Summary
Receptor-mediated interactions between neurons and astroglia are likely to play a crucial role in the growth and guidance of CNS axons. Using antibodies to neuronal cell surface proteins, we identified two receptor systems mediating neurite outgrowth on cultured astrocytes. N-cadherin, a Ca2+-dependent cell adhesion molecule, functions prominently in the outgrowth of neurites on astrocytes by E8 and E14 chick ciliary ganglion (CC) neurons. β1-class integrin ECM receptor heterodimers function less prominently in E8 and not at all in E14 neurite outgrowth on astrocytes. The lack of effect of integrin β1 antibodies on E14 neurite outgrowth reflects an apparent loss of integrin function, as assayed by E14 neuronal attachment and process outgrowth on laminin. N-CAM appeared not to be required for neurite outgrowth by either E8 or E14 neurons. Since N-cadherin and integrin β1 antibodies together virtually eliminated E8 CG neurite outgrowth on cultured astrocytes, these two neuronal receptors are probably important in regulating axon growth on astroglia in vivo.
Introduction
Neural pathways are established with a high degree of precision and reproducibility in the developing nervous system. This is accomplished initially through the directed movement of axonal growth cones to their synaptic target cells (see Bentley and Keshishian, 1982; Raper et al., 1983a, 1983b; Tosney and Landmesser, 1985). Growth cones are guided, in part, by contact with the surfaces of other neuronal and nonneuronal cells and the molecules they secrete (see Bentley and Caudy, 1983; Raper et al., 1984; Sanes et al., 1978). Molecules that influence the extent and orientation of axon growth do so by binding to specific receptors on the neuronal membrane (see Bozyczko and Horwitz, 1986; Tomaselli et al., 1986; Bixby et al., 1987; Chang et al., 1987).
Experiments in vitro have provided insights into the molecular mechanisms of neuronal process outgrowth. Two distinct classes of neurite outgrowth-promoting substrates have been described: constituents of the extracellular matrix (ECM), most notably laminin and fibronectin (see Rogers et al., 1983; Lander et al., 1983, 1985), and the surfaces of neuronal, glial, and muscle cells (Chang et al., 1987; Noble et al., 1984; Fallon, 1985a, 1985b; Tomaselli et al., 1986). Laminin and fibronectin stimulate process outgrowth from a wide variety of central and peripheral neurons (see Rogers et al., 1983; Manthorpe et al., 1983; Akers et al., 1981; Hall et al., 1987). Neuronal responses to these ECM proteins, as well as to intact ECMs, depend on the function of neuronal glycoproteins that belong to the integrin family of adhesive protein receptor heterodimers (Bozyczko and Horwitz, 1986; Tomaselli et al., 1986, 1987; Hall et al., 1987; reviewed in Hynes, 1987). Purified integrins bind directly to several ECM proteins, including laminin and fibronectin (Horwitz et al., 1985; Buck et al., 1986). Neurons use receptors that are distinct from integrins for neurite outgrowth on the surfaces of astrocytes, Schwann cells, skeletal myotubes, and other neurons (Tomaselli et al., 1986; Bixby et al., 1987; Chang et al., 1987). For example, process outgrowth by peripheral motoneurons on skeletal myotubes in vitro depends on the function of two cell-cell adhesion molecules (CAMS), N-cadherin and N-CAM, in addition to integrin β1 receptor heterodimers (Bixby et al., 1987). Each of these neuronal proteins appears capable of functioning alone in mediating neurite extension on myotubes (Bixby et al., 1987). The extension of neurites by sympathetic neurons on the surfaces of sympathetic axons depends, in part, on the function of two distinct glycoproteins, the G4 and F11 antigens (Chang et al., 1987; Rathjen et al., 1987). Thus, neurite outgrowth on the surfaces of other neurons or nonneuronal cells is mediated by several interactions involving “adhesive molecules” on the cellular substrate and specific receptors on the neuronal plasma membrane.
The goal of the present study was to identify neuronal cell surface molecules that mediate neurite outgrowth on the surfaces of astrocytes in vitro. This information is important in two contexts. First, the surfaces of astrocytes and astrocyte precursors are a prominent substrate for the growth of axons during the development of the central nervous system (CNS) and are thus likely to be important in stimulating and guiding axon elongation (see Maggs and Scholes, 1986; Letourneau et al., 1988; Silver and Rutishauser, 1984; Silver and Sidman, 1980). In vitro, astrocytes express neurite outgrowth-promoting factors to which both central and peripheral neurons can respond (Noble et al., 1984; Fallon, 1985a, 1985b; Tomaselli et al., 1986). Second, it has been suggested that the primary defect in CNS regeneration lies in the inability of the supporting glial cells to promote regrowth efficiently (see Benfey and Aguayo, 1982; Smith et al., 1986). Thus, knowledge concerning the molecular mechanisms of axon growth on astrocyte surfaces will provide a framework in which the deficits underlying unsuccessful CNS regeneration can be studied. Some of the results presented here have been reported in abstract form (Tornaselli et al., 1987, Soc. Neurosci., abstract).
Results
Astrocytes Produce Two Neurite Outgrowth-Promoting Factors
Neonatal rat cortical astrocytes produce at least two distinct factors that stimulate neurite outgrowth by dissociated embryonic chick ciliary ganglion (CG) neurons (Figure 1). One of these is secreted by astrocytes into their culture medium (astrocyte CM). When immobilized on a polycationic substrate, molecules in astrocyte CM stimulated rapid neurite outgrowth by embryonic day 8 (E8) CG neurons (Figure 1a; Table 1). Several studies have shown that the ECM protein laminin is probably the active neurite-promoting molecule in astrocyte CM (Lander et al., 1985; Tomaselli et al., 1986; Cohen et al., 1987; Selak et al., 1985). E8 CG neurite outgrowth on astrocyte CM, like that on laminin, is inhibited by either of two monoclonal antibodies, CSAT or JG22 (Figure 1c; Table 1). These antibodies will be referred to as anti-integrin β1, since they recognize the integrin β1 subunit shared by avian receptor heterodimers for laminin and fibronectin (Horwitz et al., 1985; Buck et al., 1986; Tamkun et al., 1986; see Hynes, 1987, for integrin nomenclature). A second neurite-promoting factor is associated with astrocyte surfaces (Figure 1b; Table 1). This factor is distinct from that found in astrocyte CM, since E8 CG neurons are able to grow long neurites on astrocyte surfaces in the presence of integrin β1 antibodies (Figure 1d; Table 1).
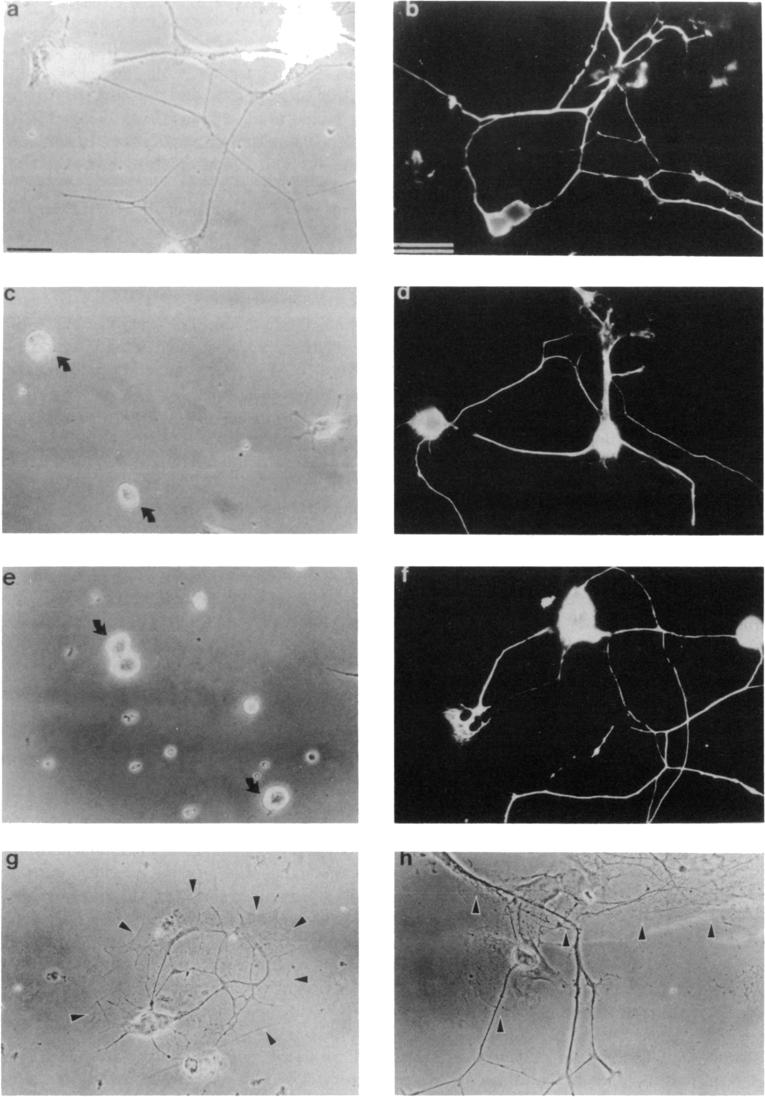
Figure 1. E8 and E14 CG Neuronal Process Outgrowth on Primary Cortical Astrocytes and on Substrates Coated with Astrocyte CM.
