Abstract
Sox3 is expressed in developing gonads and in the brain. Evolutionary evidence suggests that the X-chromosomal Sox3 gene may be the ancestral precursor of Sry, a sex-determining gene, and Sox3 has been proposed to play a role in sex determination. However, patients with mutations in SOX3 exhibit normal gonadal determination but are mentally retarded and have short stature secondary to growth hormone (GH) deficiency. We used Cre-LoxP targeted mutagenesis to delete Sox3 from mice. Null mice of both sexes had no overt behavioral deficits and exhibited normal GH gene expression. Low body weight was observed for some mice; overgrowth and misalignment of the front teeth was observed consistently. Female Sox3 null mice (−/−) developed ovaries but had excess follicular atresia, ovulation of defective oocytes, and severely reduced fertility. Pituitary (luteinizing hormone and follicle-stimulating hormone) and uterine functions were normal in females. Hemizygous male null mice (−/Y) developed testes but were hypogonadal. Testis weight was reduced by 42%, and there was extensive Sertoli cell vacuolization, loss of germ cells, reduced sperm counts, and disruption of the seminiferous tubules. We conclude that Sox3 is not required for gonadal determination but is important for normal oocyte development and male testis differentiation and gametogenesis.
Sox3 is a single-exon gene located on the X chromosome (38) and expressed in the brain and gonads. It is a member of the high-mobility-group (HMG) family of transcription factors and was cloned based on HMG-box homology to Sry (10). Sry was identified as the male-determining gene over 10 years ago (37), and there has been great interest in the subsequent genetic cascade that directs gonadal determination and development. Evolutionary evidence suggests that Sox3 may be an ancestral precursor of Sry (14), and it has been proposed that Sox3 might antagonize Sry action to influence sex determination (15). Sox3 is also expressed in the brain from the earliest stages of development (46), preceding gonadal expression, suggesting a role for Sox3 in brain formation and cognitive function.
Some of the genes implicated in the formation of the indeterminate gonad (not committed to the male or female pathway) from the urogenital ridge have been identified (e.g., Lhx9, Ftzf1/Sf-1, and Wt-1) (7, 22, 29). Several other genes have been found to play a role in testis determination and differentiation, largely based on naturally occurring mutations in humans or targeted mutagenesis of candidate genes in mice. Human heterozygous mutations in SOX9, for example, cause campomelic dysplasia (44), a skeletal abnormality that is frequently associated with ambiguous genitalia (12). Dhh is required for the normal development and function of the peritubular myoid and Leydig cells (9), and its absence creates a disorganized testis with impaired spermatogenesis. Fgf9 null mice exhibit a spectrum of testicular defects, ranging from hypoplasia to XY sex reversal (11), and the absence of Wnt4 causes abnormal vascularization of the ovary and masculinization of XX mice (18, 41).
Despite the identification of these genes, it is likely that many others are involved in early gonadal development. In particular, there remains a paucity of information about genes involved in ovarian determination. DAX1 was originally hypothesized as a female-determining gene based on the observation of sex reversal in patients with duplication of this locus (13). Studies in mice, however, have demonstrated that the absence of Dax1 causes defects in testicular development (27) and differentiation (48), a finding that has been confirmed in patients with DAX1 loss-of-function mutations (35). Absence of Dax1 also causes male-to-female sex reversal in some genetic backgrounds (28).
Sox3 is detected at the earliest stages of embryonic development (6.5 to 8 days postconception) and continues to be expressed throughout the formation of the brain and central nervous system (8, 46). The role of Sox3 in the brain, however, is not known. Recently, a screen of 17 families with X-linked mental retardation revealed 2 families harboring mutations in the SOX3 gene. In one family, an in-frame duplication of 33 bp caused an 11-amino-acid expansion in a polyalanine tract. Affected individuals had variable degrees of mental retardation, but all had short stature and complete growth hormone (GH) deficiency. In the second family, two mentally retarded boys had a 27-bp deletion in the same polyalanine tract. Surprisingly, a maternal grandfather with the same mutation was healthy. None of these patients was reported to have GH deficiency.
To further characterize the roles of Sox3 in the brain and gonads, we used Cre-LoxP targeted mutagenesis to delete Sox3 from mice. Our results demonstrate that Sox3 is not absolutely required for normal brain development or gonadal determination, perhaps reflecting functional redundancy with other Sox proteins. However, the Sox3 knockout mice exhibit variable growth, abnormal tooth development, and altered function of the somatic and germ cells in both sexes.
MATERIALS AND METHODS
Sox3 null mice.
A 16-kb genomic clone containing the entire mouse Sox3 gene was isolated from a 129Sv/J genomic library (Stratagene, La Jolla, Calif.). The targeting vector was assembled by standard recombinant techniques. A 5-kb EcoRI fragment containing the entire Sox3 gene and a 3.7-kb EcoRI fragment immediately downstream of it were cloned separately into pGEM-3z (Promega, Madison, Wis.). An XhoI/MluI fragment containing the 5′ untranslated region (UTR) was subcloned from the 5-kb clone by overlapping PCR with primers containing LoxP sequences to introduce a LoxP site 153 bp upstream of the ATG translational start codon. The resulting XhoI/MluILoxP fragment was then inserted into the 5-kb clone in place of the original sequence. A second LoxP site was inserted upstream of a neomycin resistance (Neo) cassette (48). The NeoLoxP fragment was inserted into the 5-kb Sox3 clone at a HindIII site downstream of two putative poly(A) signals. The 3.7-kb genomic Sox3 clone was inserted downstream of the Neo cassette, and a thymidine kinase cassette was inserted at the 3′ end of the entire construct. The final construct contains 1.6 kb of 5′-flanking sequence and 3.8 kb of 3′-flanking sequence followed by a thymidine kinase cassette.
