Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD, or dioxin) and dioxin-like compounds (DLCs) induce numerous toxicities, including developmental, endocrine, immunological, and multi-organ carcinogenic, in animals and/or humans. Multiple studies completed by the National Toxicology Program (NTP) focused on the effects caused in Harlan Sprague-Dawley rats by specific DLCs, among them the prototypical dioxin, TCDD. Because humans are exposed daily to a combination of DLCs, primarily via ingestion of food, the Toxic Equivalency Factor (TEF) was developed in order to evaluate health hazards caused by these mixtures. Herein we review the pathological effects reported in humans exposed to TCDD; 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126); and 2,3,4,7,8,-pentachlorodibenzofuran (PeCDF) and compare them to similar changes seen in NTP murine studies performed with the same compounds. While there were differences in specific pathologies observed, clear consistency in the target organs affected (liver, oral cavity, cardiovascular system, immune system, thyroid, pancreas, and lung) could be seen in both human studies and rodent toxicity and carcinogenicity investigations.
Keywords: Carcinogenesis, dioxin, human, PCB126, PeCDF, rodent, TCDD
Introduction
2,3,7,8-Tetrachlorodiobenzo-p-dioxin (TCDD, or dioxin) was classified in 1997 as a “Group 1 carcinogen (carcinogenic to humans, non-genotoxic carcinogen)” by the International Agency for Research on Cancer (IARC) (http://www.iarc.fr/ENG/Databases/index.php). Hazards associated with TCDD do not constitute a novel finding, because its toxicologic properties have been known since the 1950’s (Watanabe et al., 1999). The top three sources of origin of dioxins are municipal incinerators, industrial incinerators, and metal refining (Watanabe et al., 1999). Humans are exposed to dioxins on a daily basis primarily through the consumption of food and water and, to a lesser extent, by inhalation (Mandal, 2005).
Although the existence of low levels of TCDD and dioxins is widespread, only a limited number of human studies have been conducted to measure effects on various systems of the body due to the limited number of populations that have been highly exposed. TCDD became well known as a contaminant of the herbicide, Agent Orange, used in the Vietnam War and was found in Times Beach, Missouri; Love Canal, New York; and Seveso, Italy following an industrial explosion in 1976 (Bertazzi et al., 2001; Schecter et al., 2006). Most recent studies indicate that exposure is associated with an increase in all cancers combined and several specific cancers including rectal cancer, lung cancer, Hodgkin’s disease, non-Hodgkin’s lymphoma, and myeloid leukemia (ATSDR, 1998; Bertazzi et al., 2001).
3,3′,4,4′,5-Pentachlorobiphenyl (PCB 126) was produced as a component of dielectric insulating fluid for transformers and capacitors prior to 1977 (NTP, 2004a). The creation and utilization of this chemical ceased because of residue found in the environment, although it is still discharged into the air and waterways by the disposal of objects that contain it (NTP, 2004a). Bioaccumulation of PCB 126 is prevalent in human and animal tissues with biological responses comparable to those evoked by TCDD (NTP, 2004a). Information on health effects of PCBs is available from studies of exposure in the workplace, consumption of contaminated rice oil in the Japanese “Yusho Accident” and the Taiwanese “Yu-Cheng Accident,” consumption of contaminated fish, general environmental exposures, and food products of animal origin. Evidence suggests that PCBs can produce several toxicities, such as skin lesions, dental abnormalities, immune deficiency, and/or reproductive abnormalities, and increase the risk of developing cardiovascular and/or liver disease and diabetes (Carpenter, 2006; Schecter et al., 2006). PCB126 has been listed as a Group 2A carcinogen (probably carcinogenic to humans) by the IARC (http://www.iarc.fr/ENG/Databases/index.php).
2,3,4,7,8,-Pentachlorodibenzofuran (PeCDF) has been made intentionally, only for the use of scientific research, but is found in the environment due to discharge from sources of combustion and incineration (NTP, 2004c). As one of the high-potency dioxin-like compounds (DLCs), PeCDF shows such high relative toxicity that it is similar to the most potent dioxin, TCDD. Toxic effects observed following exposure to PeCDF, as well as the structurally similar polychlorinated dibenzodioxins (PCDD), include developmental and reproductive alterations, immunotoxicity, teratogenicity, carcinogenicity, and lethality (ATSDR, 1998; Birnbaum, 1994; Poland and Knutson, 1982). PeCDF is listed by the IARC as a Group 3 carcinogen that is not classifiable as to carcinogenicity in humans (http://www.iarc.fr/ENG/Databases/index.php).
Studies suggest that the similar toxic and carcinogenic effects of TCDD and DLCs including PCB126 and PeCDF are mediated through their binding to the aryl hydrocarbon receptor (AhR) (ATSDR, 1998; ATSDR, 2000), which performs three chief functions: generating cellular signal transduction, binding DNA, and activating transcription (IARC, 1997). Upon binding of TCDD, PCB126, or PeCDF to AhR in cells, AhR-dioxin complex forms, cause a cascade of events involving gene and mRNA regulation, protein synthesis, biochemical modifications, and cell-growth stimulus. The eventual result is the formation of lesions, including cancer (Mandal, 2005).
The National Toxicology Program (NTP) conducted four murine carcinogenesis bioassays of TCDD in Swiss-Webster mice (NTP, 1982a), Osborne-Mendel rats, B6C3F1mice (NTP, 1982b), and female Harlan Sprague-Dawley rats (NTP, 2004b). Two carcinogenesis studies of PCB 126 (NTP, 2004a) or PeCDF (NTP, 2004c) in female Harlan Sprague-Dawley rats were also reported by the NTP. These studies of dioxin and DLCs are listed in Table 1 (web address of NTP long-term study reports/abstracts: http://ntp.niehs.nih.gov/go/reports). In these investigations, increases occurred in incidences of neoplastic effects, such as cholangiocarcinoma and/or hepatocellular adenoma, squamous-cell carcinoma of the oral cavity and uterus, and cystic keratinizing epithelioma of the lung (Brix et al., 2004; Hailey et al., 2005; Jokinen et al., 2003; Nyska et al., 2005; Tani et al., 2004; Walker et al., 2005, 2006; Yoshizawa et al., 2005a, b).
Table 1.
Associated doses used in the NTP two-year studies of dioxin and dioxin-like compounds.
| Compounds | Structures | TEFa | Core groups (animal, dose, route) |
|---|---|---|---|
| 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Group 1 carcinogenb) |
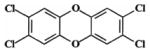
|
1 | TR201c: male & female Swiss-Webster mice; 0.001 (male) and0.005 (female) μg; dermal exposure without initiation
TR209: male & female Osborne-Mendel rats; 0.01, 0.05, 0.5 μg/kg; gavage exposure TR209: male & female B6C3F1 mice; 0.01, 0.05, 0.5 μg/kg (male), 0.04, 0.2, 2 μg/kg (female); gavage exposure TR521: female Harlan Sprague-Dawley rats; 3, 10, 22, 46, 100 ng/kg; gavage exposure |
| 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) (Group 2A carcinogenb) |
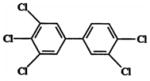
|
0.1 | TR520: female Harlan Sprague-Dawley rats; 30, 100, 175, 300, 550, 1000 ng/kg; gavage exposure |
| 2,3,4,7,8-pentachlorodibenzofuran (PeCDF) (Group 3 carcinogenb) |
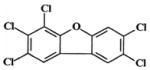
|
0.5 | TR525: female Harlan Sprague-Dawley rats; 6, 20, 44, 92, 200 ng/kg; gavage exposure |
Toxic equivalency factor (Van den Berg et al., 1998, 2006).
To humans, TCDD is classified as carcinogenic chemical, PCB126 as probable carcinogenic chemical; PeCDF is not classifiable as to carcinogenicity by International Agency for Research on Cancer (IARC) (http://monographs.iarc.fr/ENG/Classification/index.php).
National Toxicology Program (NTP) study report number.
This review, one of a series of works highlighting specific findings from these NTP dioxin Toxic-Equivalency-Factor (TEF) evaluative studies, focuses on comparison of effects observed in rodents to those reported in human epidemiologic studies induced by exposure to TCDD, PCB 126, and PeCDF. Our objective is to provide understanding of possible concordance of pathologic and neoplastic effects reported in rodents and humans.
Results and Discussion
In this literature review, we compare lesions observed in rats, mice, and humans exposed to TCDD, PCB 126, and PeCDF. Pathological changes induced by these compounds, seen in various NTP studies, are compared to effects reported in epidemiological investigations (Table 2). We systematically analyze information gleaned for target organ(s) and summarize toxicity and carcinogenicity reported within the various bodily systems.
Table 2.
Comparison of toxic effects and carcinogenesis of TCDD and PCBs in rodent NTP studies and human epidemiology.