Neurons were visualized either by phase contrast or, when grown on astrocyte monolayers, by immunofluorescent staining with the A2B5 monoclonal antibody. (a) E8 CG neurons grown 16 hr on an astrocyte CM substrate. (b) E8 CG neurons grown 16 hr on a monolayer of astrocytes. (c) E8 CG neurons grown 16 hr on an astrocyte CM substrate in the presence of 100 μg/ml anti-integrin β1 (JG22) IgG. Note process outgrowth is inhibited. (d) E8 CG neurons grown 16 hr on astrocyte surfaces in the presence of 1 μg/ml anti-integrin β1 (JG22) Fab. Note process outgrowth is not prevented as it is on astrocyte CM (e) E14 CG neurons grown 16 hr on astrocyte CM. Arrows mark E14 neurons that do not respond to astrocyte CM (f) E14 CG neurons grown 16 hr on astrocyte surfaces. Note E14 CG neurons extend long neurites on astrocyte surfaces. (g) E14 CG neurons cultured 16 hr on small astrocyte islands centered on laminin-coated, glass coverslips. Note E14 CG neurites do not extend off of the astrocyte monolayer. (h) In contrast to E14 neurites, E8 neurites often grow off the astrocyte islands onto the surrounding laminin substrates. Arrowheads in (g) and (h) mark the boundary between the astrocyte monolayer and the surrounding laminin substrate. Bars, 10 μm. Bar in (a) also applies to (c) and (e); bar in (b) also applies to (d) and (f-h).
Table 1.
Attachment and Process Outgrowth by E8 and E14 CG Neurons on Laminin and Astrocyte-Derived Neurite-Promoting Factors
| Process Outgrowth: Percentage of Neurons with Neurites | ||||||
| Substrates |
||||||
| Laminin |
Astrocyte CM Medium |
Astrocytes |
||||
| Neurons |
Control |
Anti-Integrin Fab |
Control |
Anti-Integrin IgG |
Control |
Anti-Integrin Fab |
| E8 CG | 77 ± 5 | 4 ± 1 | 55 ± 4a | 13 ± 3a | 87 ± 1 | 80 ± 2 |
| E14 CG |
2 ± 1 |
1 ± 1 |
3 ± 1 |
1 ± 0 |
45 ± 3 |
48 ± 2 |
| Attachment: Percentage of Positive Controls | ||||||
| Laminin Substrate |
||||||
| Neurons |
Control |
Anti-Integrin IgG |
||||
| E8 CG | 87 ± 4 | 4 ± 1 | ||||
| E14 CG | 25 ± 1 | 3 ± 1 | ||||
E8 and E14 CG neurons were grown 16−20 hr in the absence (control) or the presence of anti–integrin (β1 (JG22 IgG at 100 μg/ml or JG22 Fab at 0.5 mg/ml on laminin and at 1 mg/ml on astrocytes) on substrates coated with laminin or astrocyte CM or on astrocyte monolayers. Fixed neurons were scored for the percentage bearing a process ≥2 cell diameters in length. Values represent the average and range of determinations made on duplicate cultures run in parallel. At least 200 neurons were counted for each value.
E8 and E14 CG neuronal attachment to laminin-coated substrates was measured in the absence (control) or the presence of anti–integrin β1 IgG (JG22 at 50 μg/ml). The number of adherent neurons in 10 random microscope fields per well was counted and averaged. Values represent the average and range of determinations made on duplicate wells run in parallel and are expressed as percentage of attachment relative to the highly adhesive substrate polyy-d-lysine.
Data from Tomaselli et al. (1986).
Developmental Change in Neuronal Responses
The ability of CG neurons to respond to these two astrocyte-derived neurite-promoting factors is regulated during embryonic development. This is demonstrated by comparing E8 and E14 CG neurite outgrowth on astrocyte CM and on astrocyte surfaces. Unlike E8 neurons, E14 CG neurons failed to extend neurites on either astrocyte CM or laminin (Figure 1e; Table 1). The inability of E14 CG neurons to extend neurites on either of these substrates may be due to diminished laminin receptor function, since E14 neurons also attached poorly to laminin compared with E8 neurons (Table 1). However, when cultured on astrocytes, about half of the E14 CG neurons extended neurites in less than 16 hr (Figure 1f; Table 1), and this percentage was not diminished by integrin β1, antibodies (Table 1). Thus, as is also true for embryonic chick retinal neurons (Cohen et al., 1986, 1987; Hall et al., 1987), CG neurons that have lost the ability to extend neurites on either astrocyte CM or laminin remain responsive to additional factors produced by astrocytes. These factors have been postulated to be associated with the astrocyte membrane (Tomaselli et al., 1986; Cohen et al., 1986, 1987) but could also be diffusible neurite-promoting factors secreted by astrocytes (see Lindsay, 1979; Assouline et al., 1987). In support of the former possibility, neurite extension by E14 neurons was dependent on contact with astrocyte surfaces. When E14 CG neurons were grown on small “island monolayers” of astrocytes centered on laminin-coated coverslips, their growth cones did not grow off the astrocytes onto the surrounding laminin substrate (Figure 1g). In contrast, E8 CG neurons, which are capable of responding to laminin, often grew from the astrocyte monolayers onto the surrounding laminin substrates (Figure 1h).
Effects of N-Cadherin, N-CAM, and lntegrin β1 Antibodies on Neurite Outgrowth on Astrocyte Surfaces
To identify molecules involved in neuronal process outgrowth on astrocyte cell surfaces, E8 and E14 neurons were cultured for 16−20 hr on confluent monolayers of astrocytes in the presence of antibodies to neuronal cell surface proteins. These included antibodies to CAMS as well as antibodies to ECM receptors. The percentage of neurons with neurites and the lengths of neurites that grew on astrocyte surfaces in the presence or absence of these antibodies were measured and compared. Anti-N-cadherin recognizes a 130 kd Ca2+-dependent CAM expressed on the surfaces of avian neurons (Crittenden et al., 1987). When added at a concentration known to maximally inhibit N-cadherin function, anti-N-cadherin IgG had a strong inhibitory effect on both E8 and E14 CG neuronal process outgrowth on astrocyte surfaces. A 1 mg/ml concentration of anti-N-cadherin IgG reduced the number of E8 neurons that extended neurites on astrocytes by about 75% (Figures 2b and 2c; Figure 3a, closed diamonds). In addition, the average length of those neurites that were extended in the presence of anti-N-cadherin IgG was reduced by about 65% (Figure 4a). Similarly, anti-N-cadherin IgG reduced the percentage of E14 neurons with neurites by about 85% (Figures 2e and 2f; Figure 3c, open squares), with a corresponding decrease of about 80% in the average length of those neurites that grew (Figure 4b). N-cadherin antibodies did not appear to prevent the attachment of CG neurons to the astrocyte monolayers, since many neurons remained adherent to the astrocyte monolayers without extending neurites (see Figure 2). Fab’ fragments of anti-N-cadherin IgG had similar, but weaker, effects on neurite outgrowth by both E8 (Figure 3b, closed diamonds; Figure 4a) and E14 neurons (Figure 3c, closed diamonds; Figure 4b). Thus, N-cadherin plays an important role in neuronal process outgrowth on astrocyte surfaces. N-cadherin functions independently of neuronal receptors for laminin, since N-cadherin antibodies had no demonstrable inhibitory effect on neurite extension on laminin substrates (Figure 4c).
