Abstract
We have investigated a role for the amino-terminal FERM-like domain of the focal adhesion kinase (FAK) as a negative regulator of its own activity and phosphorylation state. Deletion of the first 375 amino acids from the amino terminus of FAK increases its catalytic activity in vitro, its phosphorylation when expressed in mammalian cells, and the phosphorylation of a FAK substrate, paxillin. Deletion mutants are phosphorylated in suspension, suggesting that they are no longer regulated by adhesion. The amino terminus of FAK can interact with the kinase domain of FAK in vitro and in vivo, suggesting that it might act as an autoinhibitor of FAK activity. The amino terminus of FAK can act in trans to inhibit FAK phosphorylation when expressed in mammalian cells or to directly inhibit FAK activity in vitro. Expression of the amino terminus of FAK inhibits cell cycle progression in CHO cells, consistent with its inhibition of FAK phosphorylation and function in trans. A glutathione S-transferase fusion protein containing the cytoplasmic tail of the β1 integrin stimulates FAK activity in vitro, suggesting that FAK could be regulated by molecular interactions with the amino terminus. Based on these and previous data, we propose a working model for activation of FAK in cell adhesion.
Focal adhesion kinase (FAK) is a 125-kDa tyrosine kinase whose activity and phosphorylation status are regulated by cell adhesion to the extracellular matrix through integrins (25, 28, 40). FAK is maintained in a dephosphorylated and inactive state when cells are held in suspension. Upon attachment of cells to the extracellular matrix, FAK becomes localized to focal adhesions and is activated. Such regulation positions FAK as a key enzyme in transmitting information from integrins to the various signaling pathways that coordinate events that modulate or are dependent on cell adhesion. Indeed, FAK has been implicated as an important regulator of a variety of developmental and signaling pathways, including those that regulate cellular events such as cell cycle progression, cell survival, and cell migration (20, 23, 24, 28, 40).
Previous studies suggest that a number of factors contribute to the activation of FAK by cell adhesion. FAK's ability to localize to focal adhesions appears to be critical in promoting its phosphorylation. Most FAK mutants that contain deletions or point mutations in their carboxy-terminal domains which abolish their ability to be recruited into focal adhesions no longer become strongly phosphorylated (9, 32). Autophosphorylation of FAK at Y397 is critical for many FAK-dependent functions, including phosphorylation of FAK on other residues (2, 3). Phosphorylation of Y397 creates a binding site for several SH2 domain-containing molecules, including phosphatidylinositol 3-kinase, Grb7, and Src (5, 16, 26, 39). Mutation of this residue to phenylalanine blocks phosphorylation of FAK on residues within its kinase domain and carboxy terminus (2). Clustering of chimeric molecules containing the extracellular and transmembrane domains of the interleukin-2 receptor and the cytoplasmic domain of β integrins can induce FAK phosphorylation, suggesting that aggregation of β integrin cytoplasmic domains and their associated proteins is sufficient to allow phosphorylation of FAK (1). Lastly, a recent report suggests a role for intermolecular transphosphorylation of FAK in FAK activation, as induction of FAK/gyrase B chimera dimerization led to increased FAK phosphorylation (34).
The above data are consistent with a model in which integrin-induced clustering of FAK into focal adhesions is responsible for FAK's activation by integrins. However, other data suggest that although it may be required for FAK activation, focal adhesion localization is not sufficient for FAK activation. For example, Wennerberg et al. have recently described a β1 integrin tail mutant that allows FAK's localization to focal adhesions but does not support FAK phosphorylation (37). Similarly, although the focal adhesion targeting (FAT) sequence in FAK is necessary and sufficient for FAK's localization to focal adhesions, other regions of FAK have been shown to play a role in the regulation of FAK's activity (10, 29, 34). Lastly, but perhaps most importantly, current data do not address any potential conformational changes within FAK that are likely involved in its response to factors involved in its activation by integrins.