The targeting vector was electroporated into R1 embryonic stem (ES) cells (31), which were plated onto feeder cells and selected by use of G418 and ganciclovir. Colonies were expanded and screened by Southern blotting with 5′ probes. Thirty-two targeted clones were observed, but there are 2.9 kb of Sox3 sequence between the upstream LoxP site and the Neo cassette, which allows for incorrect recombination events that exclude the upstream LoxP site. PCR amplification and sequencing of the upstream LoxP insertion site yielded 10 correctly targeted clones. DNA from these clones was digested with SphI, and Southern blot analysis confirmed correct 3′ recombination in all 10. The Northwestern University Targeted Mutagenesis Facility used two correctly targeted clones for injection into 129SvJ blastocysts. Resulting chimeric male mice were bred with wild-type (WT) 129SvJ animals, and progeny were screened by PCR for germ line transmission of the targeted allele. Two independent lines of targeted animals were established and bred with CMV-Cre transgenic mice. Progeny were screened by PCR for excision of the Sox3 locus, and correct deletion was confirmed by sequencing of the PCR product. All experiments involving animals were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Northwestern University Animal Care and Use Committee.
Animal husbandry and fertility.
Males were housed with pairs of female mice for 14 to 20 days, and then animals were separated into individual cages. Fertility was equivalent in matings between WT or Sox3 knockout (KO) (Sox3−/Y) male mice and WT females. Sox3 KO (Sox3−/−) females yielded no litters initially, and the fertility defect was quantified by housing matched pairs of WT and Sox3 KO females with a WT male for 14 to 20 days. Animals were then separated into individual cages and monitored daily for 6 weeks to allow sufficient time for delivery of pups.
To assess ovulatory quality and capacity, groups of six WT and six Sox3 KO females were subjected to ovulation induction. Animals were injected with pregnant mare’s serum gonadotropin at 1800 h on day 1 and with human chorionic gonadotropin at 1800 h 2 days later. Oocytes were harvested at 0700 h the next morning, scored for morphology, and fertilized with WT sperm. Embryos were then transferred to culture and monitored through the morula stage.
For extraction of RNA from oocytes and cumulus cells, mice were sacrificed at 12 to 14 h post-human chorionic gonadotropin injection. Oocytes were dissected from the oviduct, and the cumulus cells were disassociated from the oocytes by use of 0.3 mg of hyaluronidase (Sigma) per ml and gentle passage through a pipette tip. The cumulus cells were immediately collected, spun down, and flash-frozen on dry ice. Oocytes were washed and inspected to ensure that no cumulus cells were present and then flash-frozen on dry ice.
For sperm collection, proven breeder males were sacrificed, and the cauda epididymides and vasa deferentia were dissected and placed in human tubal fluid medium (Specialty Media, Phillipsburg, N.J.). Several small slashes were made to the cauda and the tissue was very gently squeezed, releasing sperm into the medium. The sperm suspension was centrifuged and flash-frozen on dry ice.
RT-PCR.
Total RNA (50 to 500 ng) was reverse transcribed by use of avian myeloblastosis virus reverse transcriptase (RT) (Promega). GH mRNA was amplified by PCR using IQ SYBR Green Supermix and an iCycler instrument from Bio-Rad (Hercules, Calif.) according to the manufacturer's protocol. Sox3 and RPL19 mRNAs were amplified by PCR using Taq polymerase and buffers from Promega. GH values are given as ratios to RPL19.
Histology.
Ovaries were fixed in 10% neutral buffered formalin. Testes were fixed in Bouin's fixative for hematoxylin and eosin (H&E) staining and in 10% neutral buffered formalin for immunochemistry. All samples were embedded in paraffin and cut into 5-μm sections. H&E staining was performed by standard protocols. For immunohistochemistry, paraffin was removed from sections by serial washes in xylene and ethanol followed by antigen retrieval in sodium citrate buffer (pH 6.0; microwaving for 5 min). Sections were blocked in normal serum and incubated with primary antibody overnight in antibody diluent reagent solution (Zymed, South San Francisco, Calif.). Antibodies were purchased for detection of GATA-4 (C-20; 1:200) (Santa Cruz, Santa Cruz, Calif.) and laminin (L9393; 1:100) (Sigma-Aldrich, St. Louis, Mo.). An antibody against espin (1:15) was generously provided by J. Bartles (Northwestern University, Chicago, Ill.) (5), and an antibody against Sox3 was provided by T. Edlund (Umea University, Umea, Sweden). Secondary antibodies (donkey Cy3-anti-rabbit and donkey Cy3-anti-goat; Jackson ImmunoResearch, West Grove, Pa.) were applied for 2 hours at room temperature. Sections were washed with phosphate-buffered saline containing 0.5% Triton X-100 and mounted with Vector fluorescent mounting medium (Vector Laboratories, Burlingame, Calif.).
Serum hormones.
Hormones in serum were measured by the Ligand Assay and Analysis Core of the Center for Research in Reproduction at the University of Virginia.
RESULTS
Expression of Sox3.
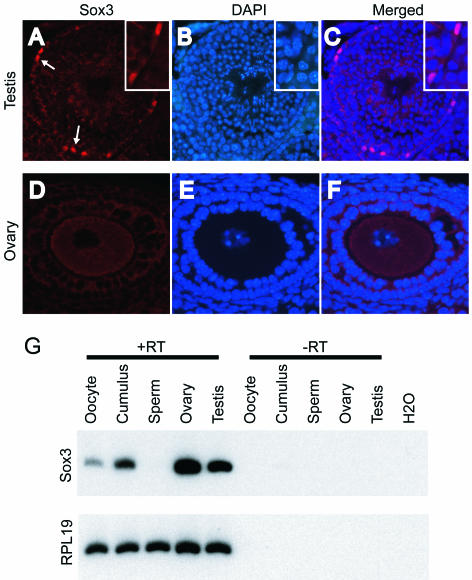
Immunochemistry was performed by use of a polyclonal Sox3 antibody. Nuclear Sox3 staining was present in multiple brain regions of WT mice but was absent in the Sox3 KO mice (data not shown) (45). In testes, Sox3 protein was abundant in the nuclei of Sertoli cells (Fig. 1A, white arrows and inset). Nuclear staining was not observed in Leydig cells or germ cells. Sox3 was not detected in ovaries by immunochemistry (Fig. 1D). However, Sox3 mRNA was detected in oocytes and cumulus granulosa cells purified after induced ovulation and in whole ovaries (Fig. 1G).
