) | t = .76 | 42 | .45 |
| Brain Volume Data (cm3) | |||||
| Superior Frontal Gray Matter | 29.96 (6.23) | 33.49 (5.35) | |||
| Anterior Cingulate Gray Matter | 7.41 (1.10) | 8.57 (1.54) | |||
| Total Orbital Frontal Gray Matter | 10.97 (2.80) | 10.86 (2.34) | |||
| Total Intracranial Volume | 1351 (87) | 1371 (163) |
Notes. Data are presented as mean ± SD in parentheses, unless otherwise indicated.
Positive and Negative Symptoms
Comparison of sample characteristics for the two genotype groups in the symptom data analysis (56 phe carriers versus 144 leu/leu homozygotes) did not reveal any significant differences in distributions of age, sex, age at first psychotic symptoms and global assessment of functioning (see Table 1). Allelic distribution for the genotype groups in the symptom data analysis did not differ significantly from Hardy-Weinberg equilibrium. ANOVA revealed a significant main effect of genotype for the positive symptom scores (F = 5.98, df = 1, 198, p = .015; 95% CI = .03 to .25). This effect was driven primarily by greater severity of hallucinations among patients who were phe carriers compared to patients who were leu/leu homozygotes (t = 2.56, df = 1, 198, p = .011; 95% CI = .04 to .32). The leu607phe polymorphism accounted for approximately 3% of the variance in hallucinations among patients. Neither the main effect of genotype for the negative symptom items nor genotype-by-negative symptom item interaction were statistically significant.
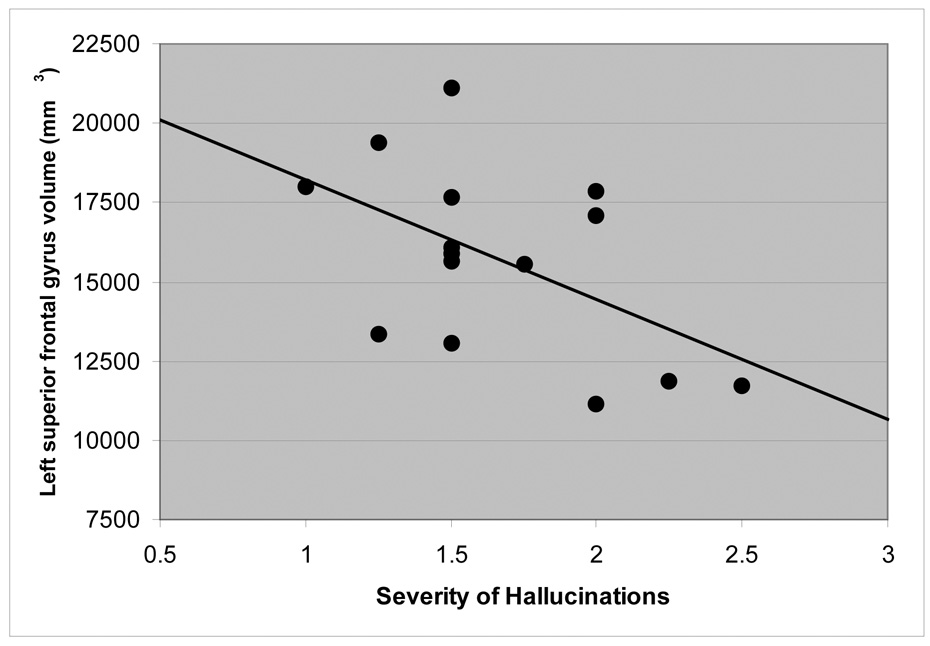
We examined the relationship between superior frontal and anterior cingulate gyrus gray matter volumes and severity of hallucinations among the subset of patients (N=15) who had both MR imaging and symptom data available. These analyses revealed that less gray matter in the left superior frontal gyrus correlated significantly with increased severity of hallucinations (r = −.53, df = 15, p =.045; Figure 5). Neither right/left anterior cingulate gyrus gray matter nor right superior frontal gray matter volumes correlated significantly with severity of hallucinations.
Figure 5.