FAK's amino-terminal domain contains a region of approximately 300 amino acids that has been suggested to have some similarity to the FERM domains of the ERM proteins and Band4.1 (14). FERM domains have been shown to be mediators of both protein-protein interactions and protein-membrane interactions. The roles of the amino-terminal domain of FAK, including its FERM-like domain, are largely not understood. Another tyrosine kinase, JAK, also contains a divergent FERM domain. Interestingly, JAK has recently been proposed to have an intramolecular interaction between its FERM domain and its functional kinase domain (42). In this case, it was proposed that the FERM domain of JAK helps to reorganize the kinase domain to make it fully active. In our report, we show that the amino-terminal domain of FAK interacts with its own kinase domain and that functionally this interaction has a negative effect on FAK's intrinsic catalytic activity. We propose that disruption of this interaction is necessary for full FAK activation and might be important for the regulation of FAK by integrins.
MATERIALS AND METHODS
Materials.
Glutathione-agarose beads, mouse antibody against BrdU, protein A-Sepharose, human plasma fibronectin (FN), and polyE4Y1 were purchased from Sigma (St. Louis, Mo.). Nickel-nitrilotriacetic acid agarose was purchased from Bio-Rad (Hercules, Calif.). Lipofectamine was purchased from Invitrogen (Carlsbad, Calif.). [γ-32P]ATP was purchased from Perkin-Elmer Life Sciences (Boston, Mass.). The mouse phosphotyrosine antibody PY20, the mouse antibody against paxillin, and the mouse antibody against FAK were purchased from Transduction Laboratories (Lexington, Ky.). The rabbit antibody against the carboxy terminus of FAK (C-20), the mouse antibody against the Myc tag (9E10), and the rabbit antibody against hemagglutinin (HA) (Y-11) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). The rabbit polyclonal FAK site-specific phosphorylation antibody, PY397, was purchased from Upstate (Charlottesville, Va.). The mouse antibody against HA (12CA5) was previously described (6).
DNA constructs.
pKH3-K, carrying the gene for the kinase domain of FAK (residues 401 to 664), and pKH3-C, carrying the gene for residues 676 to 1052, were previously described (36). pEGFP-C3-Paxillin was a gift from C. E. Turner. Recombinant baculovirus encoding HA-tagged FAK-wt (Δ29), HA-tagged Δ375-FAK (ΔN), and HA-tagged FAK-KD were previously described (6). Recombinant baculovirus encoding His-tagged full-length FAK was a gift from F. Matsumura of Rutgers University and was described previously (38). pKH3-N, carrying the gene for the FAK amino-terminal domain (residues 1 to 400), was constructed by amplifying pBS-FAK with the FAK 5′ BamHI (GTGGATCCATGGCAGCAGCTTACCTTGATCC) and FAK 400 3′ BamHI (GTGGATCCTTATATCTCTGCATACATCTG) primers by PCR. The resulting fragment was inserted into pKH3 at the BamHI site. Plasmids carrying the genes for GST-N and Myc-N were generated by cutting pKH3-N with BamHI and subcloning the resulting fragment into the pGEX2T and pHAN vectors, respectively. pGEX2T-N was transformed into BL21 cells, and pHAN-N was transformed into DH5α. The pHAN vector was previously described (17). pHAN-FAK, carrying the genes for His- and Myc-tagged FAK, was generated by replacing an EcoRI fragment of pHAN-N with that from the corresponding segment excised from pKH3-FL-FAK. pKH3-FL-FAK plasmid was generated from the pKH3-FAK (lacking the first 29 residues of FAK) described previously (7). An EcoRI fragment was excised from pKH3-N (which includes the first 29 residues of FAK) and used to replace the corresponding segments in plasmids pKH3-FAK. pKH3-Δ124 was generated by cutting pKH3-FL-FAK with EcoRI. The resulting fragment was purified and cloned into pKH3 at the EcoRI site. pKH3-Δ375 was generated by cutting pKH3-FL-FAK with MscI and EcoRV. The resulting fragment was purified and ligated into pEGFP-C3 at the SmaI site to first generate pEGFP versions of these constructs. The pEGFP constructs were then excised with EcoRI. The EcoRI-EcoRI fragment was purified and cloned into pKH3 at the EcoRI site, and the resulting plasmids were transformed into DH5α. pKH3-Δ400 was generated by amplifying pKH3-FL-FAK with the FAK 401 5′ BamHI (CAGGATCCATAGATGAAGAAGATACTTATACAATGCC) and 3′ pKH3 (GGACAAACCACAACTAGAATGCAG) primers. The resulting fragment was purified and digested with EcoRI and inserted into the pKH3 vector. The plasmid encoding GST-β1cyto was described previously (6).