| TCDD
|
PCBs
|
|||||
|---|---|---|---|---|---|---|
| Findings | Human | Rat | Mouse | PCBs Human | PCB126Rat | PeCDF Rat |
| Alimentary System Liver | ||||||
| Hepatocecellular adenoma/carcinoma/neoplastic nodules/liver cancer | Bertazzi et al., 2001 | HSDa (TR521b) OMc (TR209) Walker et al., 2005, 2006 | BCFd (TR209) | ATSDR, 2000; Golden et al., 2003; Kimbrough, 1985; McGregor et al., 1998 | HSD (TR520) Walker et al., 2005 | HSD (TR525) Walker et al., 2005 |
| Cholangioma | —f | HSD (TR521) Walker et al., 2006 | — | — | HSD (TR520) | HSD (TR525) |
| Cholangiocarcinoma/biliary duct cancer | Pesatori et al., 2003 | HSD (TR521) Walker et al., 2005, 2006 | — | ATSDR, 2000; Brown, 1987; Golden et al., 2003 | HSD (TR520) Walker et al., 2005 | HSD (TR525) Walker et al., 2005 |
| Hepatocholangioma | — | HSD (TR521) Walker et al., 2006 | — | — | HSD (TR520) | — |
| Toxic hepatopathy/toxic hepatitis/Chronic liver disease./fatal liver disease | Watanabe et al., 1999; Zober et al., 1994 | HSD (TR521) OM (TR209) Walker et al., 2006 | BCF(TR209) | ATSDR, 2000; McGregor et al., 1998; Yu et al., 1997 | HSD (TR520) | HSD (TR525) |
| Hepatocytic change (hypertrophy, multinucleated hepatocytes, fatty change, necrosis, altered foci) | ATSDR, 1998 | HSD (TR521) Walker et al., 2006 | SWe (TR201) | — | HSD (TR520) | HSD (TR525) |
| Nodular hyperplasia | — | HSD (TR521) Walker et al., 2006 | — | — | HSD (TR520) | HSD (TR525) |
| Inflammation/pigmentation/portal fibrosis | ATSDR, 1998 | HSD (TR521) Walker et al., 2006 | SW (TR201) | — | HSD (TR520) | HSD (TR525) |
| Oval cell hyperplasia/bile-duct hyperplasia/cysts | — | HSD (TR521) Walker et al., 2006 | — | — | HSD (TR520) | HSD (TR525) |
| Cholangiofibrosis | — | HSD (TR521) Walker et al., 2006 | — | — | HSD (TR520) | HSD (TR525) |
| Alimentary System Oral Cavity (gingiva), tooth | ||||||
| Squamous-cell carcinoma | —c | HSDa(TR521b) Walker et al., 2005, 2006; Yoshizawa et al., 2005a | — | — | HSD (TR520) Walker et al., 2005; Yoshizawa et al., 2005a | — |
| Squamous hyperplasia/gum swelling/gum pigmentation | — | HSD (TR521) Yoshizawa et al., 2005a | — | Asahi, 1993 ATSDR, 1998; Brouwer et al., 1998; Guo et al., 1999, 2004; Hashiguchi et al., 1995, 1997; Miller, 1985; Shimizu et al., 1992; Urabe and Asahi, 1985; Wang et al., 2003 | HSD (TR520) Yoshizawa et al., 2005a | — |
| Developmental dental aberration/broken teeth/periodontitis | Alaluusua et al., 2004 | — | — | Asahi, 1993; Brouwer et al., 1998; Guo et al., 1999, 2004; Hashiguchi et al., 1997; Miller, 1985; Shimizu et al., 1992; Wang et al., 2003 | — | — |
| Exocrine pancreas | ||||||
| Acinar adenoma/carcinoma/pancreatic cancer | — | HSD (TR521) Nyska et al 2004; Yoshizawa et al., 2005b; Walker et al., 2006 | — | ATSDR, 2000 Golden et al., 2003; Yassi et al., 1994 | — | HSD (TR525) Nyska et al., 2004 |
| Acinal changes (vacuolization, atropy)/inflammation | — | HSD (TR521) Nyska et al., 2004; Yoshizawa et al., 2005b; Walker et al., 2006 | — | — | HSD (TR520) Nyska et al., 2004 | HSD (TR525) Nyska et al., 2004 |
| Intestine | ||||||
| Gastrointestinal cancer/rectal cancer | Bertazzi et al., 2001 Pesatori et al., 2003 | — | — | ATSDR, 2000 Brown, 1987; Kimbrough, 1985 | — | — |
| Gastrointestinal signs | Geusau et al., 2001 | — | — | ATSDR, 2000 | — | — |
| Appendicitis | Zober et al., 1994 | — | — | — | — | — |
| Alimentary System Stomach | —e | — | — | — | — | — |
| Squamous hyperplasia (forestomach) | — | HSDa (TR521b) Walker et al., 2006 | — | — | — | HSD (TR525) |
| Cardiovascular System Heart | ||||||
| Cardiomyopathy/chronic circulatory diseases/ischemic heart disease/hypertension | ATSDR, 1998; Bertazzi et al., 2001; Pesatori et al., 2003; Steenland et al., 1999; Watanabe et al., 1999 | HSD (TR521) Walker et al., 2006 | — | ATSDR, 2000 | HSD (TR520) | HSD (TR525) |
| Endocrine System Adrenal cortex | ||||||
| Adenoma/carcinoma | — | — | — | — | HSD (TR520) | — |
| Hyperplasia | — | HSD (TR521) Walker et al., 2006 | — | — | — | — |
| Vacuolization | — | — | — | — | HSD (TR520) | — |
| Atrophy | — | HSD (TR521) Walker et al., 2006 | — | — | HSD (TR520) | — |
| Cystic degeneration | — | — | — | — | — | HSD (TR525) |
| Thyroid | ||||||
| Follicular-cell adenoma/carcinoma | Pesatori et al., 2003 | OMc (TR 209) | BCFd (TR209) | — | — | — |
| Follicular-cell hypertrophy/thyroid-related hormone changes (T4, T3, TSH)/thyroid disease/goiter/hyperthyroidism | ATSDR, 1998; Zober et al., 1994 | HSD (TR521) Tani et al., 2004; Walker et al., 2006 | — | ATSDR, 2000; Guo et al., 1999 | HSD (TR520) | HSD (TR525) |
| Endocrine pancreas (islets) | ||||||
| Diabetes/glucose intolerance | ATSDR, 1998; Bertazzi et al., 2001; Pesatori et al., 2003 | — | — | McGregor et al., 1998 | — | — |
| Genital System (Female) Clitoral gland | ||||||
| Cystic ducts | —d | HSDa (TR521b) Walker et al., 2006 | — | — | HSD (TR520) | — |
| Uterus Squamous-cell carcinoma | — | HSD (TR521) Walker et al., 2006 | — | — | HSD (TR520) | HSD (TR525) |
| Endometrial cyctic hyperplasia | — | — | — | — | — | HSD (TR525) |
| Inflammation | — | — | — | — | — | HSD (TR525) |
| Squamous metaplasia | — | — | — | — | — | HSD (TR525) |
| Endometriosis | Eskenazi et al., 2002; Mayani et al., 1997 | — | — | Arisawa et al., 2005; Gerhard et al., 1999; Louis et al., 2005 | — | — |
| Menstrual cycle changes | — | — | — | ATSDR, 2000 | — | — |
| Genital System (Male) | ||||||
| Hormonal changes (testosterone, FSH, LH) | ATSDR, 1998; Egeland et al., 1994 | — | — | — | — | — |
| Alterations in sexual development/shorter penis length (infant) | — | — | — | Brouwer et al., 1998; Guo et al., 2004 | — | — |
| Immune and Hematopoietic System | ||||||
| Hodgkin’s disease/non-Hodgkin’s Lymphoma/acute leukemia/myeloid leukemia/multiple myeloma/lymphaemopoietic cancer/lymphoma/leukemia | ATSDR, 1998; Bertazzi et al., 2001; Buckley et al., 1989; Huff et al., 1994; Mannetje et al., 2004; Pesatori et al., 2003; Rix et al., 1998; Viel et al., 2000 | — | BCF c (TR209) | ATSDR, 2000 Golden et al., 2003 Kimbrough et al., 1985 | — | — |
| Immunosuppressant effects | ATSDR, 1998; Halperin et al., 1998; Watanabe et al., 1999 | — | — | ATSDR, 2000 McGregor et al., 1998; Weisglas-Kuperus et al., 2000, 2004 | — | — |
| Infectious diseases/parasitic diseases/otitis media due to immunosuppression | Zober et al., 1994 | — | — | Weisglas-Kuperus et al., 2004 ATSDR, 2000 Guo et al., 2004 | — | — |
| Immune and Hematopoietic System Thymus | ||||||
| Atrophy | —d | HSDa (TR521b) Walker et al., 2006 | — | — | HSD (TR520) | HSD (TR525) |
| Spleen | ||||||
| Lymphoid follicular atrophy | — | — | — | — | HSD (TR520) | — |
| Integumentary System Skin, mammary gland | ||||||
| Fibrosarcoma | — | — | SW c(TR201) | — | — | |
| Malignant melanoma | Akhtar et al., 2005 | — | — | ATSDR, 2000; Golden et al., 2003 | — | — |
| Breast cancer | Birnbaum and Fenton, 2003; Huff et al., 1994 | — | — | ATSDR, 2000; Kimbrough, 1985 | — | — |
| Porphyria/cutanea tarda/hypertrichosis/hirsutism/hyperpigmentation | ATSDR, 1998; Wantanabe et al., 1999 | — | — | Asahi, 1993; ATSDR, 2000; Guo et al., 1999, 2004; Kimbrough, 1985; Miller, 1985; Urabe et al, 1985 | — | — |
| Choloracne/squamous metaplasia & keratinization of sebaceous glands & hair follicles/hypertrophy of Meibomian gland | ATSDR, 1998; Geusau et al., 2001; Pesatori et al., 2003; Zober et al., 1994, 1997/1998; Wantanabe et al., 1999 | — | — | Asahi, 1993; ATSDR, 2000; Guo et al., 1999, 2004; McGregor et al., 1998; Miller, 1985; Urabe et al, 1985 | — | — |
| Nervous System | ||||||
| Neurological disorders (peripheral nervous signs, lower limb weakness, muscle pains, sleepiness, headaches, dizziness, nausea) | ATSDR, 1998; Zober et al., 1994, 1997/1998; Wantanabe et al., 1999 | — | — | — | — | — |
| Delay in neurodevelopment/neurobehavioral changes (hypotony, hyperactivity, lower mean intelligence quotients, altered latencies and amplitudes of auditory event-related potentials)/mental disorders (infant/in utero exposure) | — | — | — | ATSDR, 2000; Brouwer et al., 1998; Carpenter, 2006; Guo et al., 2004 | — | — |
| Respiratory System Lung | ||||||
| Cystic keratinizing epithelioma of lung/lung cancer | ATSDR, 1998; Bertazzi et al., 2001; Fingerhut et al., 1991; Huff et al., 1994; McGregor et al., 1998; Pesatori et al., 2003 | HSDa (TR 521b) Walker et al., 2005, 2006 | — | Pavuk et al., 2004 | Brix et al., 2004 HSD (TR 520) Walker et al., 2005, 2006 | HSD (TR 525) Walker et al., 2005, 2006 |
| Metaplasia of lung alveolar epithelium (bronchiolar, squamous) | —c | HSD (TR521) | — | — | Brix et al., 2004 HSD (TR520) Walker et al., 2006 | HSD (TR525) Walker et al., 2006 |
| Chronic (obstructive) respiratory diseases/upper respiratory tract infections/bronchitis, laryngitis/hemorrhagic pleuritis | ATSDR, 1998; Bertazzi et al., 2001; Wantanabe et al., 1999; Zober et al., 1994 | — | — | Asahi, 1993; ATSDR, 2000; Brouwer et al., 1998; Guo et al., 2004 | — | — |
| Urinary System Kidney | ||||||
| Nephropathy Skeletal System | — | HSD (TR521) | — | — | HSD (TR520) | HSD (TR525) |
| Bone & Joint | ||||||
| Arthritis/herniated disks | — | — | — | Guo et al., 1999 Kuratsune, 1980 | — | — |
| Others | ||||||
| Soft-tissue sarcomas | ATSDR, 1998; Fingerhut et al., 1991; Huff et al., 1994; McGregor et al., 1998; Pesatori et al., 2003; Rix et al., 1998; Viel et al., 2000 | — | — | — | — | — |
Harlan Sprague-Dawley rats.
National Toxicology Program (NTP) study report number.
Osborne Mendel rats.
B6C3F1 mice,
Swiss-Webster mice.
No available information published.
Harlan Sprague Dawley rats.
National Toxicology Program (NTP) study report number.
No available information published.
Harlan Sprague-Dawley rats.
National Toxicology Program (NTP) study report number.
Osborne Mendel rats.
B6C3F1 mice.
No available information published.
Harlan Sprague-Dawley rats.
National Toxicology Program (NTP) study report number.
B6C3F1 mice.
No available information published.
Harlan Sprague-Dawley rats.
National Toxicology Program (NTP) study report number.
Swiss-Webster mice.
No available information published.
Harlan Sprague Dawley rats.
National Toxicology Program (NTP) study report number.
No available information published.