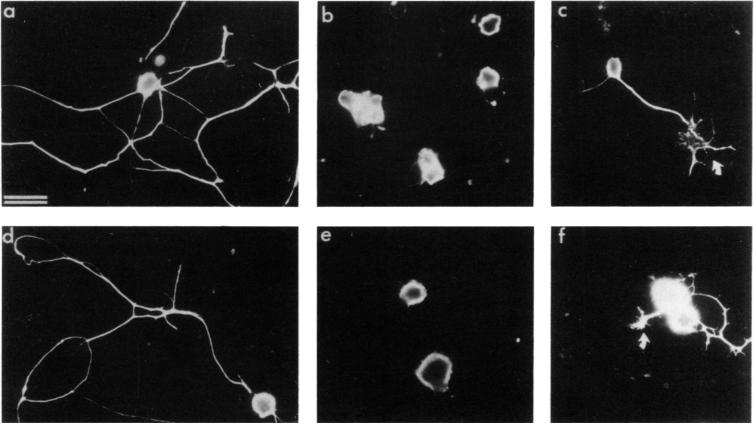
Figure 2. E8 and E14 CG Neuronal Process Outgrowth on Astrocyte Surface Is Strongly Inhibited by Anti-N-Cadherin but Not Anti-N-CAM.
Neurons were grown 16 hr on astrocyte surfaces in the presence of polyclonal anti-N-CAM IgG (a and d; 1 mg/ml), anti-N-cadherin IgG (b and c; 1 mg/ml), or anti-N-cadherin Fab’ (e and f; 1 mg/ml). The majority of E8 (a-c) and E14 (d-f) CG neurons were prevented from initiating neurites in the presence of N-cadherin antibodies. However, short neurites with enlarged growth cones were sometimes seen (arrows in c and f). Process outgrowth in the presence of anti-N-CAM appears to be unaffected (a and d). Neurons were visualized by immunofluorescent staining with the A2B5 monoclonal antibody and were readily distinguished from A2B5-positive astrocytes using morphological criteria. Bar, 10 μm.
Figure 3. Distribution of the Lengths of CG Neurites Extended on Astrocyte Surfaces in the Presence of Antibodies to N-Cadherin. N-CAM, and Integrin β1.
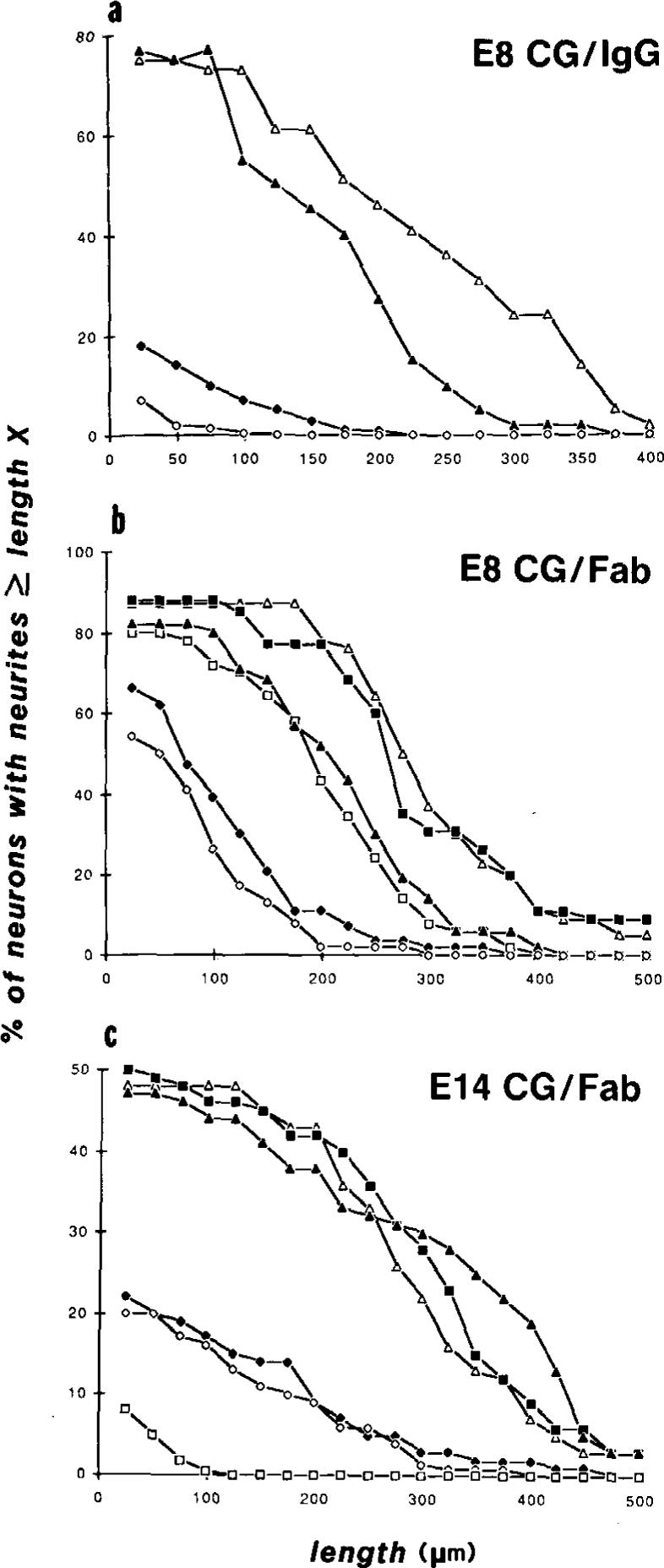
E8 (a and b) or E14 (c) CG neurons were cultured 16−20 hr on astrocyte monolayers in the presence of different antibodies, and camera lucida tracings of fixed and stained neurites were measured. The percentage of neurons with neurites greater than a certain length (vertical axis) is expressed as a function of neurite length (horizontal axis). Each graph consists of determinations made on individual cultures from the same experiment. (a) Distribution of neurite lengths of E8 CG neurons grown on astrocytes in the presence of 1A6 IgG (∼500 μg/ml; Δ), anti-integrin β1 (CSAT) IgG (100 μg/ml; ▲), anti-N-cadherin IgG (1 mg/ml; ◆), or anti-integrin β1 (CSAT) IgG plus anti-N-cadherin IgG (100 μg/ml and 1 mg/ml, respectively; ◇). In this experiment, 1A6 IgG served as a control antibody since it has no significant effect on neurite outgrowth on astrocyte surfaces (see Figures 4a and 4b).
(b) Distribution of neurite lengths of E8 CG neurons grown on astrocytes in the absence of antibodies (△) or in the presence of anti-N-CAM Fab’ (1 mg/ml; ■), anti-integrin β1 (JG22) Fab’ (1 mg/ml; ▲), anti-integrin β1 (JG22) Fab plus anti-N-CAM Fab’ (1 mg/ml each; □), anti-N-cadherin Fab’ (1 mg/ml; ◆), or anti-integrin β1 (JG22) Fab plus anti-N-cadherin Fab’ (1 mg/ml each; ◇).
(c) Distribution of neurite lengths of E14 CG neurons grown on astrocytes in the absence of antibody (Δ) or in the presence of anti-N-CAM Fab’ (1 mg/ml; ■), anti-integrin β1 (JG22) Fab (1 mg/ml; ▲), anti-N-cadherin Fab’ (1 mg/ml; ◆), anti-integrin Fab’ plus anti-integrin β1 (JG22) Fab (1 mg/ml each; ◇), or anti-N-cadherin IgG (1 mg/ml; □).
Symbol Summary: △, control; ▲, anti-integrin β1; ◆, anti-N-cadherin; ◇, anti-integrin β1 plus anti-N-cadherin; ■, anti-N-CAM; □, anti-N-CAM plus anti-integrin β1 in (b), anti-N-cadherin IgG in (c).
Figure 4. Average Total Length of Neurites Extended by E8 or E14 CG Neurons on Astrocytes or on Laminin in the Presence of Different Antibodies.
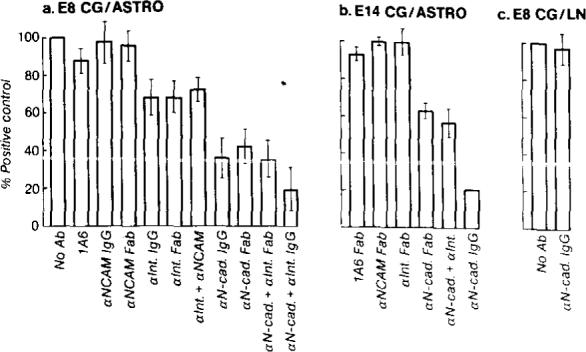
Values are expressed as percentage of the neurite length in the absence of added antibodies (positive control). In (a) error bars represent the standard error of determinations made on 30−35 neurons per condition. In (b), error bars represent the range of average neurite lengths from two different experiments (total 60−70 neurons per condition). (a) E8 CG neurons were grown 16−20 hr on astrocytes in the absence of antibodies or in the presence of 1A6 Fab (1 mg/ml), anti-N-CAM IgG (1 mg/ml), anti-N-CAM Fab’ (1 mg/ml), anti-integrin β1 (CSAT) IgG (100 μg/ml), anti-integrin β1 (JG22) Fab (1 mg/ml), anti-integrin β1 (JG22) Fab plus anti-N-CAM Fab’ (1 mg/ml each), anti-N-cadherin IgG (1 mg/ml), anti-N-cadherin Fab’ (1 mg/ml), anti-N-cadherin Fab’ plus anti-integrin β1 (JG22) Fab (1 mg/ml each), or anti-N-cadherin IgG plus anti-integrin β1 (CSAT) IgG (1 mg/ml and 100 μg/ml, respectively). (b) E14 CG neurons were grown 16−20 hr on astrocytes in the presence of 1A6 Fab (1 mg/ml), anti-N-CAM Fab’ (1 mg/ml), anti-integrin β1 (JG22) Fab (1 mg/ml), anti-N-cadherin Fab’ (1 mg/ml), anti-N-cadherin Fab’ plus anti-integrin β1 (JG22) Fab (1 mg/ml each), or anti-N-cadherin IgG (1 mg/ml). (c) E8 CG neurons were grown 16 hr on laminin substrates in the absence of added antibodies or in the presence of 1 mg/ml anti-N-cadherin IgG.
