Abstract
Cells in the brains of adult mammals continue to proliferate in the subventricular zone (SVZ) throughout the lateral wall of the lateral ventricle. Here we show, using whole mount dissections of this wall from adult mice, that the SVZ is organized as an extensive network of chains of neuronal precursors. These chains are immunopositive to the polysialylated form of NCAM, a molecule present at sites of plasticity, and TuJ1, an early neuronal marker. The majority of the chains are oriented along the rostrocaudal axis and many join the rostral migratory stream that terminates in the olfactory bulb. Using focal microinjections of DiI and transplantation of SVZ cells carrying a neuron-specific reporter gene, we demonstrate that cells originating at different rostrocaudal levels of the SVZ migrate rostrally and reach the olfactory bulb where they differentiate into neurons. Our results reveal an extensive network of pathways for the tangential chain migration of neuronal precursors throughout the lateral wall of the lateral ventricle in the adult mammalian brain.
Keywords: subventricular zone, subependymal layer, neurogenesis
Two proliferative layers give rise to most brain cells during development: (i) the ventricular zone, which is organized as a pseudostratified epithelium surrounding the lumen of the ventricles, and (ii) the subventricular zone (SVZ), which forms later in development adjacent to the ventricular zone, and for which no apparent organization has been described.
Intriguingly, proliferation continues postnatally and throughout adulthood in the SVZ of mammals (1). Most studies have concluded that the adult SVZ is a vestigial layer of dividing cells that either die or generate glia (2–7). Interestingly, other studies have suggested that neuronal precursors are found in the SVZ of postnatal and adult rodents (8–11). Recent work, which followed the fates of restricted cohorts of labeled cells, has demonstrated that SVZ cells in the anteriormost portion of the lateral ventricle of neonatal (7) and adult (12) rodents migrate to the olfactory bulb where they differentiate into granular and periglomerular neurons. A compartmentalized model of the SVZ has been proposed (7) that states that this anteriormost SVZ, named SVZa, is specialized for the production of olfactory bulb neuronal precursors, while caudally proliferating cells may die or generate glia.
The tangential migration of neuronal precursors from the SVZa to the olfactory bulb occurs along a restricted pathway called the rostral migratory stream (RMS) (8) (see Fig. 1C). Once in the olfactory bulb, cells exit the RMS and migrate radially into the granular and periglomerular layers (7, 12, 13). In adult mice, the migrating neuronal precursors in the RMS are organized into cords of cells, or chains. These chains can be visualized with antibodies that recognize the polysialylated form of NCAM (PSA-NCAM) (14), a molecule enriched in the RMS (14–16). Cells in these chains migrate closely associated to one another without radial glial or axonal guides (17).
Figure 1.
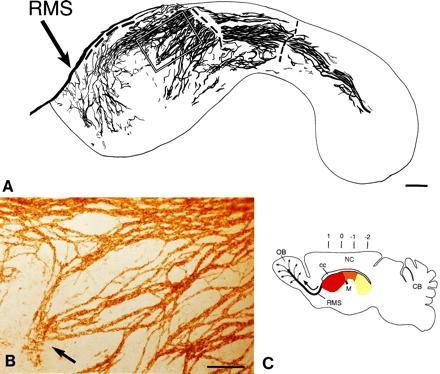
Network of PSA-NCAM immunopositive chains in the lateral wall of the lateral ventricle of adult mice. Dorsal is up and rostral is left. (A) Camera lucida drawing of PSA-NCAM immunopositive chains in whole mounts of this wall. The dorsal group of chains on the wall of the anterior horn are connected to the RMS, but the tissue broke at this point and others (dashed line) during processing. The outline corresponds to the colored area in C. The rectangle indicates the area shown in B. (B) Photomicrograph of PSA-NCAM-immunopositive chains shown in marked rectangle in camera lucida drawing in A. Notice a group of chains (arrow) that are disorganized and end in the central region of the anterior horn. (C) Schematic sagittal view of adult mouse brain showing in different colors the different regions of the lateral wall of the lateral ventricle. The medially located anterior horn (red) is connected to the laterally located inferior horn (yellow) through an intermediate bridge (orange). Black arrow indicates direction of migration of neuronal precursors in the RMS to the olfactory bulb, where cells disperse radially (thin arrows) to reach the granular and glomerular layers (12). Numbers indicate anterior-posterior (A-P) stereotaxic coordinates (measured in mm). OB, olfactory bulb; M, foramen of Monro; NC, neocortex; CB, cerebellum; cc, corpus callosum. (A, bar = 250 μm; B, bar = 100 μm.)