FIG. 1.
Expression of Sox3. Immunohistochemical staining was performed on a testis (A) (magnification, ×200; inset, ×400) and an ovary (D) (magnification, ×400). DAPI staining of testis (B) and ovary (E) was performed to identify cell nuclei, and merged images are presented for comparison (C and F). Arrows in panel A illustrate nuclear staining of Sertoli cells by Sox3. (G) Detection of Sox3 mRNA by RT-PCR, compared to the ribosomal gene RPL19. Controls are included in which RT was omitted to exclude amplification of contaminating genomic DNA.
Targeted deletion of Sox3.
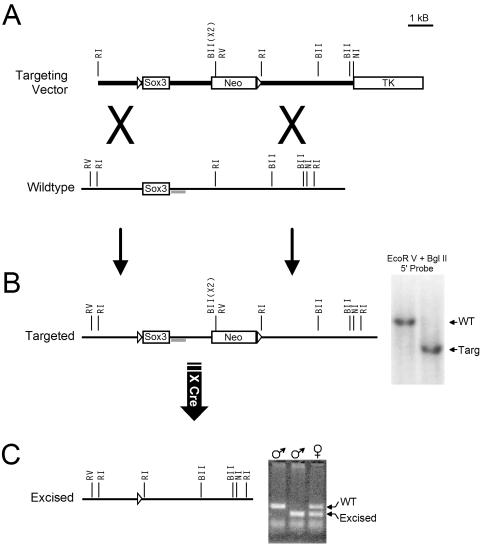
The X-chromosomal mouse Sox3 gene was targeted for deletion by homologous recombination with a construct containing LoxP sites in the 5′ UTR and downstream of the putative poly(A) signals (Fig. 2A). ES cell clones and chimeric animals were screened for correct recombination by Southern blotting (Fig. 2B), followed by PCR amplification and DNA sequencing to confirm the presence of the upstream LoxP site. Mating with CMV-Cre transgenic mice was allowed, and resultant offspring were screened by PCR for excision of the Sox3 gene (Fig. 2C). Excision was confirmed by sequencing of the PCR product. There was no evidence of embryonic lethality, and Sox3 KO mice of both sexes (Sox3−/Y and Sox3−/−) were born with the expected frequencies. Sox3 mRNA was detected in the gonads and cerebellums of WT mice of both sexes but was absent from KO mice (not shown).
FIG. 2.
Targeted mutagenesis of Sox3. (A) Sox3 is a single-exon gene located on the X chromosome. The upstream LoxP site (open triangle) was inserted into the 5′ UTR. The downstream LoxP site was inserted downstream of the poly(A) signal sequences adjacent to a Neo cassette. (B) To confirm correct 5′ recombination, genomic DNA from >350 ES cell clones was digested with EcoRV and BglII and subjected to Southern blotting with a probe (gray bar) immediately downstream of the Sox3 coding sequence. ES cells are male (XY) and Sox3 is X linked, so only a single band of either 5,040 bp (targeted) or 7,200 bp (WT) was observed for each clone. (C) Chimeric males were bred with WT females, and progeny that inherited the targeted allele were bred with CMV-Cre transgenic mice. WT and excised animal DNAs were detected by PCR as illustrated in the ethidium bromide-stained gel. The PCR products were eluted from the gel and sequenced to confirm the identities of the fragments and successful excision. Restriction enzymes are abbreviated as follows: RI, EcoRI; BII, BglII; RV, EcoRV; NI, NsiI.
Sporadic defects in growth and development of Sox3 KO mice.
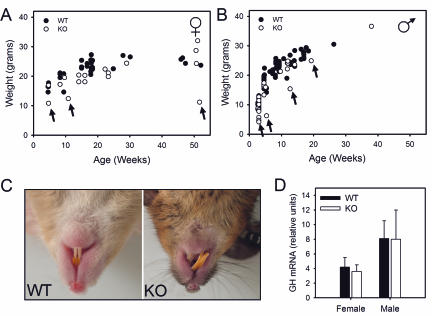
Growth curves for KO mice and WT littermates showed substantial overlap (Fig. 3A and B), and genotype was not a predictor of body weight. However, animals with low body weight were observed intermittently (arrows). Low-body-weight animals were all null for Sox3, and controlled breeding demonstrated that they were infertile. Overgrowth and misalignment of the upper and lower front teeth was observed in 50 to 70% of KO animals (Fig. 3C). There was not a strict correlation between grossly misaligned teeth and failure to grow, and animals of normal weight with tooth defects were observed. GH mRNA levels were similar for WT and KO animals (Fig. 3D).
FIG. 3.
Gross morphology of Sox3 KO mice. (A and B) Growth curves. Outliers are denoted by arrows. (C) Misaligned teeth in KO mice. (D) GH mRNA levels, presented as ratios to RPL19. n = 6 or 7.
Severely impaired fertility in female KO mice.