Alimentary System
Liver
Several case reports of hepatomegaly, alterations in hepatic enzymes, hepatitis, and/or chronic liver disease have been reported among human populations exposed accidentally and occupationally to TCDD and PCBs, including those in the BASF, Seveso, Yusho, and Yucheng accidents (Calvert et al., 1992; McGregor et al., 1998; Yu et al., 1997; Zober et al., 1994, 1997/1998); these are summarized in Table 2. Changes in liver function and structure and increased liver size have consistently been reported in murine investigations (ATSDR, 1998; ATSDR, 2000). In the NTP studies of TCDD, PCB126, and PeCDF, the incidences of several kinds of hepatocellular alterations were increased, such as toxic hepatopathy, hepatocellular hypertrophy, multinucleated hepatocytes, fatty change, necrosis, altered foci, nodular hyperplasia, inflammation, pigmentation, portal fibrosis, bile-duct changes (bile-duct hyperplasia, cysts, cholangiofibrosis) and/or oval-cell hyperplasia (Hailey et al., 2005; NTP, 2004a, b, c). The incidences of hepatocellular adenoma, carcinoma, cholangioma, cholangicarcinoma, and/or hepatocholangiocarcinoma were increased in these NTP rodent studies (Hailey et al., 2005; NTP, 1982b, 2004a, b, c; Walker et al., 2006). In epidemiologic studies of human cohorts exposed to PCBs, liver/biliary/gallbladder cancer was observed only in one study (Brown et al., 1987) in which the data failed to demonstrate a dose-response relationship. Twelve other investigations of workers exposed to PCBs at equivalent levels failed to detect an elevated risk of liver/biliary/gallbladder cancer at any exposure level (Golden et al., 2003); however, increases in hepatobiliary cancers have been observed in residents exposed to TCDD during the Seveso Accident (ATSDR, 1998; Pesatori et al., 2003).
Oral cavity and tooth
Earlier studies revealed that teeth in both mouse and rat are sensitive to TCDD and PCBs during development; broken teeth, periodontitis, gum pigmentation, and other dental aberrations were reported (Alaluusua et al., 1993; Kattainen et al., 2001; Kiukkonen et al., 2002; Lukinmaa et al., 2001; Miettinen et al., 2002; Partanen et al., 1998). Human studies revealed that childhood exposure causes dental aberrations and lesions and gingival squamous hyperplasia; they correlated with animal studies showing evidence of dental sensitivity to TCDD and PCBs (Table 2; Alaluusua et al., 2004; Guo et al., 1999, 2004; Hashiguchi et al., 1997). By comparison, following chronic exposure of adult SD rats to TCDD and PCB126 in the NTP studies, the incidences of gingival squamous hyperplasia and/or squamous-cell carcinoma in the oral cavity increased significantly (NTP, 2004a). These results indicate that dioxin and DLCs target the gingiva of the oral cavity, in particular the junctional epithelium of molars (Yoshizawa et al., 2005a). One hypothesis asserts that, during chronic exposure, the action of dioxin and DLCs involves a disruption of retinoid action leading to altered growth and differentiation of the oral gingival epithelium that results in development of gingival squamous hyperplasia and, ultimately, neoplasia (Yoshizawa et al., 2005a). Dental lesions like those seen in developmental-exposure studies were not noted in the NTP chronic studies of TCDD and PCB126.
Exocrine pancreas
Although many comparative studies of toxicities induced by dioxins in animals and humans have been published, exocrine pancreatic toxicity has not been entirely clarified in humans (Table 2). Pancreatic cancer was increased in workers exposed to PCBs at a transformer manufacturing plant in Canada (ATSDR, 2000; Yassi et al., 1994). No statistically significant increased mortality resulted from pancreatic cancer in the Seveso (Bertazzi et al., 2001), Yusho (Kuratsune et al., 1987), and Yucheng (Guo et al., 1999) accidents. In the NTP studies of TCDD, PCB126, and PeCDF, acinar-cell vacuolation, atrophy, inflammation, and/or arteritis developed at a high incidence (NTP, 2004a; Nyska et al., 2005). Rare occurrences of pancreatic acinar-cell adenomas and carcinomas were noted. These acinar-cell lesions might be related to a direct effect of TCDD on the pancreas. The increase in cytochrome-P450 1A1 and decrease in cholecystokinin-receptor expressions in vacuolated acinar cells may be involved in the pathogenesis of pancreatic lesions and tumors by initiating proliferation (Yoshizawa et al., 2005b).
Stomach and intestine
A retrospective study of BASF employees exposed to TCDD after a 1953 incident involving a chemical reactor revealed a high incidence of appendicitis (Zober et al., 1994). Clinical observations suggestive of gastrointestinal damage have been reported in workers exposed to airborne PCBs and those individuals in the Yusho accident exposed to PCBs (ATSDR, 2000). Follow-up studies of the Seveso accident that caused TCDD exposure showed an increased incidence of rectal cancer (Bertazzi et al., 2001; Pesatori et al., 2003). An elevation in rectal cancer was found in capacitor-manufacturing plants in the United States, but follow-up evaluation of 2588 total workers after 7 years of observation detected no additional deaths from this cancer (Brown, 1987). In the NTP study of TCDD (Table 2), the incidence of squamous-cell hyperplasia in the forestomach was increased, probably due to the disruption of retinoid metabolism (Van Birgelen et al., 1995). No intestinal tumors have been induced by TCDD and PCBs in the rodent models.
Cardiovascular System
Suggestive but inconclusive evidence has implicated adverse cardiovascular effects in humans exposed to relative high concentrations of dioxins (ATSDR, 1998). Increased deaths from chronic heart disease were observed among the Seveso cohort and in the medical study conducted by the National Institute for Occupational Safety and Health (NIOSH) (Bertazzi et al., 2001; Pesatori et al., 2003). Several occupational-exposure studies have investigated the possible relationship(s) between PCB exposure and increased risk of cardiovascular disease or altered blood pressure (ATSDR, 2000).
Dioxins have been found to produce effects on the cardiovascular system in the rodent models (Table 2). One study reported that rats treated with TCDD showed hemorrhages in the brain as well as the spinal cord, and an increase occurred in the incidence of mesenteric and thoracic periarteritis accompanied by thrombosis, above background levels. In the same study, with females only, the incidence of myocardial degeneration also increased above background (Kociba et al., 1978). In C57B1/6J mice, treatment with dioxins created a high mean tail-cuff blood pressure and an elevated urinary excretion of vasoactive eicosanoids (Dalton et al., 2001). When ApoE (−/−) mice were exposed to dioxins, a quicker progression occurred towards the creation of atherosclerotic lesions (Dalton et al., 2001). NTP investigations in the Harlan SD rats showed increased cardiomyopathy and chronic active arteritis, seen mostly in the mesentery and pancreas of rats dosed with TCDD, PCB 126, and PeCDF (Jokinen et al., 2003; NTP, 2004a,b,c). The cardiomyopathy and arteritis observed were similar to some lesions studied in humans (Jokinen et al., 2003). For example, upon observation of humans with idiopathic or toxic cardiomyopathy, multiple foci of myocardial fibrosis could be distinguished (Schoen et al., 1994). Rat arteritis documented in the NTP studies (NTP, 2004a; Jokinen et al., 2003) was best compared to spontaneous polyarteritis nodosa seen in humans (Schoen et al., 1994).
Endocrine System
Adrenal cortex
TCDD and PCBs have been shown to accumulate in the adrenal glands when incorporated into the body (Li and Wang, 2005). Because adrenal steroids exert profound influences on glucose tolerance, insulin sensitivity, lipid metabolism, obesity, vascular function, and cardiac remodeling, the potential association exists of adrenocortical alterations with increased diabetic and cardiovascular risk among highly exposed people (Li and Wang, 2005). In the NTP studies of TCDD, PCB126, and PeCDF (Table 2), increased incidences of adrenal cortical atrophy, cytoplasmic vacuolation, cystic degeneration, hyperplasia and/or tumors occurred (NTP, 2004a). Cortical atrophy and cytoplasmic vacuolation constituted prominent effects and may reflect continued stress in these rats, leading to depletion of corticosteroid hormones or activation of some other unknown mechanism(s) (Frith et al., 2000; Sapolsky et al., 1987). Hormonal manipulation can be used to induce cortical adenoma (Cohen et al., 1957). Cystic degeneration is considered to be a continuum of cytoplasmic vacuolation; in particular, severe cell loss appears with the resultant formation of cystic spaces. This lesion occurs predominantly in aging female, particularly Sprague-Dawley (SD), rats (Frith et al., 2000). Administration of estrogen in several forms was found to cause both benign and malignant neoplasms (Dunning et al., 1953). Clarification is needed to determine whether effects of estrogenic activity and/or antiestrogenic activity of TCDD and DLCs are related to the induction of cystic degeneration and adrenal carcinogenesis.
Thyroid gland
In our literature review, we examined effects of PCBs and dioxin on the thyroid gland, such as increases in TSH, T3, and/or T4 in various populations, including men, women, children, infants, and, particularly, veterans of the Vietnam War (Table 2; ATSDR, 2000; Pavuk et al., 2003; Zober et al., 1994, 1997/1998). An increased prevalence of combined thyroid disorders or diseases (goiter, thyrotoxicosis, hypothyroidism, and thyroid adenoma) was reported in German and American workers (ATSDR, 2000; Zober et al., 1994, 1997/1998). Overall, results of studies that examined dioxin and thyroid function have provided little and inconsistent evidence of long-term dioxin effects on the thyroid in humans. An increase in thyroid tumors was observed in a large cohort of workers exposed to chlorophenoxy herbicides, including those contaminated with TCDD (Saracci et al., 1991). Moreover, a possible association between exposure to dioxin and thyroid tumors has been implicated by epidemiological investigations related to the Seveso accident (Pesetori et al., 2003).
TCDD and PCBs are thought to disrupt thyroid hormonal homeostasis in the endocrine system by the induction of phase II enzymes, the UDP-glucuronosyltransferase(UGT)s (Kohn, 2000; Kohn et al., 1996; Schuur et al., 1997; Sewell et al., 1995; van Birgelen et al., 1995; van der Plas et al., 2001). The manufacture and discharge of thyroxine (T4) are under the direction of the thyroid-stimulating hormone (TSH) (Tani et al., 2004), which receives both negative and positive control from the hypothalamus, pituitary, and thyroid by the thyrothropin-releasing hormone, TSH itself, T4, and triiodothyronine (Tani et al., 2004). TCDD induces the production of UGT-1 mRNA through an aryl hydrocarbon receptor-dependent transcriptional mechanism (Bock et al., 1998; Yuch et al., 2003). A decrease in the serum T4 levels brought about by TCDD-induced UGT could possibly lead to a decline in the negative feedback inhibition on the pituitary gland, which would cause an increase in TSH resulting in hyperstimulation of the thyroid follicular cells (Sewall et al., 1995). Follicular hypertrophy induced by TCDD and PCBs in SD rats is characterized by smaller follicles and increased height of lining epithelial cells with no evidence of hyperplasia (Tani et al., 2004). Several studies have revealed associations among TCDD, T4, and thyroxine-binding globulin (TBG) and documented thyroid disease in humans following exposure (Zober et al., 1994).