The present report demonstrates that the SVZ is organized as a network of chains of neuronal precursors that extends throughout most of the lateral wall of the lateral ventricle of adult mice. We show that cells originating from different levels of the SVZ, including the caudal lateral wall of the lateral ventricle, migrate 5 to 8 mm rostrally to reach the olfactory bulb where they become neurons. Our results reveal an extensive tangential migration of neuronal precursors throughout the walls of the lateral ventricle of an adult mammalian brain.
MATERIALS AND METHODS
Immunocytochemistry.
Adult male mice (2–3 months old) were intracardially perfused with cold 0.9% saline and their brains were removed. The lateral walls of the lateral ventricle were dissected and the resulting whole mounts were fixed for 1.5 hr in 3% paraformaldehyde and washed overnight at 4°C in Tris-buffered saline (TBS; 0.1 M, pH 7.5). Whole mounts were immersed in methanol and acetone for 30 min each at −20°C. For PSA-NCAM staining, whole mounts were washed 3× in 0.1 M TBS containing 0.5% Triton X-100 for 15 min each, blocked for 2 hr in 10% goat serum/0.5% Triton X-100/0.1 M TBS, incubated for 72 hr at 4°C with 1:2000 dilution of anti-meningococcus group B (MEN-B) monoclonal antibody (18) (kind gift of Dr. G. Rougon, Faculté des Sciences de Luminy, Marseille, France), and developed with secondary anti-IgM peroxidase-coupled antibodies (Sigma). The staining was visualized with diaminobenzidine (0.02%) in phosphate buffer (PB; 0.1 M, pH 7.4) and H2O2 (0.01%). The stained walls were cleared in benzyl benzoate/benzyl alcohol (2:1) and mounted with Krystalon (EM Diagnostic Systems, Gibbstown, NJ) between two coverslips.
For TuJ1 staining, whole mounts were washed 3× in PBS/0.5% Triton X-100 for 15 min each, blocked for 2hr in 10% horse serum/PBS/0.5% Triton X-100, and incubated in 1:500 anti-TuJ1 monoclonal antibody (kind gift of Dr. A. Frankfurter, University of Virginia, Charlottesville) for 72 hr at 4°C. Whole mounts were washed 3× and incubated for 2 hr at room temperature with a biotinylated secondary antibody (Vector Laboratories), washed 3× and incubated with avidin fluorescein isothiocyanate (Vector Laboratories) for 1 hr, and washed 3× and visualized under fluorescence optics in Anti-Fade (Molecular Probes). Double-immunostaining for TuJ1 and PSA-NCAM was carried out using the above protocols, staining for TuJ1 first, followed by fixation for 15 min and washing before beginning the staining with PSA-NCAM antibodies. PSA-NCAM was revealed with Anti-IgM coupled to rhodamine secondary antibodies (Jackson ImmunoResearch). Controls in which individual primary antibodies were eliminated or replaced with other nonspecific monoclonal antibodies resulted in no staining for both diaminobenzidine and fluorescence conditions.
Grafts.
SVZ was dissected (9) from the lateral wall of the lateral ventricle of P10-P15 NSE-transgenic mice (kind gift of Drs. S. Forss-Petter and P. Danielson, Scripps Institute, La Jolla, CA) and cut into ≈100 μm fragments in L-15 (GIBCO) medium. At this age, the SVZ is thick, making it possible to dissect an enriched population of SVZ cells. The fragments were stereotaxically transplanted (12) into CD-1 adult mice (2–3 months) at the coordinates listed in Table 1. The animals were killed 1 month later by an overdose of pentobarbital (0.1 ml Nembutal). Animals were intracardially perfused with 0.9% NaCl followed by 3% paraformaldehyde. The brains were postfixed for 30 min and transferred to PB. Serial 50-μm thick horizontal sections were cut on a Vibratome (Lancer, St. Louis) and were incubated overnight at 37°C in 2 mM MgCl2/0.01% deoxycholic acid/0.02% Nonidet P-40/4 mM potassium ferrocyanide/4 mM potassium ferricyanide/1 mg/ml 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) (Molecular Probes) in PBS pH 7.3 to reveal β-galactosidase-positive cells. Sections were washed in PB, counterstained with Hoechst 33258, and mounted on gelatin-coated slides. The position of the graft was verified in all brains and the sections were scanned for LacZ-positive cells at ×200 magnification under the light microscope.