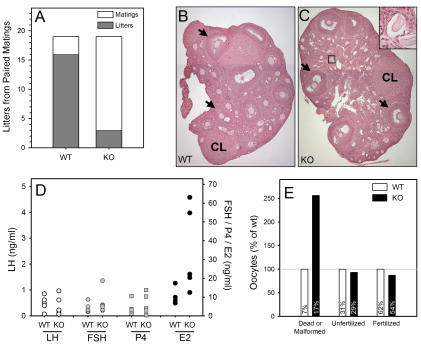
Phenotypically male and female mice were screened for Sry to detect possible sex reversal. Of 30 female and 40 male KO mice, no sex-reversed animals were observed. Male KO and heterozygous female (Sox3−/+) mice exhibited fertility rates equal to those of WT animals, with the exception of the low-body-weight animals noted above. Specifically, crosses of WT animals yielded 3.96 ± 0.53 pups per litter (means ± standard errors of the means [SEM]; n = 25), whereas matings to either heterozygous female or KO male mice averaged 4.88 ± 0.40 pups per litter (n = 26). In contrast, Sox3 KO females had a severe impairment in fertility. In 19 paired matings, 16 WT females gave birth compared to only 3 Sox3 KO females (Fig. 4A). Litter size was also larger on average for the WT animals (5.69 ± 0.62 pups per litter) than for the Sox3 KO females (3 pups per litter in each case).
FIG. 4.
Functional analysis of female Sox3 KO mice. (A) Fertility. WT and Sox3 KO females were paired and mated to WT males. (B) H&E-stained WT ovary. Magnification, ×50. Arrows, large antral follicles; CL, corpus luteum. (C) H&E-stained Sox3 KO ovary. Magnification, ×50. The small box surrounds atretic follicles that are enlarged to a higher magnification in the inset. (D) Hormone levels in sera (n = 5 to 8). (E) Ovulation induction. Six each of WT and KO females were subjected to superovulation, and ova for each genotype were harvested. Oocytes were categorized as dead-malformed or normal and then incubated with WT sperm to determine the rates of fertilization. Values within bars are percentages of the individual genotype pool (WT or KO). Within each category, data are presented as percentages of the WT level.
Ovarian defects in female KO mice.
Sox3 is expressed in the pituitary gland (unpublished observation), so gonadotropins were measured to determine if female infertility was secondary to defects in pituitary hormone secretion. However, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels were equivalent in WT and KO females (Fig. 4D). Ovaries from WT and KO animals were examined morphologically and functionally to identify other possible causes of infertility. Sox3 KO ovaries contained numerous small atretic follicles (Fig. 4C). Despite this, large antral follicles (black arrows) and corpora lutea (CL) were present with approximately equal frequencies in WT (Fig. 4B) and KO (Fig. 4C) ovaries. No atretic follicles were observed in ovaries from immature (3 to 3.5 weeks old) animals of any genotype, but they were present in ovaries from peripubertal (4.5 to 5.5 weeks old) Sox3 KO females.
Progesterone levels were normal in the Sox3 KO females, reflecting the presence of corpora lutea. However, estradiol levels were elevated in Sox3 KO females, despite normal levels of LH (Fig. 4D), consistent with the altered distribution of small follicles. To explore follicle health further, WT and KO females were subjected to ovulation induction and the harvested oocytes were fertilized with WT sperm and cultured in vitro to the morula stage. Sox3 KO females yielded fewer oocytes than WT females (148 versus 169 per six animals) and had a 2.6-fold increase in dead or malformed oocytes (Fig. 4E). The subsequent success rate for fertilization and development was similar for surviving WT and KO oocytes. No histological differences were observed in the uteri of Sox3 KO females (not shown). Thus, defects in oocyte development and maturation likely contribute to the observed loss of fertility of Sox3 KO females.
Hypogonadism in male KO mice.
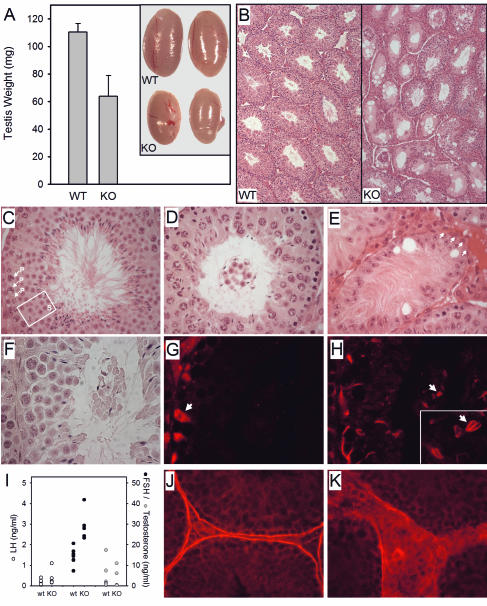
The testis weight of male KO mice (Sox3−/Y) was reduced 42% (Fig. 5A). Gross histological inspection revealed seminiferous tubules that were irregular in shape and small in diameter (Fig. 5B). On closer inspection, it was apparent that germ cell development was markedly abnormal in Sox3 KO testes. In comparison to that of a WT littermate (Fig. 5C), a mildly affected tubule from a KO mouse (Fig. 5D) has multiple layers of pachytene spermatocytes, and the round spermatids are virtually absent from the epithelium. A cluster of round and elongated spermatids is present in the lumen and is detached from the epithelium. In moderately affected tubules, the germ cell cluster is often embedded in a matrix of Sertoli cell projections (not shown), and in the most severe cases, the germ cells are lost entirely and the tubule exhibits a Sertoli-cell-only morphology (Fig. 5E). A number of large vacuoles are also present, a feature previously shown to reflect Sertoli cell dysfunction (33). Sperm counts were lower in KO (25.7 × 106 ± 4.2 × 106/ml) than in WT (63.6 × 106 ± 12.8 × 106) mice.
FIG. 5.