Endocrine pancreas
Evidence exists that environmental exposure to TCDD and PCBs contributes to the incidence of diabetes (ATSDR 1998; McGregor et al., 1998; Steenland et al., 1999). A highly significant relationship between dioxin exposure and the severity of diabetes was noted in the United States Air Force personnel who dropped Agent Orange contaminated with dioxin during the Vietnam War (Henriksen et al., 1997). Epidemiologic analysis of the Seveso accident also showed a significantly higher incidence of diabetes in exposed individuals (Bertazzi et al., 2001; Pesatori et al., 2003). In the present NTP studies of TCDD, PCB126, and PeCDF (Table 2), no related lesions in endocrine pancreas were noted (NTP, 2004a). Discrepancy exists among reports of exocrine and endocrine pathological effects in rats and humans, including the diabetic condition; however, potential long-term functional disturbances in the glucose metabolism in rats exposed to these compounds should be further investigated.
Female Genital System
In the previous NTP investigations using SD rats, the range of treatment-related changes in the uterine system comprised active inflammation, cystic endometrial hyperplasia, squamous metaplasia, and uterine carcinoma in the PeCDF study and squamous-cell carcinoma of the uterus in the TCDD study (Table 2). The span of alterations seen with the different compounds may suggest more than one mechanism, such as estrogenic activity and/or antiestrogenic activity of PeCDF and TCDD, as well as local disruption of retinoid homeostasis via vitamin A deficiency. TCDD and DLCs were documented as inducers of squamous metaplasia and squamous-cell carcinoma in other organs, including the oral cavity and lung (Walker et al, 2006; Yoshizawa et al., 2005b). Evidence shows that TCDD and DLCs can increase the incidence and severity of endometriosis in monkeys (Rier and Foster, 2002; Rier et al., 1993, 2001) and promote the growth or survival of endometrial tissue implanted in a surgically-induced murine model of endometriosis (Cummings et al., 1996, 1999; Johnson et al., 1997). The inappropriate and uncontrolled growth of endometrial cells, normally confined to the lining of the uterus, outside the womb (Birnbaum and Cummings, 2002), leads to endometriosis. In the present NTP rat studies of TCDD and DLCs, no incidences of endometriosis were noted. Although studies with sufficient statistical powers have been lacking, and non-differential misclassifications of disease status have hindered clarification, a potential connection exists between exposure to TCDD and DLCs and the increasing prevalence of endometriosis in humans, as cohort and clinical studies conducted in Israel, Italy, and the United States have demonstrated (Arisawa et al., 2005; Eskenazi et al., 2000, 2002; Louis et al., 2005). In the Seveso Women’s Health Study, the first investigation to explore the relation between TCDD exposure and endometriosis in a large population of women with a wide range of exposures, women with serum TCDD levels of 100 pg/ml (ppt) or higher showed a doubling, but stastistically insignificant, risk for endometriosis without evidence of any dose-response relationship (Eskenazi et al., 2002). Moreover, the results of an epidemiologic study in Belgium revealed no statistically significant association between exposure to DLCs and the occurrence of endometriosis in infertile women (Pauwels et al., 2001). Clear evidence of a role for dioxins in the increased incidences of endometriosis will require careful cohort studies of women with and without endometriosis, documenting exact exposures.
Male Genital System
TCDD has been responsible for altering testosterone, follicle-stimulating hormone, and luteinizing hormone in the male genital system; existing and half-life dioxin levels present within the serum have been positively associated with luteinizing hormone and inversely correlated to testosterone (Egeland et al., 1994). Essential for the continuance of libido, testosterone is responsible for the commencement and safeguarding of spermatogenesis; elevated levels of luteinizing hormone and follicle-stimulating hormone can cause low sperm counts (Morrow et al., 1986). In comparison to research observed in animals, human investigations have shown that dioxin manipulates the hypothalamic-pituitary axis as well as testosterone production (Egeland et al., 1994). In a study in rats, dioxin reduced testosterone levels (Mebus et al., 1987; Moore and Peterson, 1988). No evidence exists that decline in testosterone is a consequence of an augmentation in testosterone catabolism or secretion (Kleeman et al., 1990). On the other hand, proof has shown that dioxin reduces synthesis of testosterone (Kleeman et al., 1990). Research in rats indicated that dioxin has the ability to slow the receptiveness of the pituitary gland to testosterone and Ley-dig cells to stimulation of luteinizing hormone (Bookstaff et al., 1990; Moore et al., 1992). Data gathered from a human study suggested a similar pathway of dioxin activity due to weakened pituitary and Leydig-cell receptiveness (Egeland et al., 1994). No toxicologic data from the male reproductive system were available from the NTP studies (NTP, 1982a)
Immune and Hematopoietic Systems
The effects of TCDD and PCBs on immunotoxicity and hematopoietic toxicity in rodents, seen in the NTP studies, and humans are summarized in Table 2.
In the NTP studies of TCDD, PCB126, and/or PeCDF, thymic and/or splenic lymphoid atrophy was noted as an immunosuppressant effect (Table 2). Lymphocytes are known to be one of the primary targets of TCDD and PCB immunotoxicity via AhR (Halperin et al., 1998; Kerkvliet, 2002; Watanabe et al., 1999). Both humoral and cell-mediated immunity were suppressed following acute or chronic exposure of rodents to low levels of TCDD. Data from several host-resistance models indicated that TCDD increased the susceptibility of animals to a variety of infectious diseases, prevented the rejection of transplanted tumors, and increased tumor growth and metastasis, presumably through alteration in immune function. The greater sensitivity of lymphocytes in humans than that in mice and rats seen in an in vitro study of CYP1A1 induction suggested higher susceptibility of the human immune system to TCDD (Nohara et al., 2006). As exposure to PCBs can suppress both antibodies (immunoglobulins) and the cellular immune response, frequent infections can be a direct result (Carpenter, 2006). Studies have clearly shown that persons exposed to TCDD and PCBs exhibit a greater incidence of numerous kinds of infections, including those of the respiratory tract, skin, and ear (ATSDR, 1998, 2000; Carpenter, 2006; Guo et al., 2004; Weisglas-Kuperus et al., 2000, 2004; Zober et al., 1994).
An excess of lymphohemopoietic neoplasms was found in the Seveso population and several cohort studies, as shown in Table 2–4; the higher risk of Hodgkin’s disease, non-Hodgkin’s lymphoma, and myeloid leukemia was noted (ATSDR, 1998, 2000; Bertazzi et al., 2001; Pesatori et al., 2003). A case-control study nested in the IARC international cohort provided weak evidence of a dose-response relationship with estimated TCDD exposures (IARC, 1997). In the previous NTP rodent studies, although positive dose-related trends were observed in the incidences of either lymphoma or leukemia in female B6C3F1 mice, TCDD has not been carcinogenic for the lymphohematopoietic system (NTP, 1982b).
Integumentary System
Chloracne and skin hyperpigmentation caused by TCDD and DLCs constitute well known side effects in several dioxin-exposure accidents (Table 2), such as those at Seveso, Yusho, and Yu-Cheng (Guo et al., 1999; 2004; Schecter et al., 2006; Urabe and Asahi, 1985). Recently, a notorious event occurred when Ukraine President Viktor Yushchenko was stricken with facial chloracne resulting from deliberate poisoning with TCDD during his presidential campaign (Holt, 2005; Schecter et al.,2006; Sterling and Hanke, 2005).
In order to elucidate the effects of TCDD on the integumentary system, researchers used rhesus monkeys to study morphological changes that appeared during dosing with 500 ppt of TCDD for nine months. Upon necropsy, squamous metaplasia and keratinization were seen within the sebaceous glands and hair follicles. Keratinization was also observed in the Meibomian glands of the eyelids. Thickening of the fingernails and toenails was reported (Allen et al., 1977).
In the rodent NTP studies, TCDD was carcinogenic for female Swiss-Webster mice causing fibrosarcomas in the integumentary system (Table 2; NTP, 1982a). Few reports have documented increased incidences of fibrosarcoma in dioxin and PCB human epidemiologic studies (ATSDR, 1998, 2000). Significant associations have been reported between the occurrence of malignant melanoma and PCB exposure in humans (Golden et al., 2003). The incidence of melanoma was reportedly increased, relative to national rates, among white ranch-hand veterans who sprayed TCDD-contaminated herbicides in Vietnam. Researchers know that genetic susceptibility and environmental factors contribute to the initiation and progression of melanoma. Excessive ultraviolet exposure is considered the main etiological factor in its initiation; however, epidemiological and experimental evidence has suggested that exposure to environmental carcinogens contributes to the development of melanoma. Exposure to environmental chemicals, especially TCDD, activates the aryl-hydrocarbon-receptor pathway and contributes to melanoma progression, specifically through stimulation of the expression and activity of the matrix metalloproteinases (Villano et al., 2006).
While many small clinic-based breast cancer studies have been published (Table 2), some of which suggest a dose-dependent relation to total PCB exposure (ATSDR, 2000; Golden et al., 2003), most large retrospective epidemiologic investigations have not demonstrated a relationship between elevated total PCB levels and breast cancer. While the human data of the relationship between TCDD exposure and the occurrence of breast cancer have not been clarified, an earlier epidemiologic study of the Seveso population suggested a possible insignificant decrease in the incidence of breast cancer (Huff et al., 1994). A subsequent epidemiologic investigation demonstrated a significant increase in breast cancer in association with elevated serum levels of TCDD (Birnbaum and Fenton, 2003). Due to its antiestrogenic properties, TCDD inhibited spontaneous and carcinogen-induced mammary tumor formation and growth in rodent models (Safe et al., 1999).