Table 1.
Number of cells that reach the olfactory bulb after transplantation of NSE-lacZ transgenic SVZ grafts into nontransgenic SVZ at different A-P levels (see Fig. 1C)
| Brain | Coordinates, mm
|
No. of
LacZ+
|
|||
|---|---|---|---|---|---|
| A-P | L | D | GC | PG | |
| 1 | 0.5 | 1.2 | 2.8 | 3 | 4 |
| 2 | 0.5 | 1.2 | 2.8 | 24 | 1 |
| 3 | 0 | 1 | 1.5 | 12 | |
| 4 | 0 | 1.12 | 2.3 | 4 | |
| 5 | 0 | 1.3 | 2.3 | 36 | 1 |
| 6 | 0 | 1.5 | 2.6 | 4 | 1 |
| 7 | 0 | 1.5 | 1.5 | 1431 | 52 |
| 8 | 0 | 1.5 | 1.5 | 234 | |
| 9 | 0 | 1.5 | 2 | 12357 | 43 |
| 10 | −0.5 | 1.4 | 1.8 | 20 | |
| 12 | −0.5 | 1.7 | 1.9 | 3 | |
| 13 | −0.8 | 1.6 | 1.5 | 210 | |
| 14 | −1 | 1.8 | 1.5 | 10 | |
| 15 | −1 | 2.4 | 1.8 | 4 | |
| 16 | −1 | 2.6 | 1.6 | 12 | 1 |
| 17 | −1.5 | 2.5 | 1.8 | 133 | 8 |
| 18 | −1.5 | 2.5 | 1.8 | 790 | 4 |
| 19 | −1.5 | 3.25 | 1.8 | 9 | 3 |
Stereotaxic coordinates are with reference to bregma (A-P, anteroposterio; L, lateral; D, depth from surface of brain). LacZ-positive granular (GC) and periglomerular (PG) neurons were counted in every serial 50-μm section through the olfactory bulb.
DiI Microinjection.
Forty nanoliters of 5% DiI dissolved in dimethyl formamide and vegetable oil (Wesson, Fullerton, CA) was stereotaxically microinjected into the SVZ of adult mice at the coordinates indicated in Table 2. Mice were killed 5, 10, or 30 days after DiI injection and were perfused with 0.9% saline and 3% paraformaldehyde. Brains were postfixed in the same fixative overnight and then transferred into PB. Serial 50-μm horizontal or sagittal sections were cut on a Vibratome, counterstained with Hoechst 33258, wet mounted on slides, and coverslipped with 90% glycerol/10% PBS.
Table 2.
Results of DiI microinjections into the SVZ at different stereotaxic coordinates after three different survival times
| DiI-labeled
cells
|
|||||||
|---|---|---|---|---|---|---|---|
| Coordinates, mm
|
Survival, days | OB Core | |||||
| A-P | L | D | n | RMS | GC | ||
| 0 | 1.4 | 2 | 5 | 7 | +++ | + | − |
| −1 | 2.5 | 2 | 5 | 5 | +++ | + | − |
| −2 | 3.3 | 2 | 5 | 3 | ++ | + | − |
| −1 | 2.5 | 2 | 11 | 4 | +++ | +++ | ++ |
| −2 | 3.3 | 2 | 11 | 3 | ++ | ++ | + |
| 0 | 1.4 | 2 | 30 | 3 | +++ | ++++ | ++++ |
| −1 | 2.5 | 2 | 30 | 6 | +++ | +++ | +++ |
| −2 | 3.3 | 2 | 30 | 4 | ++ | ++ | ++ |
Sections were visually scored for relative numbers of labeled cells in the RMS and olfactory bulb (OB); ++++, Dense labeling; +++, moderate labeling; ++, sparse labeling; +, a few labeled cells; −, no labeled cells. Stereotaxic coordinates are with reference to bregma (A-P, anteroposterio; L, lateral; D, depth from the dura mater). GC, granule cell; RMS, rostral migratory stream.
RESULTS
Network of Chains of Neuronal Precursors in the Adult Mouse SVZ.