Two antibodies to the neural CAM (N-CAM) were also tested for their effects on CG neurite outgrowth on astrocytes: a monoclonal antibody, 1A6, that recognizes N-CAM but does not inhibit its function on CG neurons (Bixby et al., 1987; Watanabe et al., 1986) and a polyclonal anti-N-CAM serum that inhibits N-CAM function on chick CG neurons (Bixby and Reichardt, 1987; Bixby et al., 1987). Neither N-CAM antibody significantly diminished either the percentage of E8 or E14 CG neurons with neurites or the average length of neurites (Figures 2a and 2d; Figure 3; Figures 4a and 4b). This was true when N-CAM antibodies were added alone (Figure 3b, closed squares; Figure 3c, closed squares; Figures 4a and 4b) or in combination with integrin β1 antibodies (Figure 3b; compare open squares with closed triangle; Figures 4a and 4b).
Since astrocytes express ECM proteins on their surfaces in culture (Liesi et al., 1983; Asch et al., 1986), ECM receptor antibodies might also be expected to affect neuronal process extension on astrocytes. In contrast to the effects of N-cadherin antibodies, anti-integrin β1, IgG did not significantly diminish the percentage of E8 CG neurons that had extended neurites in 16 hr (Table 1; Figure 3a, compare closed triangles with closed diamonds). However, the average length of E8 CG neurites that grew in the presence of anti-integrin β1 IgG was consistently reduced by about 25% (Figure 3a, closed triangles; Figure 4a). Fab fragments of anti-integrin β1 IgG had similar effects on E8 neurons (Figure 3b, closed triangles; Figure 4a). In contrast, integrin β1 antibodies had little or no effect on E14 neurite outgrowth on astrocytes (Figure 3c, closed triangles; Figure 4b). Thus, in addition to N-cadherin, ECM receptors whose functions are inhibited by antibodies to the integrin β1 subunit play a role in E8 CG neurite outgrowth on astrocytes. In contrast, integrin β1 receptors appear not to be important in E14 CG neurite outgrowth on astrocytes. When added together with anti-N-cadherin IgG to cultures of E8 CG neurons, anti-integrin β1 IgG appeared to enhance the inhibitory effect seen with anti-N-cadherin alone, further reducing both the percentage of E8 neurons with neurites (Figure 3a, open diamonds) and the average length of neurites that grew (Figure 4a). This enhanced inhibitory effect was not simply a result of binding two different antibodies to the neuronal surface, since addition of both integrin β1 and N-CAM antibodies did not significantly augment the inhibition produced by anti-integrin β1 alone (Figure 3b, compare open squares with closed triangles; Figure 4a) and addition of both N-cadherin and integrin β1 antibodies to E14 CG neurons did not significantly enhance the inhibition seen with anti-N-cadherin alone (Figure 3c, compare open diamonds with closed diamonds; Figure 4b).
Discussion
By using antibodies that selectively inhibit neuronal receptors for adhesive proteins present on cell surfaces (anti-N-cadherin) or in the ECM (anti-integrin β1), we have identified two receptor systems that function in neuronal process outgrowth on astrocytes surfaces. Of the two, interactions mediated by N-cadherin are of primary importance, since N-cadherin antibodies greatly reduced both the percentage of neurons that initiated neurites and the average length of those neurites that grew. The mechanism(s) by which a small percentage of CG neurons extend short neurites in the presence of both N-cadherin and integrin β1 antibodies is unknown. This residual outgrowth could be due to incomplete inhibition by the antibodies or could reflect neurite-promoting activity by other .adhesive proteins, such as tenascin (also called cytotactin), thrombospondin, or AMOG, that are expressed by CNS glial cells in vitro (Grumet et al., 1985; Asch et al., 1986; Antonicek et al., 1987).
N-cadherin is a Ca2+-dependent CAM initially identified in avian neural retina (Grunwald et al., 1982; Crittenden et al., 1987) and mouse brain (Hatta et al., 1985) and is expressed in many tissues during embryonic development (Hatta et al., 1987). N-cadherin and the related molecules E-cadherin (Ogou et al., 1983; see also Gallin et al., 1983; Hyafil et al., 1980; Damsky et al., 1983) and P-cadherin (Nose and Takeichi, 1986) constitute a family of closely related Ca2+-dependent CAMS with differing tissue distributions (Shirayoshi et al., 1986). E-cadherin has recently been shown to mediate Ca2+-dependent cell-cell adhesion through a homophilic binding mechanism (Nagafuchi et al., 1987). It therefore seems likely that N-cadherin functions in a homophilic manner as well. The proposed roles of N-cadherin in tissue morphogenesis are many and include somite formation (Duband et al., 1987) and intercellular adherens junction formation (Volk and Geiger, 1986a, 1986b; Volk et al., 1987). The transient expression of N-cadherin in embryonic muscle during the period of motoneuron innervation suggests that N-cadherin is involved in adhesion between nerve and muscle in vivo (Hatta et al., 1987). Consistent with this hypothesis, it has recently been shown that N-cadherin functions in peripheral motoneuron outgrowth on skeletal myotube surfaces in vitro (Bixby et al., 1987). However, in contrast to the strong inhibitory effects of N-cadherin antibodies on CG neurite outgrowth on astrocytes, anti-N-cadherin, when applied alone, has subtle effects on CG neurite outgrowth on myotubes (Bixby et al., 1987). This appears to reflect the importance of several adhesive systems, including N-CAM and ECM proteins, that function in addition to N-cadherin in mediating neuronal process outgrowth on myotubes (Bixby et al., 1987).
The inhibition of neurite extension by N-cadherin antibodies appears not to be a trivial consequence of preventing neuronal attachment to the astrocyte monolayers. Instead, neurons grown on astrocytes in the presence of N-cadherin antibodies appeared to adhere efficiently to the astrocyte monolayers and, in some cases, extended short neurites with abundant filopodia and enlarged growth cones (see Figures 2c and 2f). These observations suggest that the adhesive interactions important for process extension on astrocytes may be only a subset of those that, in sum, contribute to neuron-astrocyte adhesion. For example, the glycoproteins N-CAM, Ng-CAM, AMOG, and tenascin (or cytotactin) may function in neuronal adhesion to astrocytes (Keilhauer et al., 1985; Grumet and Edelman, 1984; Antonicek et al., 1987; Kruse et al., 1985; Grumet et al., 1985). Thrombospondin, an ECM protein synthesized by astrocytes (Asch et al., 1986), may also serve similar adhesive functions (Tuszynski et al., 1987). Despite the adhesive properties of these glycoproteins, they appear not to stimulate neurite outgrowth efficiently when N-cadherin function is inhibited. Thus, although N-cadherin may be only one of several adhesive factors that mediate neuronal attachment to astrocytes, the binding of N-cadherin to its “ligand” on astrocyte surfaces, probably N-cadherin itself, appears to play a particularly prominent role in neurite extension. In contrast, our results do not support a role for the Ca2+-independent adhesion molecule, N-CAM, in CG neurite extension on astrocytes.