Scatterplot of Left Superior Frontal Gyrus Volume (mm3) and Severity of Hallucinations.
Discussion
Our data provide evidence for an association between a DISC1 polymorphism, leu607phe, and prefrontal cortical gray matter volume and positive symptoms. Specifically, carriers of the phe allele (N=11) had less cortical gray matter volume in the superior frontal gyrus and anterior cingulate gyrus compared to leu/leu homozygotes (N=33). Furthermore, in the larger cohort of patients with schizophrenia or schizoaffective disorder (N=200), carriers of the phe allele (N=56) had greater severity of hallucinations compared to patients who were leu/leu homozygotes (N=144). Finally, we also observed a significant negative correlation between superior frontal gray matter volume and severity of hallucinations among a subgroup of 15 patients. Taken together, these results provide a potential mechanism through which DISC1 may confer increased risk for schizophrenia or schizoaffective disorder.
Despite a purported role in cortical development there are surprisingly little human data documenting an association between DISC1 genetic variation and brain structure or function. In one study, Cannon and colleagues (Cannon et al 2005) reported that a 3 locus haplotype, which included the leu607phe polymorphism, was associated with lower prefrontal gray matter density among patients with schizophrenia. Support for an association between variation in DISC1 genotype and neurocognition was reported in two recent studies. Burdick and colleagues (Burdick et al 2005) indicated that DISC1 genotype was related to neurocognitive performance on measures of rapid visual search and verbal working memory in a cohort of 250 patients with schizophrenia. Also, Hennah et al (2005) reported that one haplotype of DISC1, HEP3, was linked to short-term visual memory and attention performance in affected and unaffected offspring of patients with schizophrenia. It may therefore be noteworthy that prior associations between DISC1 genetic variation and “working memory” performance (Burdick et al 2005; Hennah et al 2005) are believed to be mediated, at least in part, by gray matter in regions found in the present study to be linked to DISC1 genetic variation in patients with schizophrenia (Rasser et al 2005) and healthy volunteers (du Boisgueheneuc et al 2006).
Although the functions of DISC1 are not fully known, there is evidence that the DISC1 protein regulates several basic developmental mechanisms in the central nervous system. The involvement of DISC1 in neurite outgrowth was initially demonstrated by Ozeki et al (2003) with subsequent work indicating that the DISC1 protein participates in neurite outgrowth through interactions with FEZ1 (Miyoshi et al 2003). Studies by Morris et al (2003), Ozeki et al (2003), and Brandon et al (2004) supported this work by demonstrating a protein interaction of DISC1 with NUDEL. DISC1 has also been implicated in mitochondrial function and cAMP-signaling (Millar et al 2005), which are also relevant to the pathophysiology of schizophrenia. Moreover, both animal (Schurov et al 2004) and in-vivo (Kamiya et al 2005) studies support a role for DISC1 in brain development, which has direct relevance for neurodevelopmental models of schizophrenia that posit cortical deficits in the pathophysiology of the disorder (Bilder and Degreef, 1991; Szeszko et al 2003). Particularly noteworthy in this regard are data from Kamiya et al (2005) who reported that suppression of DISC1 may impair migration and subsequent development of the cerebral cortex through its interactions with microtubule-associated dynein motor complex.
Our study provides evidence for an association between DISC1 and gray matter volume in the superior frontal and anterior cingulate gyri, which have particular relevance for the neurobiology of schizophrenia. Structural alterations both in the anterior cingulate (Narr et al 2005) and superior frontal (Jayakumar et al 2005) gyri have been reported in patients with schizophrenia. Also, individuals at high-risk for developing schizophrenia who later became ill demonstrated abnormal anterior cingulate activity during an fMRI sentence completion task (Whalley et al 2006). We previously suggested that the “medial frontolimbic system,” in which the frontal lobes and limbic system are linked by the cingulate bundle is isomorphic with the medial/dorsal “archicortical” system (Sanides, 1969) and can be distinguished from a ventral/lateral “paleocortical” system that comprises the olfactory cortex and peri-insular and ventral neocortices including the orbital frontal cortex. A defect in the frontolimbic system or dorsal “archicortical” trend, which encompasses both the superior frontal and anterior cingulate gyri, has been hypothesized to be critical for the stable execution of internally generated, task relevant, action-oriented behaviors (Bilder and Degreef, 1991; Bilder and Szeszko, 1996; Szeszko et al 2002). Evidence for a genetically mediated defect in this integrated system in schizophrenia could play a role in neuropsychological deficits observed during executive functioning (Szeszko et al 2000; Everett et al 2001), two-choice guessing (Paulus et al 1999), verbal fluency (Schaufelberger et al 2005) and conflict monitoring (Kerns et al 2005).