Cells continue to proliferate throughout the extent of the SVZ. However, the organization of the SVZ remains unknown, in part due to the difficulty of reconstructing the curved lateral wall of the lateral ventricle from serial planar sections. We therefore dissected the entire lateral wall of the lateral ventricle from adult mice and stained the resulting whole mounts with antibodies against PSA-NCAM (18). This staining revealed that the SVZ is organized as a remarkable network of PSA-NCAM-positive chains (Fig. 1 A and B). A map of this network was constructed in camera lucida drawings from three adult male mice (Fig. 1A shows one of these maps). The distribution of chains was very similar between animals. The majority of the chains were oriented parallel to the longitudinal axis of the lateral ventricle and were more concentrated in the dorsal portion. This dorsal path of chains was continuous between the caudally and laterally located inferior horn (Fig. 1C, yellow) and the medially located anterior horn (Fig. 1C, red) through an intermediate bridge (Fig. 1C, orange). Many of these longitudinally oriented chains were observed to join the RMS. Whole mounts of the medial wall (adjacent to the septum) revealed only a few chains in the most anterior portion of the wall (data not shown). The fate of cells in the medial wall was not investigated any further.
Chain thickness varied from one to more than 10 cells. Chains ramified and rejoined other chains repeatedly, giving the impression of a laced array of linear structures. The thickest chains were localized in the dorsal aspect of the lateral wall where many thinner chains bundled together. Chains were also present, although less densely, in the ventral region of the anterior horn; some of these chains joined the RMS rostrally (Fig. 1A). The central region of the anterior horn was largely devoid of chains (Fig. 1A) and around this region were sites at which chains appeared either to begin or terminate (Fig. 1 A and B). At these sites, chains were disorganized and individual PSA-NCAM stained cells were observed.
Migrating cells in the RMS are known to be TuJ1 positive (19, 20); TuJ1 is a marker of early neuronal differentiation, recognizing class III β-tubulin (21, 22). Double staining of whole mounts with PSA-NCAM (Fig. 2B) and TuJ1 (Fig. 2A) clearly showed that cells throughout the network were both PSA-NCAM and TuJ-1 positive, indicating that the chains were composed of neuronal precursors.
Figure 2.
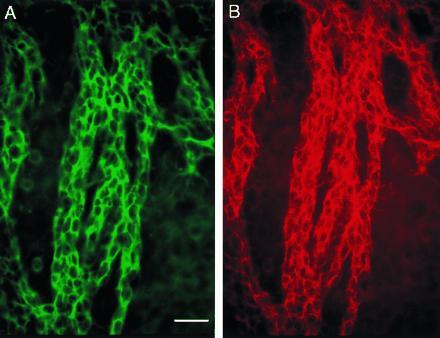
Cells in chains are neuronal precursors. Double immunostaining for TuJ1 (A, fluorescein) and PSA-NCAM (B, rhodamine) of part of the SVZ in the lateral wall of the lateral ventricle (caudal anterior horn). (Bar = 30 microns.)
SVZ Cells Grafted into Caudal SVZ Migrate Rostrally and Differentiate into Neurons.
The orientation of the PSA-NCAM/TuJ1-positive chains and their contiguity with the RMS suggested that SVZ cells migrate to the olfactory bulb even from the caudal lateral wall of the lateral ventricle. To test this hypothesis, we determined the fate of SVZ cells carrying a neuron-specific reporter gene when grafted into caudal levels of the SVZ. SVZ explants from NSE-transgenic mice in which the reporter gene lacZ is under the control of the neuron specific enolase promoter (23) were transplanted at intermediate and caudal coordinates of the SVZ of nontransgenic hosts (Fig. 3 and Table 1). Thirty days after grafting, LacZ-positive cells were found only in the olfactory bulb and at the site of transplantation. Both labeled granular and periglomerular neurons were derived from grafts placed at the different rostrocaudal locations. As observed before (12), granule neurons were far more common than periglomerular neurons.
Figure 3.