In vitro kinase assays.
Seventy percent confluent sf21 insect cells were infected with recombinant baculovirus to express either His-tagged FAK or HA-tagged FAK-wt, Δ375-FAK, or FAK-KD. His-tagged FAK was purified as previously described (38). HA-tagged FAK or its mutants were immunoprecipitated with an antibody against the HA tags (Y-11) and immobilized on protein A-Sepharose as described below. For experiments using the HA-tagged FAK constructs, the amount of FAK present in the immunoprecipitation was first equalized by adding additional protein A-Sepharose if needed. For experiments using the purified His-tagged FAK, 250 ng of FAK was incubated in all reactions. Kinase assays were carried out at 25°C for 25 min in a buffer containing 50 mM Tris (pH 7.4), 10 mM MnCl2, 2 mM MgCl2, and 10 μCi of [γ-32P]ATP as described previously (15). The peptide substrate polyE4Y1 and purified fusion proteins were added as indicated. Reactions were carried out in quadruplicate, quantified with a PhosphorImager (Amersham Biosciences), and represented as averages of four reactions ± one standard deviation.
Cell culture and transfections.
293 HEK cells and FAK−/− cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Invitrogen). CHO cells were maintained in F12 medium supplemented with 10% fetal bovine serum (Invitrogen). NIH 3T3 cells were maintained in DMEM supplemented with 10% calf serum (Invitrogen). Cells were transfected with mammalian expression plasmids, as indicated, by use of Lipofectamine following the manufacturer's instructions. Experiments were conducted 24 h after transfection unless otherwise indicated. Cells, which were replated or held in suspension, were serum starved for an additional 18 h. The cells were then briefly digested with trypsin, resuspended in DMEM containing a final concentration of 0.5 mg of soybean trypsin inhibitor (Sigma) per ml, washed twice in DMEM, and held in suspension for 60 min. The indicated cells were lysed. Cells replated with FN were added to plates coated with 10 μg of FN per ml for 60 min after being held in suspension.
Preparation of fusion proteins.
The pGEX2T constructs encoding various glutathione S-transferase (GST) fusion proteins were transformed into BL21, and the bacteria were grown to an optical density (at 600 nm) of 1.0. They were then induced with 0.5 mM isopropyl-β-thiogalactopyranoside for 4 h at 37°C. The bacteria were pelleted and lysed with a buffer containing 5 mg of lysozyme per ml in 1% Triton X-100, 5% glycerol, 200 mM NaCl, 50 mM Tris (pH 8.0), 1 mM phenylmethylsulfonyl fluoride, 0.1 trypsin inhibitor units of aprotinin per ml, 20 mM EDTA, and 20 μg of leupeptin per ml by freezing-thawing three times. The lysates were then clarified by centrifugation and immobilized on glutathione-agarose beads for 30 min at 4°C. The beads were washed four times in lysis buffers and four times in 50 mM Tris (pH 8.0)-200 mM NaCl. For in vitro kinase assays with the GST and GST-N fusion proteins, the proteins were eluted from the glutathione beads in a buffer containing 5 mM reduced glutathione, 50 mM Tris (pH 8.0), and 50 mM NaCl.
In vitro binding assays.
GST or GST-N was purified from Escherichia coli and immobilized on glutathione-agarose as described above. The concentration of fusion protein on the glutathione beads was equalized by the addition of glutathione-agarose as needed. 293 HEK cells were transfected with pKH3-K, pKH3-C, pKH3-Δ375, and pKH3-FL-FAK as described above. Cells were lysed in a buffer containing 1% Triton X-100, 5% glycerol, 50 mM Tris (pH 7.4), 200 mM NaCl, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 0.1 trypsin inhibitor units of aprotinin per ml, and 20 μg of leupeptin per ml and were centrifuged for 40 min at 4°C to remove debris. Two hundred fifty micrograms of total protein was incubated with the indicated fusion proteins for 4 h at 4°C while rotating. Unbound proteins were removed from proteins by washing four times in lysis buffer, and bound proteins were removed from the beads by adding sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and boiling for 5 min and were immunoblotted as described below.