Characterization of male Sox3 KO mice. (A) Left testicle weight (n = 6 or 7); inset, left testicles from two WT and two KO males. (B) Cross section at midpoint of testis stained with H&E (magnification, ×50). (C to H, J, and K) Structural changes in seminiferous tubules of male Sox3 KO mice. Magnification, ×400, unless otherwise noted. Panels C to E illustrate degrees of damage observed. (C) Wild-type stage VII tubule. P, pachytene spermatocytes; S (box), round spermatids. (D) Mildly affected Sox3 KO tubule with detached cluster of germ cells. (E) Severely affected Sox3 KO tubule containing Sertoli cell bodies and projections only. Arrows, region in which the structural boundary of the tubule is missing. Note the presence of large Sertoli cell vacuoles. Panels F to H are serial sections of a Sox3 KO tubule stained with H&E (F), GATA-4 (G) (Sertoli cells), and espin (H) (Sertoli cell-germ cell junctions). Luminal elongated spermatids are joined by adherens junctions (H; inset magnification, ×1,000) in the absence of Sertoli cell bodies. (I) Hormone levels in sera (n = 5 to 8). Panels J and K show laminin staining of WT (J) and Sox3 KO (K) tubules, illustrating breakdown in the tubular boundary in KO mice.
The structure of the damaged tubules was explored further by immunohistochemical staining. Adjacent sections were stained with H&E (Fig. 5F), GATA-4 (Fig. 5G; stains somatic cells, with highest affinity for Sertoli cells), or espin (Fig. 5H; stains germ cell-Sertoli cell adherens junctions). The Sertoli cell bodies remained anchored to the base of the tubule (Fig. 5G, arrow), but the Sertoli cell projections failed to retain the germ cells within the epithelium; adherens junctions were observed enveloping the elongated spermatids within the detached luminal germ cell cluster (Fig. 5H, arrow; inset, arrow). A selective rise in FSH was observed despite normal levels of testosterone (Fig. 5I), further suggesting decreased Sertoli cell function (3).
The basement membrane of the seminiferous tubule was also disrupted in Sox3 KO mice (Fig. 5E, arrows). This is most apparent after staining with laminin, which is produced by the Sertoli and peritubular myoid cells (39) and forms the structural limit of the tubule. In contrast to the continuous band of laminin that encircles the WT tubules (Fig. 5J), laminin staining was diffuse and discontinuous in Sox3 KO testes, leading to an expansion of the interstitial space (Fig. 5K).
DISCUSSION
The Sox family of proteins is identified by sequence homology within the HMG box, a 79-amino-acid DNA-binding domain. Some members of this family exhibit overlapping expression patterns, but DNA sequence specificity may confer distinct functions. For example, Sry, Sox1, Sox2, and Sox3 can all bind the same DNA sequence motif, but with different affinities (10). Each of these four proteins is expressed in the developing brain and in the urogenital ridge (10), from which the gonads develop (25, 46). With the exception of Sry, the function of these proteins is unclear, and efforts to clarify their roles have been clouded by functional redundancies (6) and embryonic lethality (4). To address the latter concern while identifying any distinct role for Sox3, we used LoxP targeting before Cre excision to delete the Sox3 gene from mice.
We observed no evidence of embryonic lethality in the absence of Sox3. Specifically, litter sizes were similar in WT crosses and crosses that involved the Sox3 KO allele; male and female Sox3 KO animals were born at the expected frequencies. Nonetheless, there was some evidence of developmental defects. The most consistent of these abnormalities was an overgrowth and misalignment of the front teeth. This observation is notable, as some patients with a SOX3 mutation were reported to have dental crowding (16) associated with other facial abnormalities.
Humans with a mutation in SOX3 also exhibit variable mental retardation, ranging from a mild level to severe impairment requiring institutional care (16). Sox3 KO mice had no apparent disabling neurological deficit. Mice exhibited normal mobility, feeding, and grooming behaviors, though we cannot rule out a more subtle behavioral defect. One of the two families with a SOX3 mutation exhibited GH deficiency (20). In the other family, two boys were mentally retarded, but their grandfather with the same mutation was not. All of the patients with a SOX3 mutation and GH deficiency had short stature. In the Sox3 KO group, stunted mice were observed with low frequency, but no examples of GH deficiency were observed. Low body weight was observed in mice as early as 3 weeks of age, prior to weaning, and does not appear to be related to tooth defects that might otherwise impede feeding. It thus appears that Sox3 contributes to, but is not absolutely required for, multiple aspects of neurological and physical development in humans and mice.
The relatively mild developmental phenotype observed in Sox3 KO mice was unexpected given the extent and early onset of Sox3 expression in the developing central nervous system. It is notable, however, that all members of the group B1 Sox genes (Sox1, Sox2, and Sox3 [34]) exhibit HMG box sequence homology and functional redundancy, and all of these genes are expressed in overlapping patterns (8, 10, 46). Targeted deletion of the Sox1 gene causes microphthalmia, but as for the Sox3 KO, the mice are viable and exhibit only mild neurological deficits (32). Thus, Sox1 and Sox3 are not absolutely required for central nervous system development or their activities are redundant with those of another protein, potentially Sox2. This hypothesis is consistent with targeted disruption of Sox2, which causes early embryonic lethality (4). It is possible that Sox3 function is also subject to the influence of modifier genes (30), as there is variable penetrance in humans with SOX3 mutations. This issue can be explored further in mice by examining the phenotypes of the Sox3 KO mice in different genetic backgrounds.
A second major finding of our study is the absence of sex reversal in Sox3 KO animals of either sex. Several members of the Sox protein family are among those involved in sex determination. Sry, which is located on the Y chromosome, evolved relatively recently (14) and is sufficient to induce testis formation in XX mice (19) and in humans with SRY translocations (42). Sox9 is also sufficient to induce testis development in XX animals (43). The consequences of SOX9 deficiency remain unclear, although haploinsufficiency causes campomelic dysplasia and is frequently associated with ambiguous genitalia (12). Based primarily on its evolutionary relationship to Sry, Sox3 has been suggested as a possible antagonist of Sry and Sox9 in the mammalian sex determination pathway (15). Specifically, Sox3 has been proposed to repress Sox9 and to be itself a target of Sry inhibition (15, 26). This model predicted that the absence of Sox3 would have no effect on males but would cause XX sex reversal by relieving the inhibition of Sox9. Our data disprove this hypothesis; however, the loss of Sox3 was not without serious consequences for the reproductive system.