Our results also suggest that genetic variation in DISC1 plays a role in positive symptoms (i.e., hallucinations) in schizophrenia. Similarly, Hennah et al (2003) reported that variation in DISC1 was associated with delusions, hallucinations, and negative symptoms among patients with schizophrenia. Strengthening the purported effect of DISC1 on brain volume and its association with positive symptoms we also observed a significant inverse correlation between superior frontal gray matter volume and severity of hallucinations among patients. In that regard our results are consistent with other studies documenting an association between severity of hallucinations and prefrontal cortical abnormalities as assessed via magnetoencephalography (Kawaguchi et al 2005) and in event related potential (Papageorgiou et al 2005), positron emission tomography (Copolov et al 2003) and functional magnetic resonance imaging (Lennox et al 2000) studies in patients with schizophrenia. Moreover, other studies indicate that the prefrontal cortex may be an important region for the development of hallucinations (Castner et al 2003) and that atypical antipsychotics that are linked with positive symptom improvement (Kane et al 1988) alter frontal dopaminergic activity (Ichikawa and Meltzer, 1999).
There were several limitations to this study that should be acknowledged. There is the caveat of examining a single SNP in relationship to multiple phenotypic traits, which may increase the likelihood of false positives. Thus, these findings should be replicated using larger sample sizes. In addition, the sample size for the MR imaging analysis was small, as reflected by the large confidence intervals, and thus, potentially underpowered to detect significant group differences in brain structure volumes. There is also the potential that case-control association studies may be influenced by undetected population stratification (Malhotra and Goldman, 1999). To limit this possibility, however, we limited the sample to Caucasians, and focused on a functional polymorphism that has been demonstrated previously to increase risk for schizophrenia and schizoaffective disorder and was part of a haplotype previously reported to be associated with gray matter density. Lastly, it would be important to consider potential gene-environment interactions in subsequent analyses.
In sum, these findings suggest that variation in frontal cortical gray matter volumes and positive symptoms may be explained, in part, by the leu607phe polymorphism in the DISC1 gene. Taken together, our data provide a potential mechanism through which DISC1 may confer increased risk for neuropsychiatric disorders such as schizophrenia or schizoaffective disorder. Future studies could focus on potential epistatic interactions of the DISC1 gene with other genes that may increase risk for schizophrenia.