The inhibitory effects of antibodies to the integrin β1 subunit on CG neurite outgrowth on astrocytes demonstrate a role for ECM receptors in mediating neuronal interactions with CNS glia. Since integrin β1 antibodies have been shown to inhibit neuronal interactions with several ECM proteins, including laminin, fibronectin, and type IV collagen (Bozyczko and Horwitz, 1986; Tomaselli et al., 1986, 1987; Hall et al., 1987), it is difficult to state conclusively which, if any, of these proteins contribute to process outgrowth on astrocyte surfaces. However, laminin expression by astrocytes or astroglial precursors in vitro (Liesi et al., 1983; Selak et al., 1985) and in vivo (Liesi, 1985a, 1985b; Liesi et al., 1984; Cohen et al., 1987; Letourneau et al., 1988), is consistent with a role for laminin in neuron-astrocyte interactions. It has been suggested that the failure of astrocytes to express laminin after injury is responsible, in part, for the inability of mammalian CNS neurons to regenerate (McLoon, 1986; Carbonetto et al., 1987; Smith et al., 1986). However, data presented here suggest that interactions involving laminin may be less effective than those involving N-cadherin in stimulating neurite outgrowth on astrocytes.
E14 CG neurons that have lost the ability to extend neurites on either laminin or astrocyte CM (or heart cell CM; see Collins and Lee, 1982) remain capable of extending neurites on astrocytes in an N-cadherin-dependent fashion (see Figures 2d and 2e). Thus, CG neuronal responses to ECM- and cell-associated neurite-promoting factors appear to be regulated independently during neural development. In this respect, CG neurons are similar to embryonic chick retinal ganglion neurons that, between E6 and E11, also lose the ability to attach to and extend neurites on laminin but remain responsive to astrocyte surfaces (Cohen et al., 1986, 1987; Hall et al., 1987). In contrast to their younger counterparts, most E14 CG axons and E12 retinal ganglion cell axons have contacted and synapsed on their target cells in the eye or optic tectum, respectively (Landmesser and Pilar, 1974a, 1974b; Goldberg, 1974). This raises the possibility that contact with synaptic targets regulates neuronal responses to laminin (see Cohen et al., 1987). The diminished capacity of both E14 CG neurons and E12 retinal neurons to attach to laminin (see Table 1; Hall et al., 1987) suggests that laminin receptor function is modified in these cells during neural development. Since integrins function as receptors for laminin (Horwitz et al., 1985; Buck et al., 1986), changes in the levels of expression and/or the functional state of neuronal integrins could account for decreased laminin-binding by CG and retinal neurons. Differences in the expression of integrin subunits have been observed between E6 and E11 avian neural retinal cells (Hall et al., 1987). Phosphorylation of the cytoplasmic domain of the avian fibroblast integrin β1 subunit reduces the binding of solubilized integrin complexes to the ECM protein fibronectin (Buck and Horwitz, 1988). Similar posttranslational modifications of neuronal integrins might account for decreased neuronal adhesion to laminin. It is also possible, however, that the loss of laminin response reflects changes in other cell surface proteins that may also participate in neuronal responses to laminin (see Smalheiser and Schwartz, 1987).
The molecular mechanisms underlying growth cone guidance are largely unknown, but have been the subject of much speculation (see Harrison, 1935; Sperry, 1963; Letourneau, 1985). It has long been suggested that growth cones express specific receptors which regulate the extent and orientation of axon growth. In the present report, we have identified two distinct neuronal receptor systems that mediate neurite outgrowth on astrocyte surfaces. Integrin β1 receptors recognize adhesive proteins in the ECM, and N-cadherin recognizes a ligand (possibly N-cadherin itself) expressed on the membranes of other cells (e.g., astrocytes and skeletal myotubes). These two receptor systems function independently (since inhibition of one does not prevent neurite outgrowth mediated by the other; see Figure 4c) but, together, contribute to process extension. N-cadherin and integrins probably represent only two of many neuronal receptors that function in neurite outgrowth (see Chang et al., 1987; Lagenaur and Lemmon, 1987; Bixby et al., 1987). The mechanisms by which growth cone guidance is affected by the binding of these and other membrane receptors to adhesive molecules associated with the growth substrate are unknown. However, the fact that both integrin β1 receptors and N-cadherin appear to interact with proteins regulating cytoskeletal function (Horwitz et al., 1986; Geiger et al., 1985; Volk et al., 1987) suggests one possible way in which growth cone morphology and motility are regulated by the binding of neuronal cell surface receptors to adhesive proteins expressed along axon pathways.
Experimental Procedures
Animals
Newborn Sprague-Dawley rats were purchased from Bantin and Kingman (Fremont, CA). Fertile White Leghorn chicks were from Feather Hill Farm (Petaluma, CA) and were incubated at 38°C and 95% humidity until use.
Chemicals and Reagents
Murine laminin was purified from Engelbreth-Helm-Swarm sarcoma tumors using published procedures (Timpl et al., 1982; Kleinman et al., 1982). DEAE cellulose (DE52) was from Whatman (Clifton, NJ). Protein A Sepharose CL-4B was from Pharmacia (Piscataway, NJ). All other chemicals were purchased from Sigma (St. Louis, MO).
Antibodies
The hybridoma cell line secreting the CSAT monoclonal antibody (Neff et al., 1982) was the generous gift of Dr. A. F. Horwitz. Hybridoma cell lines secreting the JC22 (Greve and Gottlieb, 1982) and the 224-1A6-A1 (referred to here as 1A6; Lemmon et al., 1982) monoclonal antibodies were kindly provided by Dr. D. I. Gottlieb. The 1A6 monoclonal antibody is identical to the 105 monoclonal antibody, which binds to an extracellular N-CAM epitope that is close to the cell membrane and spatially distinct from the N-CAM binding domains (Watanabe et al., 1986). The hybridoma cell line secreting the A2B5 monoclonal antibody was purchased from the American Type Culture Collection (Rockville, MD). 1A6 and JC22 IgG were purified from ascites fluid by ammonium sulfate precipitation and ion exchange chromatography on DEAE cellulose as described by Hudson and Hay (1980). CSAT IgG was purified on protein A Sepharose CL-4B as described by Neff et al. (1982). Fab fragments of 1A6, CSAT, and JC22 IgG were prepared by digestion with papain followed by chromatography on DEAE cellulose (Hudson and Hay, 1980). Anti-N-CAM serum was generated in New Zealand White rabbits against N-CAM purified from embryonic chick brain as described by Bixby and Reichardt (1987). The N-CAM antiserum recognizes all three forms of N-CAM (Id, sd, and ssd) in immunoblots of embryonic chick brain proteins (Bixby and Reichardt, 1987). The anti-N-cadherin serum was generated in New Zealand White rabbits against a purified 90 kd proteolytic fragment of a 130 kd Ca2+-dependent CAM expressed by embryonic avian neural retinal cells (Crittenden et al., 1987). The anti-N-cadherin serum recognizes a single 130 kd protein in immunoblots of chick retinal membrane proteins separated by two-dimensional gel electrophoresis (Crittenden et al., 1987). This protein, which was previously termed NcalCAM Crittenden et al., 1987; Bixby et al., 1987), cross-reacts with monoclonal antibodies to chick N-cadherin kindly provided by Dr. M. Takeichi (Lilien et al., unpublished observations). Anti-N-CAM and anti-N-cadherin IgGs were purified by ammonium sulfate precipitation and ion exchange chromatography on DEAE cellulose as described by Hudson and Hay (1980). Fab’ fragments of anti-N-CAM and anti-N-cadherin IgGs were prepared by pepsin digestion followed by reduction and alkylation as described by Hudson and Hay (1980). Antibodies to glial fibrillary acidic protein (GFAP) were kindly provided by Dr. L. F. Eng.
Isolation of Astrocytes and Neurons
Astrocytes were prepared from neonatal rat cortices following publishing procedures (Noble et al., 1984; Fallon, 1985a, 1985b). Briefly, cortices were dissected free of other brain tissue, including the olfactory bulbs and hippocampi. Following trypsinization, cortices were triturated into a single cell suspension using a Pasteur pipet. The cell suspension was filtered through sterile lens paper before plating at a density of about 5 × 106 viable cells per 25 cm2 tissue culture flask. Cells were grown until nearly confluent (approximately 7−10 days) in Dulbecco's modified Eagle's medium (DME H21; UCSF cell culture facility) supplemented with 10% FCS, 100 U/ml penicillin/streptomycin, and 2 mM glutamine. Cultures were then shaken vigorously on a platform shaker (∼120 rpm) overnight at 37°C. Adherent cells were passaged in PBS with 0.05% trypsin and 5 mM EDTA and split 1:3. After 16 hr, the cultures were pulsed twice for 48 hr each with growth medium supplemented with 10−5 M cytosine β-d-arabinofuranoside. Astrocyte cultures generated in this way were then stable for several weeks. Immunofluorescent studies demonstrated that greater than 90% of the cells in these cultures expressed the astrocyte marker GFAP. About 90% of the GFAP-positive cells were flat and polygonal in shape and were similar to type I astrocytes derived from rat optic nerve (Raff et al., 1983a, 1983b). About 5%−10% of the GFAP-positive cells were process-bearing and, like type II astrocytes, expressed the cell surface gangliosides recognized by the A2B5 monoclonal antibody (Raff et al., 1983a, 1983b).