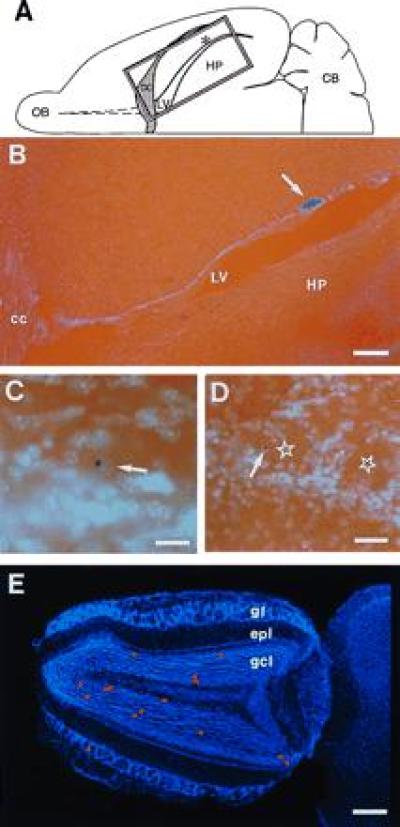
NSE-LacZ-transgenic SVZ cells grafted into caudal SVZ of nontransgenic host migrate to the olfactory bulb where they differentiate into granular and periglomerular neurons. (A) Schematic drawing of horizontal hemisection indicating the position of the graft (asterisk) in the lateral wall of the lateral ventricle (LV) and the position of photomicrograph shown in B (rectangle). (B) Horizontal section of brain 17 (A-P −1.5, Table 1) showing graft site (arrow) relative to lateral ventricle (LV), corpus callosum (cc), and hippocampus (HP). (C) LacZ-positive granule neuron in the olfactory bulb 30 days after transplantation. Arrow indicates the Hoechst counterstained nucleus. (D) LacZ-positive periglomerular neuron (arrow) in the olfactory bulb 30 days after transplantation. The centers of glomeruli are indicated by stars. (E) Hoechst-stained horizontal olfactory bulb section from brain 17 with superimposed red dots indicating the distribution of LacZ-positive granular neurons and one LacZ-positive periglomerular neuron. OB, olfactory bulb; CB, cerebellum; gl, glomerular layer; epl, external plexiform layer; gcl, granule cell layer. (B, bar = 250 μm; C, bar = 50 μm; D, bar = 120 μm; E, bar = 400 μm.)
In general, higher numbers of cells reached the olfactory bulb from transplantation into the intermediate bridge, where a high density of PSA-NCAM-positive chains was observed (Fig. 1A and Table 1). These experiments are, however, not quantitative due to the inherent variability of the grafting technique. The variability in numbers is likely due to differences in the size and viability of the grafts and, most importantly, to the number of grafted cells that become incorporated into the SVZ, a very thin target.
This experiment shows that cells grafted into caudal SVZ in the inferior horn reached the olfactory bulb and differentiated into neurons (Fig. 3 and Table 1). Grafts placed in the striatum (n = 15) away from the SVZ did not result in labeled cells in the olfactory bulb, suggesting that this tangential migration occurs only through the SVZ. In addition, striatum grafted into SVZ (n = 2) did not result in LacZ-positive cells in the olfactory bulb, indicating that this long migration is a property of SVZ cells.
Endogenous SVZ Cells Migrate to the Olfactory Bulb and Differentiate into Neurons.
To show that endogenous SVZ cells from different rostrocaudal locations migrate to the olfactory bulb, we labeled restricted cohorts of SVZ cells by focal microinjections of DiI, a vital lipophilic dye. Five days after injection, DiI-positive migrating cells were detected in the RMS from injections at all rostrocaudal levels tested (Table 2) and a few cells were observed in the core of the olfactory bulb. DiI-labeled migrating cells in the RMS were organized as chains and had the monopolar or bipolar morphology previously described (7, 12, 24). Mice killed 10 and 30 days after DiI injection (Table 2) had many labeled cells in both the migratory pathway and the granular layer of the olfactory bulb. At 30 days, many of the cells in the granular layer had the morphology of granule neurons (12, 24) (Fig. 4E). We saw no DiI-labeled glial cells in the olfactory bulb at any of the survivals studied. Fig. 4 shows DiI microinjections into the intermediate (A-P −1, Fig. 4 A–C) and caudal SVZ (A-P −1.5, Fig. 4 D and E) in two brains with 30-day survivals. The migration of DiI-labeled cells into the core of the bulb and then into adjacent layers was most robust (Fig. 4B) after injections into the intermediate bridge where the density of chains was greatest. DiI injections into adjacent striatum or cortex outside the SVZ (n = 20) at different rostrocaudal levels resulted in no labeled cells in the RMS or olfactory bulb.
Figure 4.