Nervous System
In utero exposure and exposure of infants and young children to PCBs, including those reported in the Yucheng accident, have been linked to adverse effects on intellectual functioning, (ATSDR, 2000; Carpenter, 2006; Guo et al., 2004); delay in neurodevelopment, neurobehavioral changes (hypotony, kyperactivity, lower mean intelligence quotients, altered latencies, and amplitudes of auditory event-related potentials), and mental disorders were documented. Similar changes in cognitive and motor behavior have been found in mice and rats following single-dose exposure and monkey offspring born to mothers chronically exposed during pregnancy (Brouwer et al., 1998). Symptoms of neurological disorders, such as peripheral nervous signs, lower limb weakness, muscle pains, sleepiness, headaches, dizziness, and nausea were also reported in some adult epidemiologic studies of TCDD (ATSDR, 1998; Watanabe et al., 1999; Zober et al., 1994, 1997/1998). Relatively little information is available on neurological effects of PCBs in adult humans (ATSDR, 2000). In the rodent NTP studies of TCDD, PCB126, and PeCDF, no clinical neurobehavioral changes and no neurological histopathological changes were seen (Hill et al., 2003; NTP, 1982a,b, 2004a,b,c)
Respiratory System
The respiratory system has exhibited effects induced by TCDD and PCBs, which are summarized in Table 2. The risks of pulmonary cancer were found to be increased during 20 years that passed after 1976, when numerous people were exposed accidentally to TCDD at a chemical plant in Seveso, Italy (Bertazzi et al., 2001; Pesatori et al., 2003). Additional documentation indicated pulmonary diseases and cancers following exposure to TCDD during the accident that occurred in 1953 at BASF, also a chemical company (Zober et al., 1994, 1997/1998). Not only has TCDD caused pulmonary diseases, but PCBs have also increased the risks of pulmonary diseases and cancers (ATSDR, 2000). For example, the Yusho accident in Japan and the Yucheng accident in Taiwan caused pulmonary diseases and cancers due to the contamination of rice oil with PCBs (ATSDR, 2000; Kikuchi, 1984; Nakanishi and Shigematsu, 1991); in both accidents more frequent or severe respiratory infections and chronic bronchitis accompanied by persistent cough and production of sputum were noted (ATSDR, 2000). The NTP animal study showed that treatment with TCDD, PCB 126, PeCDF, and the mixture of all three induced several forms of pulmonary diseases, such as cystic keratinizing epithelioma, bronchiolar metaplasia, and squamous metaplasia of the alveolar epithelium. Potential mechanisms leading to altered differentiation and/or proliferation of bronchiolar and alveolar epithelia might have operated via induction of CYP1A1 or disruption of retinoid metabolism; a significant toxicity to humans of TCDD, PCB 126, PeCDF, and the mixture of all three was likely indicated.
Urinary System
In humans, although abnormal urinary porphyrin patterns have been reported, few publications have implicated dioxin-induced renal effects. The porphyrogenic effect of TCDD was reported in female rats treated chronically for 45 weeks with 0.01, 0.10, and 1.00 μg/kg TCDD; the dose of 0.01 μg/kg/week resulted in significantly enhanced coproporphyrinuria after 3 months and at 10 months with a significant rise in uroporphyrin (Cantoni et al., 1981). Additionally, the previous two-year rat study showed increased urinary excretion of porphyrins in female rats exposed to 0.10 μg/kg TCDD (Kociba et al., 1978). In the present NTP studies of TCDD, PCB126, and PeCDF, the incidences and/or grade levels of nephropathy were increased (Table 2; NTP, 2004a,b,c). In humans, mechanisms and extrapolations have not been clarified; the kidney historically has not been a target of TCDD-induced neoplasia, although direct responsiveness to TCDD is known to induce renal biochemical alterations (Pegg et al., 1976; Walker et al., 1995).
Skeletal System
In the 14-year follow-up study of the Yucheng Accident (Guo et al., 1999), the incidences of arthritis and herniated disks were found to be higher in the exposed population than the control population (Table 2). In persons exposed in the Yusho accident, an elevated incidence of joint inflammation was documented (Kuratsune, 1980). Rheumatoid arthritis is characterized by proliferation of synoviocytes that produce the critical proinflammatory cytokine, IL-1beta; TCDD was shown to upregulate, via AhR, the mRNA level of IL-1beta in the human fibroblast-like line of synoviocytes (Tamaki et al., 2006). In Lewis rats, TCDD inhibited bone growth, bone modeling, and mechanical strength of bone in a dose-dependent manner (Jamsa et al., 2001) without a direct effect on increased osteoclastic bone resorption (Ilvesaro et al., 2005). No reports have been published, however, of TCDD-and DLC-induced skeletal lesions in the present NTP studies of TCDD, PCB126, and PeCDF (Table 2; NTP, 1982a,b, 2004a,b,c).
Other Systems
TCDD also has been linked to an increase in cancer mortality. In one human study, the subjects worked in twelve plants within the United States that manufactured chemicals contaminated with TCDD. Serum TCDD levels were two to three times higher than those observed in the general public. A comparison of the human study to animal research revealed that these levels were similar to those that produced cancer in animals (Steenland et al., 1999). A comparable increase in soft-tissue sarcoma was present in the Seveso population and the TCDD-exposed manufacturing workers in the United States (Fingerhut et al., 1991; Frumkin, 2003); however, no such increase was found in the other cohort studies (IARC, 1997; McGregor et al., 1998). Investigations of Vietnam veterans have not demonstrated any increase in soft-tissue sarcoma (Frumkin, 2003). Additionally, several reevaluations of occupational and accidental exposures to PCBs have not shown any excess in overall cancer mortality or mortality emanating from specific cancer sites (Bosetti et al., 2003). An excessive risk for soft-tissue sarcoma in humans, based on a small number of deaths, has been reported (Table 2–6) (ATSDR, 1998). Data for incidences of soft-tissue sarcoma were generally not available (IARC, 1997); however, no reports have been published of TCDD- and DLC-induced soft-tissue sarcoma in the present NTP studies of TCDD, PCB126, and PeCDF (Table 2–6; NTP, 1982a,b, 2004a,b,c).
Conclusions
By altering the functioning of many different organ systems, documented in rodent studies and human populations, exposures to TCDD and PCBs constitute risk factors for a large number of human diseases. In studies of human populations exposed to PCBs, exposure is to a mixtures of PCBs that includes both dioxin-like PCBs (e.g. PCB126 and PCB118) and non-dioxin like compounds (e.g. PCB153). We have summarized findings from the NTP studies and investigations reported by numerous other researchers and shown comparisons between effects manifested in rodents and humans. In the NTP murine studies, TCDD, PCB126, and/or PeCDF induced neoplastic lesions (hepatocellular and/or cholangiolar, pancreatic, thyroidal, and pulmonary) and nonneoplastic effects (hepatocellular and/or cholangiolar lesions in the liver, squamous hyperplasia in the oral cavity, cardiovascular damage, thyroidal hypertrophy, and immunosuppression) similar to those reported in humans. While differences in specific pathologies were observed, clear consistency could be seen in the target organs affected (liver, oral cavity, cardiovascular system, immune system, thyroid, pancreas, and lung) in both human and murine studies.
Acknowledgments
The authors thank all involved in the design and conduct of these NTP studies, with special appreciation expressed to Drs. John Bucher, Angelique Braen, and Milton Hejtmancik and Ms. Denise Orzech. We gratefully acknowledge Dr. Amy Brix, Dr. Susan Elmore, and Ms. JoAnne Johnson for critical review of the manuscript. The authors declare that they have no competing financial interests. This research was supported by the Intramural Research Program of the National Institutes of Health, NIEHS, Research Triangle Park, NC.
Abbreviations
- AhR
aryl hydrocarbon receptor
- ATSDR
Agency for Toxic Substances and Disease Registry
- CYP
cytochrome P450
- DLC
dioxin-like compound
- IARC
International Agency for Research on Cancer
- NTP
National Toxicology Program
- PCB 126
3,3′,4,4′5-pentachlorobiphenyl
- PCDD
polychlorinated dibenzodioxin
- PCDF
poly-chlorinated dibenzofuran
- PeCDF
2,3,4,7,8-pentachlorodibenzofuran
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TBG
thyroxine-binding globulin
- UGT
UDP-glucuronosyltransferases
References
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for chlorinated dibenzo-p-dioxins. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 1998. [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for polychlorinated biphenyls (PCBs) U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2000. [PubMed] [Google Scholar]
- Akhtar FZ, Garabrant DH, Ketchum NS, Michalek JE. Cancer in US Air Force of the Vietnam War. J Occup Environ Med. 2005;46:123–36. doi: 10.1097/01.jom.0000111603.84316.0f. [DOI] [PubMed] [Google Scholar]
- Alaluusua S, Calderara P, Gerthoux PM, Lukinmaa PL, Kovero O, Needham L, Patterson DG, Jr, Tuomisto J, Mocarelli P. Developmental dental aberrations after the dioxin accident in Seveso. Environ Health Perspect. 2004;112:1313–8. doi: 10.1289/ehp.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaluusua S, Lukinmaa PL, Pohjanvirta R, Unkila M, Tuomisto J. Exposure to 2,3,7,8-tetrachlorodibenzo-para-dioxin leads to defective dentin formation and pulpal perforation in rat incisor teeth. Toxicology. 1993;81:1–13. doi: 10.1016/0300-483x(93)90152-i. [DOI] [PubMed] [Google Scholar]
- Allen JR, Barsotti DA, Van Miller JP, Abrahamson LJ, Lalich JJ. Morphological changes in monkeys consuming a diet containing low levels of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fd Cosmet Toxicol. 1977;15:401–10. doi: 10.1016/s0015-6264(77)80004-7. [DOI] [PubMed] [Google Scholar]
- Arisawa K, Takeda H, Mikasa H. Background exposure to PCDDs/PCDFs/PCBs and its potential health effects: a review of epidemiologic studies. J Med Invest. 2005;52:10–21. doi: 10.2152/jmi.52.10. [DOI] [PubMed] [Google Scholar]