Ciliary ganglia from E8 or E14 White Leghorn chick embryos were enzymatically dissociated in Ca2+- and Mg2+-free PBS with 0.1% trypsin, and to maximize neuronal viability, dissociated neurons were cultured in the presence of 3% chick eye extract as previously described (Bixby and Reichardt, 1985; Nishi and Berg, 1981; Collins and Lee, 1982).
Substrate Preparation
Tissue culture microwells (0.28 cm2 surface area) were coated overnight at 4°C with laminin (10 μg/ml in PBS). For astrocyte CM substrates, microwells were first coated with poly-d-lysine (1 mg/ml in H2O) overnight at 4°C. After rinsing with sterile PBS, the wells were incubated with 100 μl of medium that had been conditioned for 7 days over confluent astrocyte cultures. Substrates coated with laminin or astrocyte CM were washed with sterile PBS. Dissociated neurons were plated at a density of about 50 neurons per mm2 in 100 μl of growth medium with or without antibodies and cultured 16−20 hr.
CG Neuron-Astrocyte Cocultures
Since the N-cadherin, N-CAM, and integrin β1 antibodies were all generated against avian glycoproteins, embryonic chick CG neurons were selected for culturing on rat astrocytes to study the role of these target antigens in neurite outgrowth. For neuron-astrocyte cocultures, astrocytes were passaged onto 13 mm round glass coverslips that had been precoated with 1 mg/ml poly-d-lysine in H2O. For some experiments, approximately 50,000 astrocytes were plated per coverslip and cultured at least 24 hr to yield a monolayer of cells covering the entire coverslip. In some experiments, small island monolayers of astrocytes (about 5 mm in diameter) were prepared in the center of laminin-coated coverslips as previously described (Tomaselli et al., 1986). About 5,000−10,000 CG neurons were added per coverslip and cultured 16−24 hr in the absence or presence of antibodies that had been sterile-filtered through 0.22 μm nitrocellulose filters (Millipore, Bedford, MA). Each antibody was added to cultures at concentrations previously determined to be saturating with respect to inhibition of their target antigens (see Tomaselli et al., 1986; Crittenden et al., 1987; Bixby et al., 1987).
CG Neuron Attachment Assay
The attachment of E8 and E14 CG neurons to laminin-coated substrates was measured using an assay described by Hall et al. (1987). Briefly, laminin-coated microwells were blocked for 3 hr at 25°C with PBS plus 10 mg/ml BSA. Dissociated CG neurons were resuspended in PBS plus 1 mM CaCl2 and 0.5 mM MgCl2 and allowed to attach in the absence or presence of integrin β1 antibodies for 90 min at 37°C (∼10,000 neurons per 0.28 cm2 well). Unattached cells were removed by pipetting 50 μl of PBS down each of two opposite sides of the well, and supernatants were carefully removed. Attached neurons were unambiguously identified on morphological criteria and were counted on a Zeiss IM microscope using a 40× water immersion lense. Attachment of neurons to laminin was compared with attachment to the highly adhesive substrate poly-d-lysine, to which about 80% of the .neurons attached (Hall et al., 1987). Less than 5% of the input cells attached to uncoated microwells that had been blocked with BSA.
Immunofluorescence
Neuron-astrocyte cocultures were fixed in PBS plus 3.7% paraformaldehyde and 5% sucrose, permeabilized with 0.05% saponin in PBS, and incubated 60 min at 25°C with mouse monoclonal antibody A2B5 ascites fluid diluted 11500 in PBS plus 0.05% saponin and 1% goat serum. The A2B5 antibody brightly stains the cell bodies, neurites, and growth cones of embryonic chick CG neurons (unpublished observations). After washing in PBS, cells were incubated in a 1:150 dilution of goat anti-mouse antibodies coupled to rhodamine isothiocyanate and processed as previously described (Tomaselli et al., 1986).
Analysis of Neurite Outgrowth on Astrocyte Surfaces
CG neuron-astrocyte cocultures that were fixed and stained with the A2B5 monoclonal antibody were viewed with a Zeiss IM microscope equipped with rhodamine optics. Tracings of fluorescent CG neurites in contact with the astrocyte monolayer along their entire course were drawn to scale using a camera lucida. Neurons were selected randomly, and only those neurites that could be visualized in their entire extent and were not fasciculated with other neurites were traced. Tracings were measured on a computerized digitizing pad (GTCO, Inc., Rockville, MD) and standardized with tracings of known length made previously using a stage micrometer and an eyepiece micrometer. Neurites from 30−35 neurons were measured for each experimental condition, and the total neuritic output of each neuron was computed by summing the lengths of individual neurites. The data for each experimental condition were normalized to the percentage of neurons in each culture, with neurites greater than 2 cell body diameters in length (approximately 20 μm), which had been counted earlier on the same coverslips, to yield the final length distribution curves (see Figure 3).
Acknowledgments
The authors would like to acknowledge the generous contributions of antibody-producing hybridomas from Drs. A. F. Horwitz (CSAT) and D. I. Gottlieb (JG22 and 1A6). The GFAP antiserum was the generous gift of Dr. L. F. Eng. We thank Dr. J. LaVail for use of the computerized digitizing pad, Dr. T. Large for help with the figures, Marion D. Meyerson for typing the manuscript, and Drs. Z. Hall, Y. N. Jan, S. Lisberger, G. Napolitano, and S. Crittenden for their helpful comments on the manuscript. K. J. T. is a recipient of a predoctoral Chancellor's Award from the University of California; K. M. N. is a predoctoral fellow of the National Science Foundation. This work was supported by National Institutes of Health grant NS 19090, NSF grant DCB 8511052, March of Dimes Birth Defects Foundation grant 1-774, and the Howard Hughes Medical Institute. L. F. R. is an investigator of the Howard Hughes Medical Institute.
References
- Akers RM, Mosher DF, Lilien JE. Promotion of retinal neurite outgrowth by substratum-bound fibronectin. Dev. Biol. 1981;86:179–188. doi: 10.1016/0012-1606(81)90328-6. [DOI] [PubMed] [Google Scholar]
- Antonicek H, Persohn E, Schachner M. Biochemical and functional characterization of a novel neuron-glia adhesion molecule that is involved in neuronal migration. J. Cell Biol. 1987;104:1587–1595. doi: 10.1083/jcb.104.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch ASL, Leung LK, Shapiro J, Nachman RL. Human brain glial cells synthesize thrombospondin. Proc. Natl. Acad. Sci. USA. 1986;83:2904–2908. doi: 10.1073/pnas.83.9.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assouline JG, Bosch P, Lim R, Kim IS, Jensen R, Pantagis NJ. Rat astrocytes and Schwann cells in culture synthesize nerve growth factor-like neurite-promoting factors. Brain Res. 1987;428:103–118. doi: 10.1016/0165-3806(87)90087-3. [DOI] [PubMed] [Google Scholar]
- Benfey M, Aguayo AJ. Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature. 1982;296:150–152. doi: 10.1038/296150a0. [DOI] [PubMed] [Google Scholar]
- Bentley D, Caudy M. Pioneer axons lose directed growth after selective killing of guidepost cells. Nature. 1983;304:62–65. doi: 10.1038/304062a0. [DOI] [PubMed] [Google Scholar]
- Bentley D, Keshishian H. Pathfinding by peripheral pioneer neurons in grasshoppers. Science. 1982;218:1082–1088. doi: 10.1126/science.218.4577.1082. [DOI] [PubMed] [Google Scholar]
- Bixby JL, Reichardt LF. The expression and localization of synaptic vesicle antigens at neuromuscular junctions in vitro. J. Neurosci. 1985;5:3070–3080. doi: 10.1523/JNEUROSCI.05-11-03070.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby JL, Reichardt LF. Effects of antibodies to N-CAM on the differentiation of neuromuscular junctions in vitro. Dev. Biol. 1987;119:363–372. doi: 10.1016/0012-1606(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Bixby JL, Pratt RS, Lilien J, Reichardt LF. Neurite outgrowth on muscle cell surfaces involves extracellular matrix receptors as well as Ca++-dependent and independent cell adhesion molecules. Proc. Natl. Acad. Sci. USA. 1987;84:2555–2559. doi: 10.1073/pnas.84.8.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozyczko D, Horwitz AF. The participation of a putative cell surface receptor for laminin and fibronectin in peripheral neurite extension. J. Neurosci. 1986;6:1241–1251. doi: 10.1523/JNEUROSCI.06-05-01241.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CA, Horwitz AF. Integrin, a transmembrane glycoprotein complex mediating cell-substratum adhesion. J. Cell Sci. 1988 doi: 10.1242/jcs.1987.supplement_8.13. in press. [DOI] [PubMed] [Google Scholar]