Acknowledgments
This work was supported in part by grants from NARSAD (PRS) and the National Institute of Mental Health to Dr. Szeszko (MH01990), Dr. Bilder (MH60374), Dr. Malhotra (MH01760), Dr. Kane (MH60575), Dr. Robinson (MH60004) and the NSLIJ Research Institute General Clinical Research Center (M01 RR018535).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bilder RM, Degreef G. Morphologic markers of neurodevelopmental paths to schizophrenia. In: Mednick SA, Cannon TD, Barr CE, LaFosse JM, editors. Developmental Neuropathology of Schizophrenia. New York: Plenum Press; 1991. pp. 167–190. [Google Scholar]
- Bilder RM, Szeszko PS. Structural neuroimaging and neuropsychological impairments. In: Pantellis C, Nelson HE, Barnes TRE, editors. The Neuropsychology of Schizophrenia. Sussex UK: John Wiley & Sons; 1996. pp. 279–298. [Google Scholar]
- Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorders--cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood DH, Muir WJ. Clinical phenotypes associated with DISC1, a candidate gene for schizophrenia. Neurotox Res. 2004;6:35–41. doi: 10.1007/BF03033294. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Handford EJ, Schurov I, Rain JC, Pelling M, Duran-Jimeniz B, et al. Disrupted in Schizophrenia 1 and Nudel form a neurodevelopmentally regulated protein complex: implications for schizophrenia and other major neurological disorders. Mol Cell Neurosci. 2004;25:42–55. doi: 10.1016/j.mcn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Hodgkinson CA, Szeszko PR, Lencz T, Ekholm JM, Kane JM, et al. DISC1 and neurocognitive function in schizophrenia. Neuroreport. 2005;16:1399–1402. doi: 10.1097/01.wnr.0000175248.25535.f6. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Hennah W, van Erp TG, Thompson PM, Lonnqvist J, Huttunen M, et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch Gen Psychiatry. 2005;62:1205–1213. doi: 10.1001/archpsyc.62.11.1205. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Thompson PM, van Erp TG, Toga AW, Poutanen VP, Huttunen M, et al. Cortex mapping reveals regionally specific patterns of genetic and disease- specific gray-matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci U S A. 2002;99:3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS. Amphetamine sensitization of hallucinatory-like behaviors is dependent on prefrontal cortex in nonhuman primates. Biol Psychiatry. 2003;54:105–110. doi: 10.1016/s0006-3223(03)00292-0. [DOI] [PubMed] [Google Scholar]
- Christensen BK, Bilder RM. Dual cytoarchitectonic trends: an evolutionary model of frontal lobe functioning and its application to psychopathology. Can J Psychiatry. 2000;45:247–256. doi: 10.1177/070674370004500303. [DOI] [PubMed] [Google Scholar]
- Copolov DL, Seal ML, Maruff P, Ulusoy R, Wong MT, Tochon-Danguy HJ, et al. Cortical activation associated with the experience of auditory hallucinations and perception of human speech in schizophrenia: a PET correlation study. Psychiatry Res. 2003;122:139–152. doi: 10.1016/s0925-4927(02)00121-x. [DOI] [PubMed] [Google Scholar]
- DeRosse P, Hodgkinson CA, Lencz T, Burdick KE, Kane JM, Goldman D, Malhotra AK. Disrupted in schizophrenia I genotype and positive symptoms in schizophrenia. Biol Psych. 2007;15:1208–1210. doi: 10.1016/j.biopsych.2006.07.023. [DOI] [PubMed] [Google Scholar]
- du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, et al. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006;129:3315–3328. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- Erkwoh R, Sabri O, Steinmeyer EM, Bull U, Sass H. Psychopathological and SPECT findings in never-treated schizophrenia. Acta Psychiatr Scand. 1997;96:51–57. doi: 10.1111/j.1600-0447.1997.tb09904.x. [DOI] [PubMed] [Google Scholar]
- Everett J, Lavoie K, Gagnon JF, Gosselin N. Performance of patients with schizophrenia on the Wisconsin Card Sorting Test (WCST) J Psychiatry Neurosci. 2001;26:123–130. [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders-Patient Edition (SCID-I/P) New York: New York State Psychiatric Institute; 1994. [Google Scholar]
- Hennah W, Tuulio-Henriksson A, Paunio T, Ekelund J, Varilo T, Partonen T, et al. A haplotype within the DISC1 gene is associated with visual memory functions in families with a high density of schizophrenia. Mol Psychiatry. 2005;10:1097–1103. doi: 10.1038/sj.mp.4001731. [DOI] [PubMed] [Google Scholar]