Immunoprecipitation and immunoblotting.
Subconfluent cells were washed twice with ice-cold phosphate-buffered saline and then lysed with 1% Nonidet P-40 lysis buffer (20 mM Tris [pH 8.0], 137 mM NaCl, 1% Nonidet P-40, 10% glycerol, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 10 mg of aprotinin per ml, and 20 μg of leupeptin per ml) unless otherwise indicated. Lysates were cleared by centrifugation, and total protein concentration was determined by the Bio-Rad protein assay. Immunoprecipitations were carried out by incubating cell lysates with appropriate antibodies for 3 h at 4°C, followed by incubation for 1 h with protein A-Sepharose 4B beads (Sigma). After washing, immune complexes were resolved by SDS-PAGE. Immunoblotting was carried out with horseradish peroxidase-conjugated immunoglobulin G as a secondary antibody and the Amersham Biosciences ECL system for detection.
BrdU incorporation assays.
pKH3-FL-FAK, pKH3-Δ375, or pKH3-N was transiently expressed in CHO cells. Immediately after transfection, the cells were serum starved for 18 h to arrest the cells in G0. BrdU incorporation assays were conducted as described previously (41). Briefly, cells were released from G0 by replating the cells in 10% fetal bovine serum and 150 μM BrdU. After the indicated time of growth, the cells were fixed, treated with DNase I, and processed for immunofluorescence against the HA epitope and BrdU. The percentages of nontransfected and transfected cells that had incorporated BrdU are represented as means of three experiments of 50 to 100 cells counted per condition. The error bars represent 1 standard deviation.
Immunofluorescence staining.
Cells were processed for immunofluorescence staining as described previously (41). The primary antibodies used were anti-HA polyclonal antibody Y-11 (1:200), anti-FAK paxillin monoclonal antibody (1:100), and Texas Red-conjugated phalloidin (1:50; Molecular Probes, Eugene, Oreg.). Texas Red-conjugated goat anti-mouse antibody (1:100; Jackson ImmunoResearch Laboratory, West Grove, Pa.) and fluorescein isothiocyanate-conjugated goat anti-rabbit antibody (1:100; Jackson ImmunoResearch Laboratory) were used as the secondary antibodies.
RESULTS
Deletion of FAK's amino-terminal domain increases its activity and phosphorylation state.
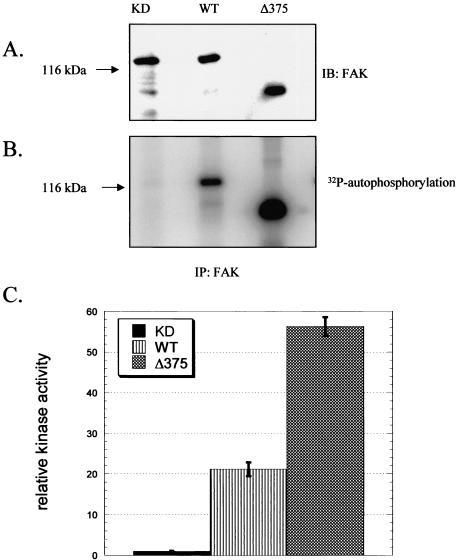
To investigate the potential role of FAK's amino-terminal domain in the regulation of its activity, we first performed in vitro kinase assays using FAK and its mutants expressed and purified from a recombinant insect cell expression system. Triple-HA-tagged wild-type (wt), kinase-dead (KD), and amino-terminal truncation mutant Δ375 FAK were expressed and immunoprecipitated from sf21 insect cells by use of an antibody against the HA tag of each construct and were subjected to in vitro kinase assays. We found that deletion of 375 amino acids from the amino terminus of FAK increased its autophosphorylation severalfold over that of FAK-wt (Fig. 1A and B). Similarly, the Δ375 mutant of FAK exhibited an approximately threefold increase in activity toward the peptide substrate E4Y1 compared to that of FAK-wt (Fig. 1C). These results suggest that the amino-terminal domain has some inhibitory effect on FAK's catalytic activity.