For male mice, we found that Sox3 is necessary for the normal formation of seminiferous tubules and for normal Sertoli cell function. The Sox3-deficient mice exhibit small, frequently fused seminiferous tubules with large vacuoles. Lumens are often filled with Sertoli cell cytoplasmic whorls and immature germ cells that have become detached from the epithelium. In the most severe cases, germ cell loss is complete. We observed numerous atretic follicles in female mice. Nonetheless, the presence of corpora lutea argues that these animals still undergo reproductive cycles and ovulate. Superovulation yielded relatively high numbers of dead or malformed oocytes, but those that remained could be fertilized with nearly normal efficiency and developed to the morula stage in culture. The Sox3 KO females delivered very few pups, however, suggesting a defect subsequent to the earliest stages of embryonic development. Histological examination of uteri revealed no differences between WT and Sox3 KO females. Thus, the picture that emerges is one in which oocytes from Sox3 KO mice are unhealthy from the earliest stages of ovarian development, leading to loss of efficiency at multiple points in the reproductive process.
Little evidence is available regarding a gonadal phenotype in humans with SOX3 mutations. For women, the data are limited to an analysis of eight patients with 46,XX sex reversal, which uncovered no mutations in the coding and flanking regions of SOX3 (21). A male patient with a genomic deletion that included SOX3 exhibited mental retardation and primary testicular failure, suggesting a possible role for SOX3 in human testis differentiation or function (38), but the gene involved was not determined. In the two families harboring SOX3 mutations described above, only males (SOX3−/Y) were affected owing to the X-chromosomal location of SOX3. A single heterozygous female was not reported to have any symptoms. In the larger family, which had X-linked mental retardation and GH deficiency, the four postpubertal males had normal LH, FSH, and testosterone levels, testis volume, and external genitalia (16). However, no histological examination of the testes was performed. In a different model of gonadal dysgenesis, deletion of Ahch (Dax1) caused a severe testicular phenotype in mice (48) that was only subsequently confirmed in humans (35). Thus, the possibility of gonadal defects in humans with SOX3 mutations remains open.
In chickens, Sox3 was detected in the primordial germ cells of both sexes (40). Expression studies of Sox3 in mouse embryonic gonadal ridges demonstrate that it is still present in gonads lacking germ cells, consistent with expression in somatic cells, probably the same pre-Sertoli cells that express Sry (10). This finding is supported by studies in adult mouse testes (36). Our data indicate that Sox3 protein is located in the nuclei of Sertoli cells and is not present in the germ cells.
Although Sox3 expression has not been localized in developing or adult mouse ovaries, there is evidence that the Sertoli and granulosa cell lineages are derived from a common precursor (1), predicting somatic (granulosa) cell expression. In the present study, we did not detect staining of Sox3 in ovaries (Fig. 1D), although Sox3 mRNA was present in ovulated oocytes and cumulus cells as well as in whole ovaries (Fig. 1G). These data suggest that Sox3 may be subject to translational control or that protein levels may be below the sensitivity of the Sox3 antibody. Sox3 mRNA levels were lower in the germ (oocyte) cells than in the somatic (cumulus) cells.
A functional requirement for Sox3 in supporting cells of the gonads (Sertoli cells and granulosa cells) is consistent with the pathologies observed in our Sox3 null mice. For both sexes, there is a loss of germ cells, and the critical roles of Sertoli and granulosa cells in maintaining the germ cells are well established (23, 24). In male mice, basal lamina breakdown can be attributed to Sertoli cell defects (39), and histological features such as vacuolization are common in syndromes of Sertoli cell dysfunction. In addition, the selective elevation of FSH is consistent with impaired Sertoli cells, likely reflecting decreased inhibin B secretion (2, 17).
In both the brain and the gonads, Sox3 is presumed to act as a transcription factor (10). We observed nuclear staining in the Sertoli cells (Fig. 1) and in the brain (not shown), consistent with this cellular activity. Nonetheless, there are no known target genes of Sox3, and at least one potential target has been shown not to be regulated by Sox3 (36). A survey of the genes known to be involved in germ cell function provides a number of potential candidates for Sox3 action (23, 24). Similarly, a review of chromosomal abnormalities present in mentally retarded patients suggests that a broad array of genes, mostly uncharacterized, underlie this syndrome as well (47). More studies will be required before the complex regulatory networks that support gonadal determination and brain differentiation are deciphered.
In summary, we have deleted Sox3 from mice to test the hypothesis that it is involved in the genetic cascade that leads to gonadal determination and to provide a model for further studies of human SOX3 mutations. Brain development was grossly normal, and our data further demonstrate that both male and female gonads develop according to genotype in the absence of Sox3. However, mild developmental defects were observed and the competence of the gonads is compromised in both sexes, probably due to failure of the Sertoli and granulosa cells to fully support their dependent germ cells.
Acknowledgments
This research was supported by NIH grants HD21921, HD28934, and HD043425.
We thank S. Pillai and T. Russell for valuable assistance, A. Nagy, R. Nagy, and W. Abramow-Newerly for providing the ES cells, and T. Edlund for providing the Sox3 antibody.