- Hennah W, Varilo T, Kestila M, Paunio T, Arajarvi R, Haukka J, et al. Haplotype transmission analysis provides evidence of association for DISC1 to schizophrenia and suggests sex-dependent effects. Hum Mol Genet. 2003;12:3151–3159. doi: 10.1093/hmg/ddg341. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Goldman D, Jaeger J, Persaud S, Kane JM, Lipsky RH, et al. Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet. 2004;75:862–872. doi: 10.1086/425586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa J, Meltzer HY. Relationship between dopaminergic and serotonergic neuronal activity in the frontal cortex and the action of typical and atypical antipsychotic drugs. Eur Arch Psychiatry Clin Neurosci. 1999;249:90–98. doi: 10.1007/pl00014190. [DOI] [PubMed] [Google Scholar]
- Jayakumar PN, Venkatasubramanian G, Gangadhar BN, Janakiramaiah N, Keshavan MS. Optimized voxel-based morphometry of gray matter volume in first-episode, antipsychotic-naive schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:587–591. doi: 10.1016/j.pnpbp.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- Kawaguchi S, Ukai S, Shinosaki K, Ishii R, Yamamoto M, Ogawa A, et al. Information processing flow and neural activations in the dorsolateral prefrontal cortex in the Stroop task in schizophrenic patients: A spatially filtered MEG analysis with high temporal and spatial resolution. Neuropsychobiology. 2005;51:191–203. doi: 10.1159/000085594. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Johnson MK, Stenger VA, Aizenstein H, et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162:1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Lennox BR, Park SB, Medley I, Morris PG, Jones PB. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res. 2000;100:13–20. doi: 10.1016/s0925-4927(00)00068-8. [DOI] [PubMed] [Google Scholar]
- Lim KO, Zipursky RB, Watts MC, Pfefferbaum A. Decreased gray matter in normal aging: an in vivo magnetic resonance study. J Gerontol. 1992;47:B26–B30. doi: 10.1093/geronj/47.1.b26. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Goldman D. Benefits and pitfalls encountered in psychiatric genetic association studies. Biol Psychiatry. 1999;45:544–550. doi: 10.1016/s0006-3223(98)00365-5. [DOI] [PubMed] [Google Scholar]
- MEDx. Sterling, VA: Sensor Systems; 1998. [Google Scholar]
- Millar JK, Christie S, Anderson S, Lawson D, Hsiao-Wei Loh D, Devon RS, Arveiler B, et al. Genomic structure and localisation within a linkage hotspot of Disrupted In Schizophrenia 1, a gene disrupted by a translocation segregating with schizophrenia. Mol Psychiatry. 2001;6:173–178. doi: 10.1038/sj.mp.4000784. [DOI] [PubMed] [Google Scholar]
- Millar JK, James R, Christie S, Porteous DJ. Disrupted in schizophrenia 1 (DISC1): subcellular targeting and induction of ring mitochondria. Mol Cell Neurosci. 2005;30:477–484. doi: 10.1016/j.mcn.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;90:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Honda A, Baba K, Taniguchi M, Oono K, Fujita T, et al. Disrupted-In-Schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol Psychiatry. 2003;8:685–694. doi: 10.1038/sj.mp.4001352. [DOI] [PubMed] [Google Scholar]
- Morris JA, Kandpal G, Ma L, Austin CP. DISC1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation. Hum Mol Genet. 2003;12:1591–1608. doi: 10.1093/hmg/ddg162. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–114. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathy CD. Atlas of the Cerebral Sulci. Stuttgart: Georg Thieme Verlag; 1990. [Google Scholar]
- Otsu NA. Thresholding selection method from gray-level histogram. IEEE. 1979;9:62–66. [Google Scholar]
- Ozeki Y, Tomoda T, Kleiderlein J, Kamiya A, Bord L, Fujii K, et al. Disrupted-in-Schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci USA. 2003;100:289–294. doi: 10.1073/pnas.0136913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou C, Lykouras L, Alevizos B, Ventouras E, Mourtzouchou P, Uzunoglu N, et al. Psychophysiological differences in schizophrenics with and without delusional misidentification syndromes: a P300 study. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:593–601. doi: 10.1016/j.pnpbp.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA, Braff DL. Long-range correlations in choice sequences of schizophrenic patients. Schizophr Res. 1999;35:69–75. doi: 10.1016/s0920-9964(98)00108-x. [DOI] [PubMed] [Google Scholar]