FIG. 1.
Deletion of FAK's amino-terminal domain increases its activity in vitro. HA-tagged FAK-wt, FAK-KD, and Δ375 FAK were immunoprecipitated from insect cells. Immunoprecipitates were analyzed by immunoblotting with anti-FAK antibody (A) or were subjected to an in vitro kinase assay in the absence (B) or presence (C) of E4Y1. Panel B shows the incorporation of 32P into FAK as visualized on a phosphorimager. In panel C, incorporation of 32P into E4Y1 was quantified by use of a phosphorimager. For convenience, the signal for FAK-KD was defined as 1, and other values are expressed as percent increases over this signal.
Next, we examined the phosphorylation state of several different amino-terminal deletions of FAK when transiently expressed in mammalian cells. Full-length FAK or the amino-terminal FAK deletion mutants Δ29, Δ124, Δ375, and Δ400 (Fig. 2A) were transiently transfected into FAK−/− cells and immunoprecipitated from cell lysates by use of an antibody against the carboxy-terminal region of FAK. They were then analyzed for tyrosine phosphorylation by immunoblotting with the antiphosphotyrosine antibody PY20. As shown in Fig. 2B, deletion of 124 amino acids or more from the amino terminus of FAK led to increased phosphorylation of the FAK mutants when compared to full-length FAK. Deletion of 29 amino acids, however, did not increase FAK phosphorylation over that of full-length FAK. Interestingly, the Δ400 mutant lacking the FAK autophosphorylation site of Y397 was also highly phosphorylated, indicating that the increased phosphorylation observed for the deletion mutants may be attributed to increased phosphorylation of sites other than Y397. Similar results were obtained when these mutants were analyzed in 293 and CHO cells (data not shown).
FIG. 2.
Deletion of FAK's amino-terminal domain increases its phosphorylation content and activity in mammalian cells. (A) pKH3-FL-FAK and the other pKH3 constructs shown were expressed in FAK−/− cells. (B) FAK was immunoprecipitated by using the C-20 antibody against FAK, and the immunoprecipitates were immunoblotted with PY20 or a mouse antibody against FAK, as indicated. (C and D) 293 cells were cotransfected with GFP-paxillin and pKH3-FL-FAK, pKH3-Δ375, or pKH3-Δ400 (C) or just the various FAK constructs, as indicated (D). Paxillin was immunoprecipitated from the cells, and paxillin immunoprecipitates were immunoblotted with PY20 (top) or a mouse antibody against paxillin (middle). Aliquots of whole-cell lysates (WCL) were immunoblotted with the Y-11 antibody against the HA tag of the exogenously expressed FAK (bottom). Molecular mass markers (in kilodaltons) are shown on the left for panel D.
To see whether the increased phosphorylation and activity of the amino-terminal deletions were capable of increasing the phosphorylation of a FAK substrate in mammalian cells, we cotransfected 293 cells with a plasmid carrying the genes for GFP-paxillin and full-length FAK or the FAK mutant Δ375 or Δ400 and compared the level of paxillin phosphorylation. Figure 2C shows that expression of Δ375 or Δ400 FAK increased the phosphorylation of paxillin about twofold over the increase caused by full-length FAK. Similar results were also obtained in FAK−/− cells (data not shown). Furthermore, the FAK amino-terminal domain truncation mutants also induced more tyrosine phosphorylation of endogenous paxillin than did full-length FAK (Fig. 2D). Taken together, these data suggest that the amino-terminal domain of FAK has an inhibitory effect on FAK's activity that is relieved when the domain is removed.