- Asahi S. Clinical features and pathogenesis of Yusho (PCB poisoning) J UOEH. 1993;15:1–11. doi: 10.7888/juoeh.15.1. (Abstract in English and text in Japanese) [DOI] [PubMed] [Google Scholar]
- Bertazzi PA, Consonni D, Bachetti S, Rubagotti M, Baccarelli A, Zocchetti C, Pesatori AC. Health effects on dioxin exposure: a 20-year mortality study. Am J Epidemiol. 2001;153:1031–44. doi: 10.1093/aje/153.11.1031. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS. The mechanism of dioxin toxicity: relationship to risk assessment. Environ Health Perspect. 1994;102:157–67. doi: 10.1289/ehp.94102s9157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum LS, Cummings AM. Dioxin and endometriosis: a plausible hypothesis. Environ Health Perspect. 2002;110:15–21. doi: 10.1289/ehp.0211015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum LS, Fenton SE. Cancer and developmental exposure to endocrine disruptors. Environ Health Perspect. 2003;111:389–94. doi: 10.1289/ehp.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock KW, Gschaidmeier H, Lehmkoster T, Munzel PA, Raschko F, Bock-Hennig B. Ah receptor-controlled transcriptional regulation and function of rat and human UDP-glucuronosyltransferase isoforms. Adv Enzyme Regul. 1998;38:207–22. doi: 10.1016/s0065-2571(97)00013-7. [DOI] [PubMed] [Google Scholar]
- Bookstaff RC, Kamel F, Moore RW, Bjerke DL, Peterson RE. Altered regulation of pituitary gonadotropin-releasing hormone (GnRH) receptor number and pituitary responsiveness to GnRH in 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated male rats. Toxicol Appl Pharmacol. 1990;105:78–92. doi: 10.1016/0041-008x(90)90360-7. [DOI] [PubMed] [Google Scholar]
- Bosetti C, Negri E, Fattore E, La Vecchia C. Occupational exposure to polychlorinated biphenyls and cancer risk. Eur J Cancer Prev. 2003;12:251–2. doi: 10.1097/00008469-200308000-00002. [DOI] [PubMed] [Google Scholar]
- Brix AE, Jokinen MP, Walker NJ, Sells DM, Nyska A. Characterization of bronchiolar metaplasia of the alveolar epithelium in female Sprague-Dawley rats exposed to 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) Toxicol Pathol. 2004;32:333–7. doi: 10.1080/01926230490431817. [DOI] [PubMed] [Google Scholar]
- Brouwer A, Ahlborg UG, Rolaf ven Leeuwen FX, Feeley MM. Report of the WHO working group on the assessment of health risks for human infants from exposure to PCDDS, PCDFS and PCBS. Chemosphere. 1998;37:1627–43. doi: 10.1016/s0045-6535(98)00230-6. [DOI] [PubMed] [Google Scholar]
- Brown DP. Mortality of workers exposed to polychlorinated biphenyls–an update. Arch Environ Health. 1987;42:333–9. doi: 10.1080/00039896.1987.9934355. [DOI] [PubMed] [Google Scholar]
- Buckley JD, Robinson LL, Sworinsky R, Garabrant DH, LeBeau M, Manchester P, Nesbit ME, Odom L, Peters JM, Woods WG. Occupational exposures of parents of children with acute non-lymphocytic leukemia: a report from the Children’s Cancer Study Group. Cancer Res. 1989;49:403–37. [PubMed] [Google Scholar]
- Calvert GM, Homung RW, Sweeney MH, Fingerhut MA, Halperin WE. Hepatic and gastrointestinal effects in an occupational cohort exposed to 2,3,7,8-tetrachlorodibenzo-para-dioxin. JAMA. 1992;267:2209–14. [PubMed] [Google Scholar]
- Cantoni L, Salmona M, Rizzardini M. Porphyrogenic effect of chronic treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin in female rats. Dose-effect relationship following urinary excretion of porphyrins. Toxicol Appl Pharmacol. 1981;57:156–63. doi: 10.1016/0041-008x(81)90275-1. [DOI] [PubMed] [Google Scholar]
- Carpenter DO. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev Environ Health. 2006;21:1–23. doi: 10.1515/reveh.2006.21.1.1. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Furth J, Buffett RF. Histologic and physiologic characteristics of hormone-secreting transplantable adrenal tumors in mice and rats. Am J Pathol. 1957;33:631–51. [PMC free article] [PubMed] [Google Scholar]
- Cummings AM, Hedge JM, Birnbaum L. Effect of prenatal exposure to TCDD on the promotion of endometriotic lesion growth by TCDD in adult female rats and mice. Toxicol Sci. 1999;52:45–9. doi: 10.1093/toxsci/52.1.45. [DOI] [PubMed] [Google Scholar]
- Cummings AM, Metcalf JL, Birnbaum L. Promotion of endometriosis by 2,3,7,8-tetrachloroidibenzo-p-dioxin in rats and mice: time-dose dependence and species comparison. Toxicol Appl Pharmacol. 1996;138:131–9. doi: 10.1006/taap.1996.0106. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Kerzee JK, Wang B, Miller M, Dieter MZ, Lorenz JN, Shertzer HG, Nerbert DW, Puga A. Dioxin exposure is an environmental risk factor for ischemic heart disease. Cardiovasc Toxicol. 2001;1:285–98. doi: 10.1385/ct:1:4:285. [DOI] [PubMed] [Google Scholar]
- Dunning WF, Curtis MR, Segaloff A. Strain differences in response to estrone and the induction of mammary gland, adrenal, and bladder cancer in rats. Cancer Res. 1953;13:147–52. [PubMed] [Google Scholar]
- Egeland GM, Sweeney MH, Fingerhut MA, Wille KK, Schnorr TM, Halperin WE. Total serum testosterone and gonadotropins in workers exposed to dioxin. Am J Epidemiol. 1994;139:272–81. doi: 10.1093/oxfordjournals.aje.a116994. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, Needham L, Patterson D, Brambilla P. Seveso Women’s Health Study: a study of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive health. Chemosphere. 2000;40:1247–53. doi: 10.1016/s0045-6535(99)00376-8. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, Needham LL, Patterson DG, Jr, Brambilla P, Gavoni N, Casalini S, Panazza S, Turner W, Gerthoux PM. Serum dioxin concentrations and endometriosis: a cohort study in Seveso, Italy. Environ Health Perspect. 2002;110:629–34. doi: 10.1289/ehp.02110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerhut MA, Halperin WE, Marlow D, Piacitelli LA, Honchar PA, Sweeney MH, Greife AL, Dill PA, Steenland K, Suruda AJ. Cancer mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. N Engl J Med. 1991;324:212–8. doi: 10.1056/NEJM199101243240402. [DOI] [PubMed] [Google Scholar]
- Frith CH, Botts S, Jokinen MP, Eighmy JJ, Hailey JR, Morgan SJ, Chandra M. Non-proliferative lesions of the endocrine system in rats. In: Guides for Toxicologic Pathology. STP/ARP/AFIP; Washington, DC: 2000. [Google Scholar]
- Frumkin H. Agent orange and cancer: an overview for clinicians. CA Cancer J Clin. 2003;53:245–55. doi: 10.3322/canjclin.53.4.245. [DOI] [PubMed] [Google Scholar]
- Gao Y, Sahiberg C, Alaluusua S, Pohjanvirta R, Tuomisto J, Lukinmas PL. Lactational exposure of rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin impairs tooth biomineralization. J Dent Res. 2004;83:139–44. doi: 10.1177/154405910408300211. [DOI] [PubMed] [Google Scholar]
- Gerhard I, Monqa B, Krahe J, Runnebaum B. Chlorinated hydrocarbons in infertile women. Environ Res. 1999;80:299–310. doi: 10.1006/enrs.1998.3890. [DOI] [PubMed] [Google Scholar]
- Geusau A, Abraham K, Geissler K, Sator MO, Stingl G, Tschachler E. Severe 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) intoxication: clinical and laboratory effects. Environ Health Perspect. 2001;109:865–9. doi: 10.1289/ehp.01109865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden R, Doull J, Waddell W, Mandel J. Potential human cancer risks from exposure to PCBs: a tale of two evaluations. Crit Rev Toxicol. 2003;33:543–80. [PubMed] [Google Scholar]
- Guo YL, Lambert GH, Hsu CC, Hsu MML. Yucheng: health effects of prenatal exposure to polychlorinated biphenyls and dibenzofurans. Int Arch Occup Environ Health. 2004;77:153–8. doi: 10.1007/s00420-003-0487-9. [DOI] [PubMed] [Google Scholar]
- Guo YL, Yu ML, Hsu CC, Rogan WJ. Chloracne, goiter, arthritis, and anemia after polychlorinated biphenyl poisoning: 14-year follow-up of the Taiwan Yucheng cohort. Environ Health Perspect. 1999;107:715–9. doi: 10.1289/ehp.99107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailey JR, Walker NJ, Sells DM, Brix AE, Jokinen MP, Nyska A. Classification of proliferative hepatocellular lesions in Harlan Sprague-Dawley rats chronically exposed to dioxin-like compounds. Toxicol Pathol. 2005;33:165–74. doi: 10.1080/01926230590888324. [DOI] [PubMed] [Google Scholar]
- Halperin W, Vogt R, Sweeney MH, Shopp G, Fingerhut M, Petersen M. Immunological markers among workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Occup Environ Med. 1998;55:742–9. doi: 10.1136/oem.55.11.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi I, Anan H, Maeda K, Akamine A, Fukuyama H, Okumura H. An epidemiologic examination on the prevalence of the periodontal diseases and oral pigmentation in Yusho patients in 1996. Fukuoka Igaku Zasshi. 1997;88:226–30. (Abstract in English, text in Japanese) [PubMed] [Google Scholar]
- Henriksen GL, Ketchum NS, Michalek JE, Swaby JA. Serum dioxin and diabetes mellitus in veterans of Operation Ranch Hand. Epidemiology. 1997;8:252–8. doi: 10.1097/00001648-199705000-00005. [DOI] [PubMed] [Google Scholar]
- Hill A, Howard CV, Strahle U, Cossins A. Neurodevelopmental defects in zebrafish (Danio rerio) at environmentally relevant dioxin (TCDD) concentrations. Toxicol Sci. 2003;76:392–9. doi: 10.1093/toxsci/kfg241. [DOI] [PubMed] [Google Scholar]
- Holt E. Doctor sues clinic over Yushchenko poisoning claims. Lancet. 2005;365:1375. doi: 10.1016/S0140-6736(05)66354-4. [DOI] [PubMed] [Google Scholar]
- Huff J, Lucier G, Tritscher A. Carcinogenicity of TCDD: experimental, mechanistic, and epidemiologic evidence. Annu Rev Pharmacol Toxicol. 1994;34:343–72. doi: 10.1146/annurev.pa.34.040194.002015. [DOI] [PubMed] [Google Scholar]
- Ilvesaro J, Pohjanvirta R, Tuomisto J, Viluksela M, Tuukkanen J. Bone resorption by aryl hydrocarbon receptor-expressing osteoclasts is not disturbed by TCDD in short-term ccultures. Life Sci. 2005;77:1351–66. doi: 10.1016/j.lfs.2005.01.027. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. Monographs on the evaluation of carcinogenic risks to humans. Polychlorinated dibenzo-para-dioxins and polycholorinated dibenzofurans. IARC Scientific Publications No. 69; Lyon, France: 1997. [PMC free article] [PubMed] [Google Scholar]
- Jamsa T, Viluksela M, Tuomisto JT, Tuomisto J, Tuukkanen J. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on bone in two rat strains with different aryl hydrocarbon receptor structures. J Bone Miner Res. 2001;16:1812–20. doi: 10.1359/jbmr.2001.16.10.1812. [DOI] [PubMed] [Google Scholar]