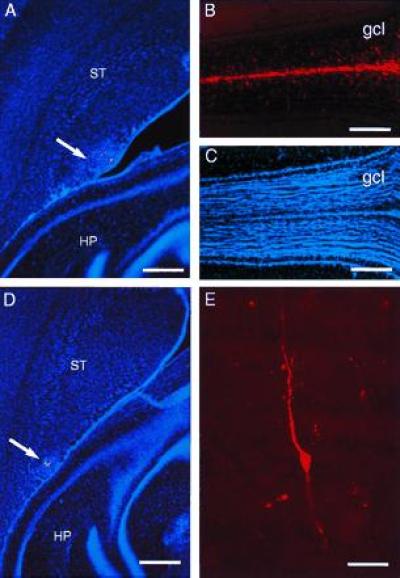
Microinjection of DiI into caudal SVZ results in labeled cells in the olfactory bulb. (A) DiI microinjection site at A-P −1 (arrow) in the SVZ at the level of the anterior hippocampus (horizontal section). (B) In this same brain, 30 days after injection, many DiI-labeled cells have reached the core of the olfactory bulb (the rostral extension of the RMS) and have migrated into the granule cell layer. (C) Hoechst 33258 counterstaining of section shown in B, to indicate olfactory bulb anatomy. (D) DiI microinjection site at A-P −1.5 (arrow) in the SVZ at the level of the hippocampus. (E) DiI-labeled granule neuron in the olfactory bulb of brain shown in D. HP, hippocampus, ST, striatum, gcl, granule cell layer. (A and D, bar = 450 microns; B and C, bar = 400 microns; bar = 20 microns.)
We found no DiI-labeled mitral or tufted cells (olfactory bulb output neurons), indicating that after injection of DiI into caudal SVZ, DiI was brought into the olfactory bulb by the migrating neuronal precursors and not by retrograde transport. Labeling of migrating cells in the RMS and olfactory bulb was not due to diffusion or leakage of DiI from the caudal SVZ to the anterior SVZ through the ventricles. Animals with labeled cells in the ependymal layer were discarded from further analysis. In addition, control injections into the ventricle that resulted in heavy labeling of ependymal layer throughout the ventricular system did not result in DiI-labeled migrating cells in the RMS or olfactory bulb.
DISCUSSION
We show here that the SVZ of the adult mouse brain is organized as a tangential array of chains of neuronal precursors. These chains are immunopositive for both PSA-NCAM and TuJ1. Many studies have described ultrastructurally immature cells (3, 5), proliferating cells (1, 2, 6, 8), and cells that express PSA-NCAM (14–16) in the SVZ of adult mammals. Here we show that the SVZ cells on the lateral wall of the lateral ventricle form a network of interconnected chains. The extent of this network is only revealed when the lateral wall of the lateral ventricle is viewed en face. These results provide a new anatomical and functional perspective of the adult SVZ, suggesting that an extensive tangential chain migration of neuronal precursors occurs through this region.
Transplantation of SVZ cells carrying a neuron-specific marker into different rostrocaudal levels of the host SVZ did not reveal migration of grafted cells to destinations other than the olfactory bulb. This suggests that the network of tangential chains described here carries neuronal precursors from regions throughout the SVZ to the olfactory bulb. Likewise, SVZ cells labeled with DiI at different rostrocaudal levels reach the RMS and the olfactory bulb. These results do not support the concept of a specialized compartment in the SVZa that gives rise to the olfactory bulb neuronal precursors (7). Instead, SVZa seems to be a region through which precursors originating from more caudal levels of the SVZ join the RMS en route to the olfactory bulb. Retroviral labeling experiments postulating the existence of the SVZa were done in neonate rats (7). It is possible that the neonatal animal or the rat differs from the adult mouse in the organization of the SVZ. However, we have detected a similar array of longitudinally oriented chains in mice as early as P10 (data not shown), suggesting that this pattern of migration is established earlier in development.
The network of chains described here is complex and extends over most of the lateral wall of the lateral ventricle. We did not study the fate of cells at all possible locations in this network and therefore our results do not exclude the possibility that SVZ cells may migrate to destinations other than the olfactory bulb. In vitro experiments (9, 11) and retroviral labeling experiments in vivo (4, 7) have suggested that SVZ cells can also give rise to glial cells. The present findings do not exclude this possibility. However, TuJ1 immunostaining indicated that the majority, if not all, of the cells in the network of chains were neuronal precursors. This implies that SVZ glial progenitors are not part of the network of chains described here.