- Buck CA, Shea E, Duggan K, Horwitz AF. Integrin (the CSAT antigen): functionality requires oligomeric integrity. J. Cell Biol. 1986;103:2421–2428. doi: 10.1083/jcb.103.6.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetto S, Evans D, Cochard P. Nerve fiber growth in culture on tissue substrata from central and peripheral nervous systems. J. Neurosci. 1987;7:610–620. doi: 10.1523/JNEUROSCI.07-02-00610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Rathjen FG, Raper JA. Extension of neurites on axons is impaired by antibodies against specific neural cell surface glycoproteins. J. Cell Biol. 1987;104:355–362. doi: 10.1083/jcb.104.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Burne JF, Winter J, Bartlett P. Retinal ganglion cells lose response to laminin with maturation. Nature. 1986;322:465–467. doi: 10.1038/322465a0. [DOI] [PubMed] [Google Scholar]
- Cohen J, Burne JF, McKinley C, Winter J. The role of laminin and the laminin/fibronectin receptor complex in the outgrowth of retinal ganglion cell axons. Dev. Biol. 1987;122:407–418. doi: 10.1016/0012-1606(87)90305-8. [DOI] [PubMed] [Google Scholar]
- Collins F, Lee MR. A reversible developmental change in the ability of ciliary ganglion neurons to extend neurites in culture. J. Neurosci. 1982;2:424–430. doi: 10.1523/JNEUROSCI.02-04-00424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden SL, Pratt RS, Cook JH, Balsamo J, Lilien J. Immunologically unique and common domains within a family of proteins related to the retina Ca++-dependent cell adhesion molecule. NcalCAM. Development. 1987;101:729–740. doi: 10.1242/dev.101.4.729. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Richa J, Solter D, Knudsen K, Buck CA. Identification and purification of a cell surface glycoprotein mediating intercellular adhesion in embryonic and adult tissue. Cell. 1983;34:455–466. doi: 10.1016/0092-8674(83)90379-3. [DOI] [PubMed] [Google Scholar]
- Duband J-L, Dufour S, Hatta K, Takeichi M, Edelman GM, Thiery JP. Adhesion molecules during somitogenesis in the avian embryo. J. Cell Biol. 1987;104:1361–1374. doi: 10.1083/jcb.104.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JR. Preferential outgrowth of central nervous system neurites on astrocytes and Schwann cells as compared with nonglial cells in vitro. J. Cell Biol. 1985a;100:198–207. doi: 10.1083/jcb.100.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JR. Neurite guidance by non-neuronal cells in culture: preferential outgrowth of peripheral neurite, on glial as compared to non-glial cell surfaces. J. Neurosci. 1985b;5:3169–3177. doi: 10.1523/JNEUROSCI.05-12-03169.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin WJ, Edelman GM, Cunningham BA. Characterization of L-CAM, a major cell adhesion molecule from embryonic liver cells. Proc. Natl. Acad. Sci. USA. 1983;80:1038–1042. doi: 10.1073/pnas.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Asvnur Z, Volberg T, Volk T. Molecular domains of adherens junctions. In: Edelman GM, Thiery J-P, editors. The Cell in Contact. Neuroscience Institute Publ.; New York: 1985. pp. 461–490. [Google Scholar]
- Goldberg S. Studies on the mechanics of development of the visual pathways in the chick embryo. Dev. Biol. 1974;36:24–43. doi: 10.1016/0012-1606(74)90188-2. [DOI] [PubMed] [Google Scholar]
- Greve JM, Gottlieb DI. Monoclonal antibodies which alter the morphology of cultured chick myogenic cells. J. Cell Biochem. 1982;18:221–229. doi: 10.1002/jcb.1982.240180209. [DOI] [PubMed] [Google Scholar]
- Grumet M, Edelman GM. Heterotypic binding between neuronal membrane vesicles and glial cells is mediated by a specific cell adhesion molecule. J. Cell Biol. 1984;98:1746–1756. doi: 10.1083/jcb.98.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M, Hoffman S, Crossin KL, Edelman GM. Cytotactin, an extracellular matrix protein of neural and non-neural tissue that mediates glia-neuron interactions. Proc. Natl. Acad. Sci. USA. 1985;82:8075–8079. doi: 10.1073/pnas.82.23.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald GB, Pratt RS, Lilien J. Enzymatic dissection of embryonic cell adhesive mechanisms. III. Immunological identification of a component of the calcium-dependent adhesive system of embryonic chick neural retinal cells. J. Cell Sci. 1982;55:69–83. doi: 10.1242/jcs.55.1.69. [DOI] [PubMed] [Google Scholar]
- Hall DE, Neugebauer KM, Reichardt LF. Embryonic neural retinal cell response to extracellular matrix proteins: developmental changes and effects of the CSAT antibody. J. Cell Biol. 1987;104:623–634. doi: 10.1083/jcb.104.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RG. On the origin and development of the nervous system studied by the methods of experimental embryology. The Croonian Lecture. Proc. R. Soc. Lond. (Biol) 1935;118:155–196. [Google Scholar]
- Hatta K, Okada TS, Takeichi M. A monoclonal antibody disrupting calcium-dependent cell-cell adhesion of brain tissues. Possible role of its target antigen in animal pattern formation. Proc. Natl. Acad. Sci. USA. 1985;82:2789–2793. doi: 10.1073/pnas.82.9.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K, Takagi S, Fujisawa H, Takeichi M. Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev. Biol. 1987;120:215–227. doi: 10.1016/0012-1606(87)90119-9. [DOI] [PubMed] [Google Scholar]
- Horwitz A, Duggan K, Greggs R, Dekker C, Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J. Cell Biol. 1985;101:2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A, Duggan K, Buck C, Beckerle M, Burridge M. Interaction of plasma membrane fibronectin receptor with talin-a transmembrane linkage. Nature. 1986;320:531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Hudson L, Hay FC. Practical Immunology. Blackwell Scientific Publishers; Palo Alto, California: 1980. Isolation and structure of immunoglobulins; pp. 156–202. [Google Scholar]
- Hyafil F, Morello D, Babinet C, Jacob F. A cell surface glycoprotein involved in the compaction of embryonal carcinoma cells and cleavage stage embryos. Cell. 1980;21:927–934. doi: 10.1016/0092-8674(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Keilhauer G, Faissner A, Schachner M. Differential inhibition of neurone-neurone, neurone-astrocyte and astrocyte-astrocyte adhesion by L1, L2 and N-CAM antibodies. Nature. 1985;316:728–730. doi: 10.1038/316728a0. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Kruse J, Keilhauer G, Faissner A, Timp R, Schachner M. The J1 glycoprotein: a novel nervous system cell adhesion molecule of the L2/HNK-1 family. Nature. 1985;316:146–148. doi: 10.1038/316146a0. [DOI] [PubMed] [Google Scholar]
- Lagenaur C, Lemmon V. An L1-like molecule, the 8D9 antigen, is a potent substrate for neurite extension. Proc. Natl. Acad. Sci. USA. 1987;84:7753–7757. doi: 10.1073/pnas.84.21.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander AD, Tomaselli K, Calof AL, Reichardt LF. Studies on extracellular matrix components that promote neurite outgrowth. Cold Spring Harbor Symp. Quant. Biol. 1983;48:611–623. doi: 10.1101/sqb.1983.048.01.065. [DOI] [PubMed] [Google Scholar]
- Lander AD, Fujii DK, Reichardt LF. Laminin is associated with the neurite outgrowth-promoting factors found in conditioned media. Proc. Natl. Acad. Sci. USA. 1985;82:2183–2187. doi: 10.1073/pnas.82.7.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L, Pilar G. Synapse formation during embryogenesis on ganglion cells lacking a periphery. J. Physiol. 1974a;241:715–736. doi: 10.1113/jphysiol.1974.sp010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L, Pilar G. Synaptic transmission and cell death during normal ganglionic development. J. Physiol. 1974b;241:737–749. doi: 10.1113/jphysiol.1974.sp010681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon V, Staros EB, Perry HE, Gottlieb DI. A monoclonal antibody which binds to the surface of chick brain cells and myotubes: cell selectivity and the properties of the antigen. Dev. Brain Res. 1982;3:349–360. doi: 10.1016/0165-3806(82)90003-7. [DOI] [PubMed] [Google Scholar]
- Letourneau PC. Axonal growth and guidance. In: Edelman GM, Gall WE, Cowan WM, editors. Molecular Bases of Neural Development. John Wiley & Sons, Inc.; New York: 1985. pp. 269–294. [Google Scholar]
- Letourneau PC, Madsen AM, Palm SL, Furcht LT. Immunoreactivity for laminin in the developing ventral longitudinal pathway of the brain. Dev. Biol. 1988;125:135–144. doi: 10.1016/0012-1606(88)90066-8. [DOI] [PubMed] [Google Scholar]