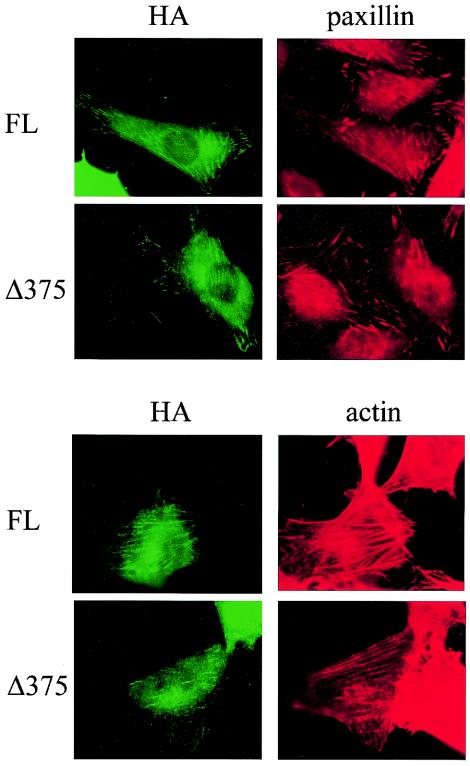
Previous studies suggested that the focal adhesion targeting sequence is within the carboxy-terminal domain of FAK (18). Consistent with this, we found that deletion of the amino-terminal domain of FAK did not prevent its localization to focal contacts (Fig. 3). These studies also showed that expression of the Δ375 FAK mutant did not alter cell adhesion, focal contact formation, or paxillin localization to focal contacts. Similar results were obtained in NIH 3T3 cells (Fig. 3) and CHO cells (data not shown).
FIG. 3.
Subcellular localization of FAK amino-terminal domain truncation mutant. NIH 3T3 cells were transfected with plasmids encoding HA-tagged full-length FAK or Δ375 mutant, as indicated. Two days after transfection, the cells were fixed and costained with anti-HA and anti-paxillin antibodies (top four panels) or anti-HA antibody and phalloidin (lower four panels), as indicated.
The FAK truncation mutants are phosphorylated in suspended cells.
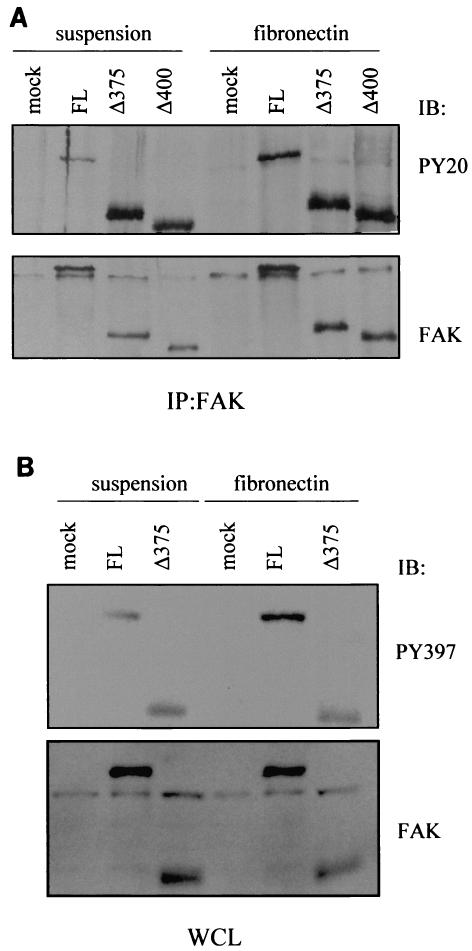
FAK's phosphorylation state is closely regulated by the adhesion state of the cell. Our data suggest that deletion of the amino-terminal region of FAK increases its activity. This increased activity could allow the FAK truncation mutants to escape their normal regulation. To test this possibility, full-length, Δ375, or Δ400 FAK was transiently expressed in CHO cells. After transfection, cells were lysed after being placed in suspension for 60 min or lysed after being replated on FN for 60 min. Full-length FAK or its truncation mutants were immunoprecipitated with an antibody that recognizes the carboxy-terminal region of FAK and were immunoblotted with either anti-FAK or PY20. Figure 4A shows that full-length FAK has a decreased phosphorylation content in suspended cells compared to that in cells replated on FN. Interestingly, cell suspension had no effect on either of the FAK truncation mutants. Δ375 and Δ400 FAK remained highly phosphorylated in both suspended and replated cells. To confirm that the autophosphorylation of the FAK amino-terminal truncation mutant is independent of cell adhesion, lysates from cells that had been transfected with vectors encoding full-length or Δ375 mutant FAK were immunoblotted with an antibody against FAK PY397. Figure 4B shows that, as expected, full-length FAK had reduced autophosphorylation at Y397 in suspended cells compared to cells adhered to FN. In contrast, the autophosphorylation of the Δ375 mutant was similar in both suspended and attached cells. Together, these results suggest that truncation of FAK's amino-terminal domain leads to constitutive activation of FAK.