- Porteous DJ, Thomson P, Brandon NJ, Millar JK. The genetics and biology of DISC1--an emerging role in psychosis and cognition. Biol Psychiatry. 2006;60:123–131. doi: 10.1016/j.biopsych.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Galaburda AM, Kennedy DN, Filipek PA, Caviness VS. Human cerebral cortex: Localization, parcellation, and morphometry with magnetic resonance imaging. J Cog Neurosci. 1992;4:352–374. doi: 10.1162/jocn.1992.4.4.352. [DOI] [PubMed] [Google Scholar]
- Rasser PE, Johnston P, Lagopoulos J, Ward PB, Schall U, Thienel R, et al. Functional MRI BOLD response to Tower of London performance of first-episode schizophrenia patients using cortical pattern matching. Neuroimage. 2005;26:941–951. doi: 10.1016/j.neuroimage.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, et al. Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry. 2000;57:471–480. doi: 10.1001/archpsyc.57.5.471. [DOI] [PubMed] [Google Scholar]
- Sanides F. Comparative architectonics of the neocortex of mammals and their evolutionary interpretation. Annals of the New York Academy of Sciences. 1969;167:404–423. [Google Scholar]
- SAS vsn 9.1. Cary, NC, USA: Copyright © 2002–2003 by SAS Institute Inc.; [Google Scholar]
- Schachter SC, Ransil BJ, Geschwind N. Associations of handedness with hair color and learning disabilities. Neuropsychologia. 1987;25:269–276. doi: 10.1016/0028-3932(87)90137-0. [DOI] [PubMed] [Google Scholar]
- Schaufelberger M, Senhorini MC, Barreiros MA, Amaro E, Jr, Menezes PR, Scazufca M, et al. Frontal and anterior cingulate activation during overt verbal fluency in patients with first episode psychosis. Rev Bras Psiquiatr. 2005;27:228–232. doi: 10.1590/s1516-44462005000300013. [DOI] [PubMed] [Google Scholar]
- Schurov IL, Handford EJ, Brandon NJ, Whiting PJ. Expression of disrupted in schizophrenia 1 (DISC1) protein in the adult and developing mouse brain indicates its role in neurodevelopment. Mol Psychiatry. 2004;9:1100–1110. doi: 10.1038/sj.mp.4001574. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams D. Structured Clinical Interview for Diagnoses-Nonpatient Version. New York: New York State Psychiatric Institute; 1998. [Google Scholar]
- St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G, et al. Association within a family of a balanced autosomal translocation with major mental illness. Lancet. 1990;336:13–16. doi: 10.1016/0140-6736(90)91520-k. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Bilder RM, Lencz T, Ashtari M, Goldman RS, Reiter G, et al. Reduced anterior cingulate gyrus volume correlates with executive dysfunction in men with first-episode schizophrenia. Schizophr Res. 2000;43:97–108. doi: 10.1016/s0920-9964(99)00155-3. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Bilder RM, Lencz T, Pollack S, Alvir JM, Ashtari M, et al. Investigation of frontal lobe subregions in first-episode schizophrenia. Psychiatry Res. 1999;90:1–15. doi: 10.1016/s0925-4927(99)00002-5. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Goldberg E, Gunduz-Bruce H, Ashtari M, Robinson D, Malhotra AK, et al. Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:2190–2197. doi: 10.1176/appi.ajp.160.12.2190. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, MacMillan S, McMeniman M, Chen S, Baribault K, Lim KO, et al. Brain structural abnormalities in psychotropic drug-naive pediatric patients with obsessive-compulsive disorder. Am J Psychiatry. 2004;161:1049–1056. doi: 10.1176/appi.ajp.161.6.1049. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Strous RD, Goldman RS, Ashtari M, Knuth KH, Lieberman JA, et al. Neuropsychological correlates of hippocampal volumes in patients experiencing a first episode of schizophrenia. Am. J. Psychiatry. 2002;159:217–226. doi: 10.1176/appi.ajp.159.2.217. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Simonotto E, Moorhead W, McIntosh A, Marshall I, Ebmeier KP, et al. Functional imaging as a predictor of schizophrenia. Biol. Psychiatry. 2006;60:454–462. doi: 10.1016/j.biopsych.2005.11.013. [DOI] [PubMed] [Google Scholar]