- Liesi P. Do neurons in the vertebrate CNS migrate on laminin? EMBO J. 1985a;4:1163–1170. doi: 10.1002/j.1460-2075.1985.tb03755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P. Laminin-immunoreactive glia distinguish regenerative adult CNS systems from non-regenerative ones. EMBO J. 1985b;4:2505–2511. doi: 10.1002/j.1460-2075.1985.tb03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P, Dahl D, Vaheri A. Laminin is produced by early rat astrocytes in primary culture. J. Cell Biol. 1983;96:920–924. doi: 10.1083/jcb.96.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P, Kaakkola S, Dahl D, Vaheri A. Laminin is induced in astrocytes of adult brain by injury. EMBO J. 1984;3:683–686. doi: 10.1002/j.1460-2075.1984.tb01867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RM. Adult rat brain astrocytes support survival of both NGF-dependent and NCF-independent neurons. Nature. 1979;282:80–82. doi: 10.1038/282080a0. [DOI] [PubMed] [Google Scholar]
- Maggs A, Scholes J. Glial domains and nerve fiber patterns in the fish retinotectal pathway. J. Neurosci. 1986;6:424–438. doi: 10.1523/JNEUROSCI.06-02-00424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthorpe M, Engvall E, Ruoslahti E, Longo FM, Davis GE, Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J. Cell Biol. 1983;97:1882–1890. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoon SC. Response of astrocytes in the visual system to Wallerian degeneration: an immunohistochemical analysis of laminin and glial fibrillary acidic protein (GFAP). Exp. Neural. 1986;91:613–621. doi: 10.1016/0014-4886(86)90056-7. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987;329:341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Neff NT, Lowrey C, Decker C, Tovar A, Damsky C, Buck C, Horwitz AF. A monoclonal antibody detaches embryonic skeletal muscle from extracellular matrices. J. Cell Biol. 1982;95:654–666. doi: 10.1083/jcb.95.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi R, Berg DK. Two components from eye tissue that differentially stimulate the growth and development of ciliary ganglion neurons in cell culture. J. Neurosci. 1981;1:505–513. doi: 10.1523/JNEUROSCI.01-05-00505.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M, Fok-Seang J, Cohen J. Glia are a unique substrate for the in vitro growth of central nervous system neurons. J. Neurosci. 1984;4:1892–1903. doi: 10.1523/JNEUROSCI.04-07-01892.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A, Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J. Cell Biol. 1986;103:2649–2658. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogou S, Yoshida-Noro C, Takeichi M. Calcium-dependent cell-cell adhesion molecules common to hepatocytes and teratocarcinoma stem cells. J. Cell Biol. 1983;97:944–948. doi: 10.1083/jcb.97.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983a;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Raff MC, Abney ER, Cohen J, Lindsay R, Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J. Neurosci. 1983b;3:1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper JA, Bastiani MJ, Goodman CS. Pathfinding by neuronal growth cones in grasshopper embryos. I. Divergent choices made by the growth cones of sibling neurons. J. Neurosci. 1983a;3:20–30. doi: 10.1523/JNEUROSCI.03-01-00020.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper JA, Bastiani MJ, Goodman CS. Pathfinding by neuronal growth cones in grasshopper embryos. II. Selective fasciculation onto specific axonal pathways. J. Neurosci. 1983b;3:31–41. doi: 10.1523/JNEUROSCI.03-01-00031.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper JA, Bastiani MJ, Goodman CS. Pathfinding by neuronal growth cones in grasshopper embryos. IV. The effects of ablating the A and P axons upon the behavior of the G growth cone. J. Neurosci. 1984;4:2329–2345. doi: 10.1523/JNEUROSCI.04-09-02329.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen FG, Wolff JM, Frank R, Bonhoeffer F, Rutishauser U. Membrane glycoproteins involved in neurite fasciculation. J. Cell Biol. 1987;103:343–353. doi: 10.1083/jcb.104.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Letourneau PC, Palm SL, McCarthy J, Furcht LT. Neurite extension by peripheral and central nervous system neurons in response to substratum-bound fibronectin and laminin. Dev. Biol. 1983;98:212–220. doi: 10.1016/0012-1606(83)90350-0. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Marshall LM, McMahan UJ. Reinnervation of muscle fiber basal lamina after removal of myofibers. J. Cell Biol. 1978;78:176–198. doi: 10.1083/jcb.78.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak I, Foidart JM, Moonen G. Laminin promotes cerebellar granule cells migration in vitro and is synthesized by cultured astrocytes. Dev. Neurosci. 1985;7:278–285. doi: 10.1159/000112296. [DOI] [PubMed] [Google Scholar]
- Shirayoshi Y, Hatta K, Hosoda M, Tsunasawa S, Sukiyama F, Takeichi M. Cadherin cell adhesion molecules with distinct binding specificities share a common structure. EMBO J. 1986;5:2485–2488. doi: 10.1002/j.1460-2075.1986.tb04525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Sidman RS. A mechanism for the guidance and topographic patterning of retinal ganglion cell axons. J. Comp. Neural. 1980;189:101–111. doi: 10.1002/cne.901890106. [DOI] [PubMed] [Google Scholar]
- Silver J, Rutishauser U. Guidance of optic axons in vivo by a preformed adhesive pathway on neuroepithelial endfeet. Dev. Biol. 1984;106:485–499. doi: 10.1016/0012-1606(84)90248-3. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR, Schwartz NB. Cranin: a laminin binding protein of cell membranes. Proc. Natl. Acad. Sci. USA. 1987;84:6457–6461. doi: 10.1073/pnas.84.18.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GM, Miller RH, Silver J. Changing role of forebrain astrocytes during development, regenerative failure, and induced regeneration upon transplantation. J. Comp. Neural. 1986;251:23–43. doi: 10.1002/cne.902510103. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc. Natl. Acad. Sci. USA. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamkun JW, DeSimone DW, Fonda D, Patel RS, Buck C, Horwitz AF, Hynes RO. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986;46:271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- Timpl R, Rohd H, Ristidi L, Ott U, Robey DG, Martin GR. Laminin. Meth. Enzymol. 1982;82:831–838. doi: 10.1016/0076-6879(82)82104-6. [DOI] [PubMed] [Google Scholar]
- Tomaselli KJ, Reichardt LF, Bixby JL. Distinct molecular interactions mediate neuronal process outgrowth on extracellular matrices and non-neuronal cell surfaces. J. Cell Biol. 1986;103:2659–2672. doi: 10.1083/jcb.103.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli KJ, Damsky CH, Reichardt LF. Interactions of a neuronal cell line (PC12) with laminin, collagen IV and fibronectin: identification of integrin-related glycoproteins involved in attachment and process outgrowth. J. Cell Biol. 1987;105:2347–2358. doi: 10.1083/jcb.105.5.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosney KW, Landmesser LT. Development of the major pathways for neurite outgrowth in the chick hindlimb. Dev. Biol. 1985;109:193–214. doi: 10.1016/0012-1606(85)90360-4. [DOI] [PubMed] [Google Scholar]
- Tuszynski GP, Rothman V, Murphy A, Siegler K, Smith L, Smith S, Karczewski J, Knudsen KA. Thrombospondin promotes cell-substratum adhesion. Science. 1987;236:1570–1573. doi: 10.1126/science.2438772. [DOI] [PubMed] [Google Scholar]
- Volk T, Geiger B. A-CAM: a 135kD receptor of intercellular adherens junctions. I. Immunoelectron microscope localization and biochemical studies. J. Cell Biol. 1986a;103:1441–1450. doi: 10.1083/jcb.103.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T, Geiger B. A-CAM: a 135kD receptor of intercellular adherens junctions. II. Antibody-mediated modulation of junction formation. J. Cell Biol. 1986b;103:1451–1464. doi: 10.1083/jcb.103.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T, Cohen O, Geiger B. Formation of heterotypic adherens-type junctions between L-CAM-containing liver cells and A-CAM-containing lens cells. Cell. 1987;50:987–994. doi: 10.1016/0092-8674(87)90525-3. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Frelinger AL, III, Rutishauser U. Topography of N-CAM structural and functional determinants. I. Classification of monoclonal antibody epitopes. J. Cell Biol. 1986;103:1721–1728. doi: 10.1083/jcb.103.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]