FIG. 4.
FAK amino-terminal truncation mutants are phosphorylated in suspension. CHO cells were transfected with various vectors encoding full-length FAK, amino-terminal truncation FAK mutants, or vector control (mock), as indicated. After serum starvation, cells were placed in suspension for 60 min, and half of the cells were replated onto FN for 60 min prior to being lysed. (A) FAK was immunoprecipitated from suspended and replated cells with the C20 antibody against FAK. Immunoprecipitates were immunoblotted with PY20 or a monoclonal FAK antibody as indicated. (B) Cell lysates were immunoblotted directly with anti-PY397 or anti-FAK antibody as indicated. WCL, whole-cell lysates.
The amino-terminal domain of FAK can bind to its kinase domain.
Recent studies showed that the amino-terminal domain of JAK3, which, like FAK, contains a divergent FERM domain, can interact with its functional kinase domain and regulate its activity (42). Since the amino-terminal domain of FAK also contains a divergent FERM domain (13), we asked whether the amino-terminal domain of FAK is capable of interacting with the other regions of FAK. A GST fusion protein containing the amino-terminal domain of FAK (residues 1 to 400; GST-N) (Fig. 5A) was expressed and purified from bacteria by use of glutathione-agarose beads. The association of GST-N with FAK and a number of its fragments (Fig. 5A) was examined by an in vitro binding assay. Figure 5B shows that GST-N bound full-length FAK, Δ375 FAK, and the kinase domain of FAK but not the FAK carboxy-terminal domain. The specificity of the binding is supported by the lack of binding between GST alone and FAK or its fragments (GST lanes). The presence of similar amounts of GST and GST-N in the samples was verified by Ponceau S staining of the same membrane (bottom panel). Similar expression levels of FAK and its fragments were verified by immunoblotting aliquots of the starting materials with an antibody against the construct's HA tag (Fig. 5C). These results suggest that the amino-terminal domain of FAK can interact with its kinase domain in vitro.
FIG. 5.
The amino-terminal domain of FAK can bind to its kinase domain. GST or GST-N immobilized on glutathione-agarose was used to probe cell lysates prepared from 293 cells that had been transiently transfected with vectors encoding full-length FAK or its fragments (A). (B) Bound proteins were immunoblotted with the HA antibody Y-11 (top), and GST fusion proteins were visualized by staining the same membrane with Ponceau S (PS) (bottom). To verify similar expression levels of FL-FAK or its fragments, 10 μg of total protein from the 293 cell lysates was immunoblotted with the HA antibody Y-11 (C). The positions of full-length FAK, Δ375 FAK, and the C and K fragments of FAK are indicated by the arrows to the left.
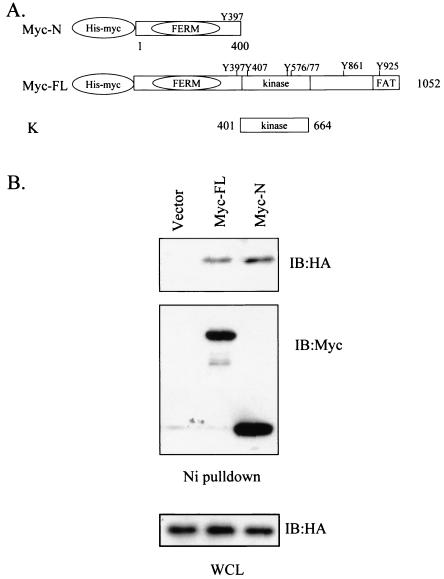
To evaluate whether the FAK amino-terminal and kinase domains could interact in vivo, 293 cells were cotransfected with a plasmid carrying the gene for the HA-tagged kinase domain of FAK and pHAN (with both Myc and His tags) vectors carrying the gene for the amino-terminal domain of FAK, full-length FAK, or vector alone as a control (Fig. 6A). Full-length FAK and the amino-terminal domain of FAK were precipitated from cell lysates by use of Ni-agarose beads and were analyzed by Western blotting with anti-Myc antibody (Fig. 6B, middle panel). Western blot analysis of the precipitates with anti-HA antibody shows that the kinase domain of FAK was coprecipitated with both the full-length FAK and the amino-terminal domain of FAK, but not with the pHAN vector control (top panel). Similar expression levels of the HA-tagged FAK kinase domain were verified by Western blotting of the whole-cell lysates (bottom panel). Together with the in vitro binding data, these results suggest the presence of an intramolecular interaction within the FAK molecule in which the amino-terminal domain of FAK associates with its kinase domain.