The molecular mechanisms underlying chain migration are unknown. Cells in this network of chains and in the RMS (14) express PSA-NCAM on their cell surface. Mutations of the NCAM gene (25, 26) or the enzymatic removal of PSA (27) hamper the tangential migration of SVZ cells along the RMS while having apparently little or no effect on radial migration. PSA-NCAM mutant cells can migrate in the RMS of wild-type mice (28), highlighting the importance of cell–cell interactions during chain migration and suggesting that PSA-NCAM is required in only some of the cells in the chains. The present results indicate that chain migration occurs not only along the RMS, but throughout most of the rostrocaudal extent of the lateral ventricle. It will be interesting to determine how this extensive net of pathways for chain migration is affected in the NCAM mutant mice.
The discovery of this extensive network of pathways raises questions about the mechanisms responsible for the directional migration of neuronal precursors toward the olfactory bulb. Recently, a chemorepulsive activity capable of repelling cells from SVZ neonatal explants in culture was described (29). This factor is, however, derived from the caudal septum, on the medial side of the lateral ventricle. We show here that, in the adult mouse, it is the SVZ of the lateral wall that is a major source of olfactory bulb precursors. It is unlikely that a factor produced in the septum can influence the migration of cells on the opposite wall of the ventricle. A similar repulsive activity may be found on the lateral side of the lateral ventricle at the caudal aspect of the network of chains we describe here. However, the complex paths that chains follow are difficult to explain based on gradients of chemorepulsive or chemoattractive factors alone. The direction of traffic within individual chains and the direction in which cells flow at sites of intersection of multiple chains will provide important information about possible mechanisms underlying directional migration.
Neural stem cells are thought to be present in SVZ of the adult mammalian brain (ref. 10; for reviews, see refs. 30 and 31). The network of chains may function as collection pathways for the progeny of these putative stem cells. Stem cells may be widely distributed or localized in discrete regions. At some points in the SVZ network, there are sites at which chains appear to terminate. These regions may correspond to sites of birth of migrating young neurons and are perhaps rich in stem cells. Alternatively, these dead ends may correspond to sites where migrating cells die.
The tangential dispersal of neuronal precursors has been described in several regions of the developing brain (32–37). However, the mechanisms and pathways underlying the tangential dispersal of neuronal precursors in the embryo are poorly understood. The network of chains throughout the adult SVZ that we describe here may be the remnant of a more extensive set of tangential pathways already present in development. As such, chain migration may be a general mechanism for the tangential dispersal of neuronal precursors.
Our work demonstrates a network of chains in the SVZ that carries neuronal precursors from caudal SVZ to rostral parts of the adult brain. It will be important to determine what guides migration through this SVZ network and which other vertebrate brains contain this array of pathways. Equally intriguing is the biological significance of such an extensive network of neuronal precursors, apparently many of them destined for a single brain region, the olfactory bulb.
Acknowledgments
We thank S. Forss-Petter and P. Danielson for the NSEp transgenic mice; G. Rougon for the anti-MEN-B antibody; A. Frankfurter for the TuJ1 antibody; B. Haripal and J. Einbond for histological processing; P. Rousselot for help with photographs; A. Hemmati-Brivanlou for advice on clearing brain tissue; and S. Liu, C. Lois, F. Nottebohm, C. Scharff, and D. Thaler for comments on the manuscript. This work was supported by National Institutes of Health Grants NICHD-NS32116 and NINDS-NS28478.
Footnotes
Abbreviations: NSE, neuron-specific enolase; PSA-NCAM, polysialylated neural cell adhesion molecule; RMS, rostral migratory stream; SVZ, subventricular zone; SVZa, anteriormost SVZ; A-P, anterior-posterior.