REFERENCES
- 1.Albrecht, K. H., and E. M. Eicher. 2001. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev. Biol. 240:92-107. [DOI] [PubMed] [Google Scholar]
- 2.Anawalt, B. D., R. A. Bebb, A. M. Matsumoto, N. P. Groome, P. J. Illingworth, A. S. McNeilly, and W. J. Bremner. 1996. Serum inhibin B levels reflect Sertoli cell function in normal men and men with testicular dysfunction. J. Clin. Endocrinol. Metab. 81:3341-3345. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, R. A., and R. M. Sharpe. 2000. Regulation of inhibin production in the human male and its clinical applications. Int. J. Androl. 23:136-144. [DOI] [PubMed] [Google Scholar]
- 4.Avilion, A. A., S. K. Nicolis, L. H. Pevny, L. Perez, N. Vivian, and R. Lovell-Badge. 2003. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 17:126-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartles, J. R., A. Wierda, and L. Zheng. 1996. Identification and characterization of espin, an actin-binding protein localized to the F-actin-rich junctional plaques of Sertoli cell ectoplasmic specializations. J. Cell Sci. 109:1229-1239. [DOI] [PubMed] [Google Scholar]
- 6.Bergstrom, D. E., M. Young, K. H. Albrecht, and E. M. Eicher. 2000. Related function of mouse SOX3, SOX9, and SRY HMG domains assayed by male sex determination. Genesis 28:111-124. [PMC free article] [PubMed] [Google Scholar]
- 7.Birk, O. S., D. E. Casiano, C. A. Wassif, T. Cogliati, L. Zhao, Y. Zhao, A. Grinberg, S. Huang, J. A. Kreidberg, K. L. Parker, F. D. Porter, and H. Westphal. 2000. The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature 403:909-913. [DOI] [PubMed] [Google Scholar]
- 8.Brunelli, S., E. Silva Casey, D. Bell, R. Harland, and R. Lovell-Badge. 2003. Expression of Sox3 throughout the developing central nervous system is dependent on the combined action of discrete, evolutionarily conserved regulatory elements. Genesis 36:12-24. [DOI] [PubMed] [Google Scholar]
- 9.Clark, A. M., K. K. Garland, and L. D. Russell. 2000. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol. Reprod. 63:1825-1838. [DOI] [PubMed] [Google Scholar]
- 10.Collignon, J., S. Sockanathan, A. Hacker, M. Cohen-Tannoudji, D. Norris, S. Rastan, M. Stevanovic, P. N. Goodfellow, and R. Lovell-Badge. 1996. A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development 122:509-520. [DOI] [PubMed] [Google Scholar]
- 11.Colvin, J. S., R. P. Green, J. Schmahl, B. Capel, and D. M. Ornitz. 2001. Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell 104:875-889. [DOI] [PubMed] [Google Scholar]
- 12.Foster, J. W., M. A. Dominguez-Steglich, S. Guioli, G. Kowk, P. A. Weller, M. Stevanovic, J. Weissenbach, S. Mansour, I. D. Young, P. N. Goodfellow, et al. 1994. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372:525-530. [DOI] [PubMed] [Google Scholar]
- 13.Goodfellow, P. N., and G. Camerino. 1999. DAX-1, an “antitestis” gene. Cell. Mol. Life Sci. 55:857-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graves, J. A. 1998. Evolution of the mammalian Y chromosome and sex-determining genes. J. Exp. Zool. 281:472-481. [PubMed] [Google Scholar]
- 15.Graves, J. A. 1998. Interactions between SRY and SOX genes in mammalian sex determination. Bioessays 20:264-269. [DOI] [PubMed] [Google Scholar]
- 16.Hamel, B. C., A. P. Smits, B. J. Otten, B. van den Helm, H. H. Ropers, and E. C. Mariman. 1996. Familial X-linked mental retardation and isolated growth hormone deficiency: clinical and molecular findings. Am. J. Med. Genet. 64:35-41. [DOI] [PubMed] [Google Scholar]
- 17.Illingworth, P. J., N. P. Groome, W. Byrd, W. E. Rainey, A. S. McNeilly, J. P. Mather, and W. J. Bremner. 1996. Inhibin-B: a likely candidate for the physiologically important form of inhibin in men. J. Clin. Endocrinol. Metab. 81:1321-1325. [DOI] [PubMed] [Google Scholar]
- 18.Jeays-Ward, K., C. Hoyle, J. Brennan, M. Dandonneau, G. Alldus, B. Capel, and A. Swain. 2003. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development 130:3663-3670. [DOI] [PubMed] [Google Scholar]
- 19.Koopman, P., J. Gubbay, N. Vivian, P. Goodfellow, and R. Lovell-Badge. 1991. Male development of chromosomally female mice transgenic for Sry. Nature 351:117-121. [DOI] [PubMed] [Google Scholar]
- 20.Laumonnier, F., N. Ronce, B. C. Hamel, P. Thomas, J. Lespinasse, M. Raynaud, C. Paringaux, H. Van Bokhoven, V. Kalscheuer, J. P. Fryns, J. Chelly, C. Moraine, and S. Briault. 2002. Transcription factor SOX3 is involved in X-linked mental retardation with growth hormone deficiency. Am. J. Hum. Genet. 71:1450-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim, H. N., G. D. Berkovitz, I. A. Hughes, and J. R. Hawkins. 2000. Mutation analysis of subjects with 46, XX sex reversal and 46, XY gonadal dysgenesis does not support the involvement of SOX3 in testis determination. Hum. Genet. 107:650-652. [DOI] [PubMed] [Google Scholar]
- 22.Luo, X., Y. Ikeda, and K. L. Parker. 1994. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481-490. [DOI] [PubMed] [Google Scholar]
- 23.Matzuk, M. M., K. H. Burns, M. M. Viveiros, and J. J. Eppig. 2002. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 296:2178-2180. [DOI] [PubMed] [Google Scholar]