- Johnson KL, Cummings AM, Birnbaum LS. Promotion of endometriosis in mice by polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls. Environ Health Perspect. 1997;105:750–5. doi: 10.1289/ehp.97105750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen MP, Walker NJ, Brix AE, Sells DM, Haseman JK, Nyska A. Increase in cardiovascular pathology in female Sprague-Dawley rats following chronic treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin and 3,3′,4,4′,5-pentachlorobiphenyl. Cardiovasc Toxicol. 2003;4:299–310. doi: 10.1385/CT:3:4:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattainen H, Tuukkanen J, Simanainen U, Tuomisto J, Kovero O, Lukinmaa PL, Alaluusua S, Tuomisto J, Viluksela M. In utero/lactational treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure impairs molar tooth development in rats. Toxicol Appl Pharmacol. 2001;174:216–24. doi: 10.1006/taap.2001.9216. [DOI] [PubMed] [Google Scholar]
- Kerkvliet NI. Recent advances in understanding the mechanisms of TCDD immunotoxicity. Int Immunopharmacol. 2002;2:277–91. doi: 10.1016/s1567-5769(01)00179-5. [DOI] [PubMed] [Google Scholar]
- Kikuchi M. Autopsy of patients with Yusho. Prog Clin Biol Res. 1984;137:19–30. [PubMed] [Google Scholar]
- Kimbrough RD. Laboratory and human studies on polychlorinated biphenyls (PCBs) and related compounds. Environ Health Perspect. 1985;59:99–106. doi: 10.1289/ehp.59-1568093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiukkonen A, Viluksela M, Sahlberg C, Alaluusua S, Tuomisto JT, Tuomisto JT, Tuomisto J, Lukinmaa PL. Response of the incisor tooth to with 2,3,7,8-tetrachlorodibenzo-p-dioxin in a dioxin-resistant and a dioxin-sensitive with 2,3,7,8-tetrachlorodibenzo-p-dioxin rat strain. Toxicol Sci. 2002;69:482–9. doi: 10.1093/toxsci/69.2.482. [DOI] [PubMed] [Google Scholar]
- Kleeman JM, Moore RW, Peterson RE. Inhibition of testicular steriodogenesis in 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated rats: evidence that the key lesion occurs prior to or during pregnenolone formation. Toxicol Appl Pharmacol. 1990;106:112–5. doi: 10.1016/0041-008x(90)90111-7. [DOI] [PubMed] [Google Scholar]
- Kociba RJ, Keyes DG, Beyer JE, Carreon RM, Wade CE, Dittenber DA, Kalnins RP, Frauson LE, Park CN, Barnard SD, Hummel RA, Humiston CG. Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol Appl Pharmacol. 1978;46:279–303. doi: 10.1016/0041-008x(78)90075-3. [DOI] [PubMed] [Google Scholar]
- Kohn MC. Effects of TCDD on thyroid hormone homeostasis in the rat. Drug Chem Toxicol. 2000;23:259–77. doi: 10.1081/dct-100100114. [DOI] [PubMed] [Google Scholar]
- Kohn MC, Sewall CH, Lucier GW, Portier CJ. A mechanistic model of effects of dioxin on thyroid hormones in the rat. Toxicol Appl Pharmacol. 1996;136:29–48. doi: 10.1006/taap.1996.0004. [DOI] [PubMed] [Google Scholar]
- Kuratsune M. Yusho. In: Kinbrough RD, editor. Halogenated Biphenyls, Terphenyls, Napththalenes, Dibenzodioxins and Related Products. Elsevier North Holland; Amsterdam: 1980. pp. 287–92. [Google Scholar]
- Kuratsune M, Ikeda M, Nakamura Y, Hirohata T. A cohort study on mortality of “Yusho” patients: a preliminary report. Princess Takamatsu Symp. 1987;18:61–6. [PubMed] [Google Scholar]
- Li LA, Wang PW. PCB126 induces differential changes in androgen, cortisol, and aldosterone biosynthesis in human adrenocortical H295R cells. Toxicol Sci. 2005;85:530–40. doi: 10.1093/toxsci/kfi105. [DOI] [PubMed] [Google Scholar]
- Louis GM, Weiner JM, Whitcomb BW, Sperrazza R, Schisterman EF, Lobdell DT, Crickard K, Greizerstein H, Kostyniak PJ. Environmental PCB exposure and risk of endometriosis. Hum Reprod. 2005;20:279–85. doi: 10.1093/humrep/deh575. [DOI] [PubMed] [Google Scholar]
- Lukinmaa PL, Sahlberg C, Leppaniemi A, Partanen AM, Kovero O, Pohjanvirta R, Tuomisto J, Alaluusua S. Arrest of rat molar teeth development by lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 2001;173:38–47. doi: 10.1006/taap.2001.9155. [DOI] [PubMed] [Google Scholar]
- Mandal PK. Dioxin: a review of its environmental effects and aryl hydrocarbon receptor biology. J Comp Physiol B. 2005;175:221–30. doi: 10.1007/s00360-005-0483-3. [DOI] [PubMed] [Google Scholar]
- Mannetje A, McLean D, Cheng S, Boffetta P, Colin D, Pearce N. Mortality in New Zealand workers exposed to phenoxy herbicides and dioxins. Occup Environ Med. 2004;62:34–40. doi: 10.1136/oem.2004.015776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayani A, Barel S, Soback S, Almaqor M. Dioxin concentrations in women with endometriosis. Hum Reprod. 1997;12:373–5. doi: 10.1093/humrep/12.2.373. [DOI] [PubMed] [Google Scholar]
- McGregor DB, Partensky C, Wilbourn J, Rice JM. An IARC evaluation of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans as risk factors in human carcinogenesis. Environ Health Perspect. 1998;106:755–60. doi: 10.1289/ehp.98106755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebus CA, Reddy VR, Piper WN. Depression of rat testicular 17-hydroxylase and 17,20-lyase after administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Biochem Pharmacol. 1987;36:727–31. doi: 10.1016/0006-2952(87)90726-x. [DOI] [PubMed] [Google Scholar]
- Miettinen HM, Alaluusua S, Tuomisto J, Viluksela M. Effect of in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on rat molar development: the role of exposure time. Toxicol Appl Pharmacol. 2002;184:57–66. [PubMed] [Google Scholar]
- Miller RW. Congenital PCB poisoning: a reevaluation. Environ Health Perspect. 1985;60:211–4. doi: 10.1289/ehp.8560211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RW, Bookstaff RC, Mably TA, Peterson RE. Differential effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on responsiveness of male rats to androgens, 17β-estradiol, luteinizing hormone, gonadotropin releasing hormone, and progesterone. Chemosphere. 1992;25:91–4. [Google Scholar]
- Moore RW, Peterson RE. Androgen catabolism and excretion in 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated rats. Biochem Pharmacol. 1988;37:560–2. doi: 10.1016/0006-2952(88)90232-8. [DOI] [PubMed] [Google Scholar]
- Morrow AF, Baker G, Burger HG. Different testosterone and LH relationships in infertile men. J Androl. 1986;7:310–5. doi: 10.1002/j.1939-4640.1986.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Shigematsu N. Carcinogenic effect of polychlorinated biphenyls (PCBs) and their derivatives, including carcinogenicity to the lung. Fukuoka Igaku Zasshi. 1991;82:251–5. [PubMed] [Google Scholar]
- National Toxicology Program. Carcinogenesis bioassay of 2,3,7,8-tetrachlorodibenzo-p-dioxin (CAS No. 1746-01-6) in Swiss Webster mice (dermal study) NIEHS; Research Triangle Park, NC: 1982a. NTP TR 201, NIH publication No. 82–1757. [PubMed] [Google Scholar]
- National Toxicology Program. Carcinogenesis bioassay of 2,3,7,8-tetrachlorodibenzo-p-dioxin (CAS No. 1746-01-6) in Osborne-Mendel rats and B6C3F1 mice (gavage study) NIEHS; Research Triangle Park, NC: 1982b. NTP TR 209, NIH publication No. 82–1765. [PubMed] [Google Scholar]
- National Toxicology Program. Toxicology and carcinogenesis studies of 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) (CAS No. 57465-28-8) in female Harlan Sprague-Dawley rats (gavage studies) NIEHS; Research Triangle Park, NC: 2004a. NTP TR 520, NIH publication No. 04–4454. [PubMed] [Google Scholar]
- National Toxicology Program. Toxicology and carcinogenesis studies of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (CAS No. 1746-01-6) in female Harlan Sprague-Dawley rats (gavage studies) NIEHS; Research Triangle Park, NC: 2004b. NTP TR 521, NIH publication No. 04–4455. [PubMed] [Google Scholar]
- National Toxicology Program. Toxicology and carcinogenesis studies of 2,3,4,7,8,-pentachlorodibenzofuran (PeCDF) (CAS No. 57117-31-4) in female Harlan Sprague-Dawley rats (gavage studies) NIEHS; Research Triangle Park, NC: 2004c. NTP TR 525, NIH publication No. 04–4461. [PubMed] [Google Scholar]
- Nohara K, Ao K, Miyamoto Y, Ito T, Suzuki T, Toyoshiba H, Tohyama C. Comparison of the 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced CYP1A1 gene expression profile in lymphocytes from mice, rats, and humans: most potent induction in humans. Toxicology. 2006;225:204–13. doi: 10.1016/j.tox.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Nyska A, Jokinen MP, Brix AE, Sells DM, Wyde ME, Orzech DP, Haseman JK, Flake G, Walker NJ. Exocrine pancreatic pathology in female Harlan Sprague-Dawley rats after chronic treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin and dioxin-like compounds. Environ Health Perspect. 2004;112:903–9. doi: 10.1289/ehp.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyska A, Yoshizawa K, Jokinen MP, Brix AE, Sells DM, Wyde ME, Orzech DP, Kissling GE, Walker NJ. Olfactory epithelial metaplasia and hyperplasia in female Harlan Sprague-Dawley rats following chronic treatment with polychlorinated biphenyls. Toxicol Pathol. 2005;33:371–7. doi: 10.1080/01926230590930209. [DOI] [PubMed] [Google Scholar]
- Partanen AM, Alaluusua S, Miettinen P, Thesleff I, Tuomisto J, Pohjanvirta R, Lukinmaa PL. Epidermal growth factor receptor as a mediator of developmental toxicity of dioxin in mouse embryonic teeth. Lab invest. 1998;78:1473–81. [PubMed] [Google Scholar]
- Pauwels A, Schepens PJC, Hooghe TD, Delbeke L, Dhont M, Brouwer A, Weyler J. The risk of endometriosis and exposure to dioxin and polychlorinated biphenyls: a case- control study of infertile women. Hum Reprod. 2001;16:2050–5. doi: 10.1093/humrep/16.10.2050. [DOI] [PubMed] [Google Scholar]
- Pavuk M, Cerhan JR, Lynch CF, Schecter A, Petrik J, Chovancova J, Kocan A. Environmental exposure to PCBs and cancer incidence in eastern Slovakia. Chemoshere. 2004;54:1509–20. doi: 10.1016/j.chemosphere.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Pavuk M, Schecter AJ, Akhtar FZ, Michalek AE. Serum 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) levels and thyroid function in air force veterans of the Vietnam war. Ann Epidemiol. 2003;13:335–43. doi: 10.1016/s1047-2797(02)00422-2. [DOI] [PubMed] [Google Scholar]
- Pegg DG, Hewitt WR, McComack KM, Hook JB. Effects of 2,3,7,8-tetrachlorodibenzo-rho-dioxin on renal function in the rat. J Toxicol Environ Health. 1976;2:55–65. doi: 10.1080/15287397609529417. [DOI] [PubMed] [Google Scholar]
- Pesatori AC, Consonni D, Bachetti S, Zocchetti C, Bonzini M, Baccarelli A, Bertazzi PA. Short- and long-term morbidity and mortality in the population exposed to dioxin after the “Seveso Accident. Ind Health. 2003;41:127–138. doi: 10.2486/indhealth.41.127. [DOI] [PubMed] [Google Scholar]