References
- 1.Allen E. J Comp Neurol. 1912;22:547–568. [Google Scholar]
- 2.Smart I. J Comp Neurol. 1961;116:325–348. [Google Scholar]
- 3.Privat A, Leblond C P. J Comp Neurol. 1972;146:277–302. doi: 10.1002/cne.901460302. [DOI] [PubMed] [Google Scholar]
- 4.Levison S W, Goldman J E. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- 5.Blakemore W F, Jolly D R. J Neurocytol. 1972;1:69–84. doi: 10.1007/BF01098647. [DOI] [PubMed] [Google Scholar]
- 6.Morshead C M, Van der Kooy D. J Neurosci. 1992;12:249–256. doi: 10.1523/JNEUROSCI.12-01-00249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luskin M B. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 8.Altman J. J Comp Neurol. 1969;137:433–458. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 9.Lois C, Alvarez-Buylla A. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morshead C M, Reynolds B A, Craig C G, McBurney M W, Staines W A, Morassutti D, Weiss S, Van der Kooy D. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 11.Kirschenbaum B, Goldman S A. Proc Natl Acad Sci USA. 1995;92:210–214. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lois C, Alvarez-Buylla A. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 13.Corotto F S, Henegar J A, Maruniak J A. Neurosci Lett. 1993;149:111–114. doi: 10.1016/0304-3940(93)90748-a. [DOI] [PubMed] [Google Scholar]
- 14.Rousselot P, Lois C, Alvarez-Buylla A. J Comp Neurol. 1995;351:51–61. doi: 10.1002/cne.903510106. [DOI] [PubMed] [Google Scholar]
- 15.Seki T, Arai Y. Neurosci Res. 1993;17:265–290. doi: 10.1016/0168-0102(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 16.Bonfanti L, Theodosis D T. Neuroscience. 1994;62:291–305. doi: 10.1016/0306-4522(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 17.Lois C, Garcia-Verdugo J M, Alvarez-Buylla A. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 18.Rougon G, Dubois C, Buckley N, Magnani J L, Zollinger W. J Cell Biol. 1986;103:2429–2437. doi: 10.1083/jcb.103.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menezes J R L, Luskin M B. J Neurosci. 1994;14:5399–5416. doi: 10.1523/JNEUROSCI.14-09-05399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas L B, Gates M A, Steindler D A. Glia. 1996;17:1–14. doi: 10.1002/(SICI)1098-1136(199605)17:1<1::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Lee M K, Tuttle J B, Rebhun L I, Cleveland D W, Frankfurter A. Cell Motil Cytoskeleton. 1990;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- 22.Easter S S, Ross L S, Frankfurter A. J Neurosci. 1993;13:285–299. doi: 10.1523/JNEUROSCI.13-01-00285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forss-Petter S, Danielson P E, Catsicas S, Battenberg E, Price J, Nerenberg M, Sutcliffe J G. Neuron. 1990;5:187–197. doi: 10.1016/0896-6273(90)90308-3. [DOI] [PubMed] [Google Scholar]
- 24.Kishi K. J Comp Neurol. 1987;258:112–124. doi: 10.1002/cne.902580109. [DOI] [PubMed] [Google Scholar]
- 25.Tomasiewicz H, Ono K, Yee D, Thompson C, Goridis C, Rutishauser U, Magnuson T. Neuron. 1993;11:1163–1174. doi: 10.1016/0896-6273(93)90228-j. [DOI] [PubMed] [Google Scholar]
- 26.Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, Barthels D, Rajewsky K, Wille W. Nature (London) 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- 27.Ono K, Tomasiewicz H, Magnuson T, Rutishauser U. Neuron. 1994;13:595–609. doi: 10.1016/0896-6273(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 28.Hu H, Tomasiewicz H, Magnuson T, Rutishauser U. Neuron. 1996;16:735–743. doi: 10.1016/s0896-6273(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 29.Hu H, Rutishauser U. Neuron. 1996;16:933–940. doi: 10.1016/s0896-6273(00)80116-6. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez-Buylla A, Lois C. Stem Cells. 1995;13:263–272. doi: 10.1002/stem.5530130307. [DOI] [PubMed] [Google Scholar]
- 31.Gage F H, Ray J, Fisher L J. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 32.Walsh C, Cepko C L. Science. 1992;255:434–440. doi: 10.1126/science.1734520. [DOI] [PubMed] [Google Scholar]
- 33.O’Rourke N A, Dailey M E, Smith S J, McConnell S K. Science. 1992;258:299–302. doi: 10.1126/science.1411527. [DOI] [PubMed] [Google Scholar]
- 34.Fishell G, Mason C A, Hatten M E. Nature (London) 1993;362:636–638. doi: 10.1038/362636a0. [DOI] [PubMed] [Google Scholar]
- 35.Tan S-S, Breen S. Nature (London) 1993;362:638–639. doi: 10.1038/362638a0. [DOI] [PubMed] [Google Scholar]
- 36.Halliday A L, Cepko C L. Neuron. 1992;9:15–26. doi: 10.1016/0896-6273(92)90216-z. [DOI] [PubMed] [Google Scholar]
- 37.Leber S M, Sanes J R. J Neurosci. 1995;15:1236–1248. doi: 10.1523/JNEUROSCI.15-02-01236.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