- 24.Matzuk, M. M., and D. J. Lamb. 2002. Genetic dissection of mammalian fertility pathways. Nat. Cell Biol. Nat. Med. [DOI] [PubMed]
- 25.Mayer, A., G. Mosler, W. Just, C. Pilgrim, and I. Reisert. 2000. Developmental profile of Sry transcripts in mouse brain. Neurogenetics 3:25-30. [DOI] [PubMed] [Google Scholar]
- 26.McElreavey, K., E. Vilain, N. Abbas, I. Herskowitz, and M. Fellous. 1993. A regulatory cascade hypothesis for mammalian sex determination: SRY represses a negative regulator of male development. Proc. Natl. Acad. Sci. USA 90:3368-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meeks, J. J., S. E. Crawford, T. A. Russell, K. I. Morohashi, J. Weiss, and J. L. Jameson. 2003. Dax1 regulates testis cord organization during gonadal differentiation. Development 130:1029-1036. [DOI] [PubMed] [Google Scholar]
- 28.Meeks, J. J., J. Weiss, and J. L. Jameson. 2003. Dax1 is required for testis determination. Nat. Genet. 34:32-33. [DOI] [PubMed] [Google Scholar]
- 29.Morrish, B. C., and A. H. Sinclair. 2002. Vertebrate sex determination: many means to an end. Reproduction 124:447-457. [DOI] [PubMed] [Google Scholar]
- 30.Nadeau, J. H. 2001. Modifier genes in mice and humans. Nat. Rev. Genet. 2:165-174. [DOI] [PubMed] [Google Scholar]
- 31.Nagy, A., J. Rossant, R. Nagy, W. Abramow-Newerly, and J. C. Roder. 1993. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA 90:8424-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishiguchi, S., H. Wood, H. Kondoh, R. Lovell-Badge, and V. Episkopou. 1998. Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 12:776-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell, L., R. Ettlin, A. Sinha Hikim, and E. Clegg. 1990. Histological and histopathological evaluation of the testis. Cache River Press, Clearwater, Fla.
- 34.Schepers, G. E., R. D. Teasdale, and P. Koopman. 2002. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev. Cell 3:167-170. [DOI] [PubMed] [Google Scholar]
- 35.Seminara, S. B., J. C. Achermann, M. Genel, J. L. Jameson, and W. F. Crowley, Jr. 1999. X-linked adrenal hypoplasia congenita: a mutation in DAX1 expands the phenotypic spectrum in males and females. J. Clin. Endocrinol. Metab. 84:4501-4509. [DOI] [PubMed] [Google Scholar]
- 36.Shen, J. H., and H. A. Ingraham. 2002. Regulation of the orphan nuclear receptor steroidogenic factor 1 by Sox proteins. Mol. Endocrinol. 16:529-540. [DOI] [PubMed] [Google Scholar]
- 37.Sinclair, A. H., P. Berta, M. S. Palmer, J. R. Hawkins, B. L. Griffiths, M. J. Smith, J. W. Foster, A. M. Frischauf, R. Lovell-Badge, and P. N. Goodfellow. 1990. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346:240-244. [DOI] [PubMed] [Google Scholar]
- 38.Stevanovic, M., R. Lovell-Badge, J. Collignon, and P. N. Goodfellow. 1993. SOX3 is an X-linked gene related to SRY. Hum. Mol. Genet. 2:2013-2018. [DOI] [PubMed] [Google Scholar]
- 39.Tung, P. S., M. K. Skinner, and I. B. Fritz. 1984. Cooperativity between Sertoli cells and peritubular myoid cells in the formation of the basal lamina in the seminiferous tubule. Ann. N. Y. Acad. Sci. 438:435-446. [DOI] [PubMed] [Google Scholar]
- 40.Uchikawa, M., Y. Kamachi, and H. Kondoh. 1999. Two distinct subgroups of group B Sox genes for transcriptional activators and repressors: their expression during embryonic organogenesis of the chicken. Mech. Dev. 84:103-120. [DOI] [PubMed] [Google Scholar]
- 41.Vainio, S., M. Heikkila, A. Kispert, N. Chin, and A. P. McMahon. 1999. Female development in mammals is regulated by Wnt-4 signalling. Nature 397:405-409. [DOI] [PubMed] [Google Scholar]
- 42.Van der Auwera, B., N. Van Roy, A. De Paepe, J. R. Hawkins, I. Liebaers, S. Castedo, J. Dumon, and F. Speleman. 1992. Molecular cytogenetic analysis of XX males using Y-specific DNA sequences, including SRY. Hum. Genet. 89:23-28. [DOI] [PubMed] [Google Scholar]
- 43.Vidal, V. P., M. C. Chaboissier, D. G. de Rooij, and A. Schedl. 2001. Sox9 induces testis development in XX transgenic mice. Nat. Genet. 28:216-217. [DOI] [PubMed] [Google Scholar]
- 44.Wagner, T., J. Wirth, J. Meyer, B. Zabel, M. Held, J. Zimmer, J. Pasantes, F. D. Bricarelli, J. Keutel, E. Hustert, et al. 1994. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79:1111-1120. [DOI] [PubMed] [Google Scholar]
- 45.Wilson, S. I., A. Rydstrom, T. Trimborn, K. Willert, R. Nusse, T. M. Jessell, and T. Edlund. 2001. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature 411:325-330. [DOI] [PubMed] [Google Scholar]
- 46.Wood, H. B., and V. Episkopou. 1999. Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre-gastrulation to early somite stages. Mech. Dev. 86:197-201. [DOI] [PubMed] [Google Scholar]
- 47.Xu, J., and Z. Chen. 2003. Advances in molecular cytogenetics for the evaluation of mental retardation. Am. J. Med. Genet. 117C:15-24. [DOI] [PubMed] [Google Scholar]
- 48.Yu, R. N., M. Ito, T. L. Saunders, S. A. Camper, and J. L. Jameson. 1998. Role of Ahch in gonadal development and gametogenesis. Nat. Genet. 20:353-357. [DOI] [PubMed] [Google Scholar]