- Poland A, Knutson JC. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–54. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Rier SE, Foster WG. Environmental dioxins and endometriosis. Toxicol Sci. 2002;70:161–70. doi: 10.1093/toxsci/70.2.161. [DOI] [PubMed] [Google Scholar]
- Rier SE, Martin DC, Bowman RE, Dmowski WP, Becker JL. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fund Appl Toxicol. 1993;21:433–41. doi: 10.1006/faat.1993.1119. [DOI] [PubMed] [Google Scholar]
- Rier SE, Turner WE, Martin DC, Morris R, Lucier GW, Clark GC. Serum levels of TCDD and dioxin-like chemicals in rhesus monkeys chronically exposed to dioxin: correlation of increased serum PCB levels with endometriosis. Toxicol Sci. 2001;59:147–59. doi: 10.1093/toxsci/59.1.147. [DOI] [PubMed] [Google Scholar]
- Rix BA, Villadsen E, Engholm G. Hodgkin disease, pharyngeal cancer, and soft tissue sarcomas in Danish paper mill workers. Occup Environ Med. 1998;40:55–62. doi: 10.1097/00043764-199801000-00011. [DOI] [PubMed] [Google Scholar]
- Safe S, Qin C, McDougal A. Development of selective aryl hydrocarbon receptor modulators for treatment of breast cancer. Exper Opin Investig Drugs. 1999;8:1385–96. doi: 10.1517/13543784.8.9.1385. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Armanini M, Packan D, Tombaugh G. Stress and glucocorticoids in aging. Endocrinol Metab Clin North Am. 1987;16:965–80. [PubMed] [Google Scholar]
- Saracci R, Kogevinas M, Winkelmann MA, Bertazzi PA, Bueno de Mesquita BH, Coggon D, Green LM, Kauppinen T, L’Abbe KA, Littorin M, Lynge E, Mathews JD, Neuberger M, Osman J, Pearce N. Cancer mortality in workers exposed to chlorophenoxy herbicides and chlorophenols. Lancet. 1991;338:1027–32. doi: 10.1016/0140-6736(91)91898-5. [DOI] [PubMed] [Google Scholar]
- Schecter A, Birnbaum L, Ryan JJ, Constable JD. Dioxins: an overview. Environ Res. 2006;101:419–28. doi: 10.1016/j.envres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Schoen FJ. The heart. In: Contran RS, Kumar V, Robbins SL, editors. Robbins Pathologic Basis of Disease. W. B. Saunders; Philadelphia: 1994. pp. 517–82. [Google Scholar]
- Schuur AG, Boekhorst FM, Brouwer A, Visser TJ. Extrathyroidal effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on thyroid hormone turnover in male Sprague-Dawley rats. Endocrinology. 1997;138:3727–34. doi: 10.1210/endo.138.9.5386. [DOI] [PubMed] [Google Scholar]
- Sewall CH, Flagler N, Vanden Heuvel JP, Clark GC, Tritscher AM, Maronpot RR, Lucier GW. Alterations in thyroid function in female Sprague-Dawley rats following chronic treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1995;132:237–44. doi: 10.1006/taap.1995.1104. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Nakata S, Murakami T, Tamari K, Takahama Y, Akamine A, Aono M. Long-term occlusal guidance of a severely in toxicated patient with yusho (PCB poisoning): a case report. Am J Orthod Dentofacial Orthop. 1992;101:393–402. doi: 10.1016/0889-5406(92)70111-M. [DOI] [PubMed] [Google Scholar]
- Steenland K, Piacitelli L, Deddens J, Fingerhut M, Chang LI. Cancer, heart disease, and diabetes in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Natl Cancer Inst. 1999;91:779–86. doi: 10.1093/jnci/91.9.779. [DOI] [PubMed] [Google Scholar]
- Sterling JB, Hanke CW. Dioxin toxicity and chloracne in the Ukraine. J Drugs Dermatol. 2005;4:148–50. [PubMed] [Google Scholar]
- Tamaki A, Hayashi H, Nakajima H, Takii T, Katagiri D, Miyazawa K, Hirose K, Onozaki K. Polycyclic aromatic hydrocarbon increases mRNA level for interleukin 1 beta in human fibroblast-like synoviocyte line via aryl hydrocarbon receptor. Biol Pharm Bull. 2006;27:407–10. doi: 10.1248/bpb.27.407. [DOI] [PubMed] [Google Scholar]
- Tani Y, Maronpot RR, Foley JF, Haseman JK, Walker NJ, Nyska A. Follicular epithelial cell hypertrophy induced by chronic oral administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin in female Harlan Sprague-Dawley rats. Toxicol Pathol. 2004;32:441–9. doi: 10.1080/01926230490260952. [DOI] [PubMed] [Google Scholar]
- Urabe H, Asahi M. Past and current dermatological status of Yusho patients. Environ Health Perspect. 1985;59:11–5. doi: 10.1289/ehp.59-1568099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Birgelen AP, Smit EA, Kampen IM, Groeneveld CN, Fase KM, Van der Kolk J, Poiger H, Van den Berg M, Koeman JH, Brouwer A. Subchronic effects of 2,3,7,8-TCDD or PCBs on thyroid hormone metabolism: use in risk assessment. Eur J Pharmacol. 1995;293:77–85. doi: 10.1016/0926-6917(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Van Birgelen AP, Van der Kolk J, Fase KM, Bol I, Poiger H, Brouwer A, Van den Berg M. Subchronic dose-response study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in female Sprague-Dawley rats. Toxicol Appl Pharmacol. 1995;132:1–13. doi: 10.1006/taap.1995.1080. [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum L, Bosveld ATC, Brunstrom B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, Kubiak T, Larsen JC, van Leeuwen FX, Liem AK, Nolt C, Peterson RE, Poellinger L, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Waern F, Zacharewski T. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106:775–92. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum L, Denison M, De Vito M, Farland W, Feely M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. The 2005 World Health Organization re-evaluation of human and mammalian toxic equivalency factors for dioxin and dioxin-like compounds. Toxicol Sci. 2006;93:223–41. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Plas SA, Lutkeschipholt I, Spenkelink B, Brouwer A. Effects of subchronic exposure to complex mixtures of dioxin-like and non-dioxin-like polyhalogenated aromatic compounds on thyroid hormone and vitamin A levels in female Sprague-Dawley rats. Toxicol Sci. 2001;59:92–100. doi: 10.1093/toxsci/59.1.92. [DOI] [PubMed] [Google Scholar]
- Viel JF, Arverux P, Baverel J, Cahn JY. Soft-tissue sarcoma and non-Hodgkin’s lymphoma clusters around a municipal solid waste incinerator with high dioxin emission levels. Am J Epidem. 2000;152:13–9. doi: 10.1093/aje/152.1.13. [DOI] [PubMed] [Google Scholar]
- Villano CM, White LA. The aryl hydrocarbon receptor-signaling pathway and tissue remodeling: insights from the zebrafish (Danio rerio) model system. Toxicol Sci. 2006;92:1–4. doi: 10.1093/toxsci/kfj215. [DOI] [PubMed] [Google Scholar]
- Walker NJ, Crockett PW, Nyska A, Brix AE, Jokinen MP, Sells DM, Hailey JR, Easterling M, Haseman JK, Yin M, Wyde ME, Bucher JR, Portier CJ. Dose-additive carcinogenicity of a defined mixture of “dioxin-like compounds. Environ Health Perspect. 2005;113:43–8. doi: 10.1289/ehp.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker NJ, Gastel JA, Costa LT, Clark GC, Lucier GW, Sutter TR. Rat CYP1B1: an adrenal cytochrome P450 that exhibits sex-dependent expression in livers and kidneys of TCDD-treated animals. Carcinogenesis. 1995;16:1319–27. doi: 10.1093/carcin/16.6.1319. [DOI] [PubMed] [Google Scholar]
- Walker NJ, Wyde ME, Fischer LJ, Nyska A, Bucher JR. Comparison of chronic toxicity and carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in 2-year bioassays in female Sprague-Dawley rats. Mol Nutr Food Res. 2006;50:934–44. doi: 10.1002/mnfr.200600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Chen TT, Hsu JF, Hsu CC, Chang LW, Ryan JJ, Guo YL, Lambert GH. Neonatal and childhood teeth in relation to perinatal exposure to polychlorinated biphenyls and dibenzofurans: observations of the Yuchang children in Taiwan. Environ Res. 2003;93:131–7. doi: 10.1016/s0013-9351(03)00040-9. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kitamura K, Nagahashi M. Effects of dioxins on human health: A review. J Epidemiol. 1999;9:1–13. doi: 10.2188/jea.9.1. [DOI] [PubMed] [Google Scholar]
- Weisglas-Kuperus N, Patandin S, Berbers GAM, Sas TCJ, Mulder PGH, Sauer PJJ, Hooijkaas H. Immunologic effects of background exposure to polychlorinated biphenyls and dioxins in Dutch preschool children. Environ Health Perspect. 2000;108:1203–7. doi: 10.1289/ehp.001081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisglas-Kuperus N, Vreugdenhil HJI, Mulder PGH. Immunological effects of environmental exposure to polychlorinated biphenyls and dioxins in Dutch school children. Toxicol Lett. 2004;149:281–5. doi: 10.1016/j.toxlet.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Yassi A, Tate R, Fish D. Cancer mortality in workers employed at a transformer manufacturing plant. Am J Ind Med. 1994;25:425–37. doi: 10.1002/ajim.4700250310. [DOI] [PubMed] [Google Scholar]
- Yoshizawa K, Marsh T, Foley JF, Cai B, Peddada S, Walker NJ, Nyska A. Mechanims of exocrine pancreatic toxicity induced by oral treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin in female Harlan Sprague-Dawley rats. Toxicol Sci. 2005b;85:594–606. doi: 10.1093/toxsci/kfi121. [DOI] [PubMed] [Google Scholar]
- Yoshizawa K, Walker NJ, Jokinen MP, Brix AE, Sells DM, Marsh T, Wyde ME, Orzech D, Haseman JK, Nyska A. Gingival carcinogenicity in female Harlan Sprague-Dawley rats following two-year oral treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin and dioxin-like compounds. Toxicol Sci. 2005a;83:64–77. doi: 10.1093/toxsci/kfi016. [DOI] [PubMed] [Google Scholar]
- Yu ML, Guo YL, Hsu CC, Rogan WJ. Increased mortality from chronic liver disease and cirrhosis 13 years after the Taiwan “Yucheng” (“oil disease”) incident. Am J Ind Med. 1997;31:172–5. doi: 10.1002/(sici)1097-0274(199702)31:2<172::aid-ajim6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Yuch MF, Huang YH, Hiller A, Chen S, Nguyen N, Tukey RH. Involvement of the xenobiotic response element (XRE) in Ah receptor-mediated induction of human UDP-glucuronosyltransferase 1A1. J Biol Chem. 2003;278:15001–6. doi: 10.1074/jbc.M300645200. [DOI] [PubMed] [Google Scholar]
- Zober A, Messerer P, Ott MG. BASF Studies: Epidemiological and clinical investigations on dioxin-exposed chemical workers. Teratog Carcinog Mutagen. 19971998;17:249–56. [PubMed] [Google Scholar]
- Zober A, Ott MG, Messerer P. Morbidity follow up study of BASF employees exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) after a 1953 chemical reactor incident. Occup Environ Med. 1994;51:479–86. doi: 10.1136/oem.51.7.479. [DOI] [PMC free article] [PubMed] [Google Scholar]