FIG. 6.
Association of the amino-terminal and kinase domains of FAK in vivo. CHO cells were cotransfected with plasmid carrying the gene for the HA-tagged kinase domain of FAK (designated K) (A) and the pHAN vector encoding His- and Myc-tagged full-length FAK or amino-terminal FAK (designated Myc-FL and Myc-N, respectively), as indicated. (B) The lysates were precipitated with nickel beads and blotted with anti-HA (top) or anti-Myc (middle) antibody. Aliquots of lysates were also blotted with anti-HA antibody directly (bottom). WCL, whole-cell lysates.
The amino-terminal domain of FAK can act in trans to inhibit FAK.
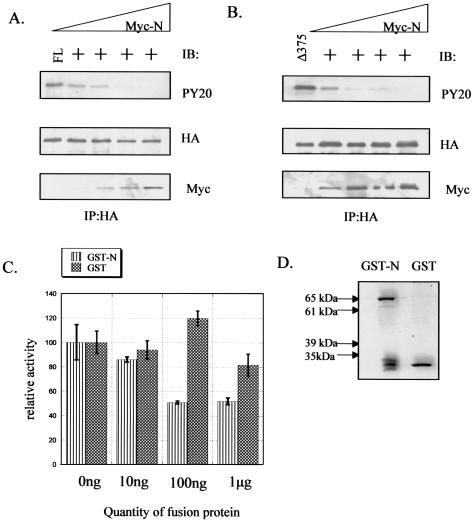
We next determined whether an interaction between the amino-terminal domain of FAK and its kinase domain could down regulate FAK activity in mammalian cells. 293 cells were cotransfected with full-length or Δ375 FAK and increasing amounts of an epitope-tagged amino-terminal domain of FAK (residues 1 to 400; Myc-N). Full-length FAK or the Δ375 FAK mutant was precipitated from cell lysates, and the immunoprecipitates were subjected to immunoblotting with PY20 to determine their tyrosine phosphorylation levels. As shown in Fig. 7A and B, expression of Myc-N reduced the tyrosine phosphorylation of both the coexpressed full-length FAK and the Δ375 mutant of FAK in a dose-dependent manner (top and middle panels). The amount of Myc-N that coprecipitated with full-length FAK or its mutant also increased with the amount of the Myc-N plasmid that was transfected (bottom panel). We also observed that coexpression of a slightly smaller version of the amino-terminal domain of FAK (residues 1 to 376) with full-length FAK inhibited FAK phosphorylation (data not shown). Since this fragment lacks the autophosphorylation site at Y397, it is unlikely that the cotransfected amino-terminal domains inhibited FAK phosphorylation by acting as a competing substrate. The ability of Myc-N to inhibit the phosphorylation of the Δ375 FAK mutant also suggests that the FAK amino-terminal domain inhibited FAK's phosphorylation through direct binding to the kinase domain rather than through interfering with an upstream signaling pathway.
FIG. 7.
The amino-terminal domain of FAK can inhibit FAK in trans. 293 cells were cotransfected with pKH3-FL-FAK (A) or pKH3-Δ375 (B) and increasing amounts of expression vector encoding Myc-N. The amount of DNA transfected was equalized by the addition of the pHAN vector control. FL-FAK or Δ375 FAK was immunoprecipitated from these cells by use of the HA antibody Y-11. The immunoprecipitates were immunoblotted with PY20 or a monoclonal antibody against HA (12CA5). The lower-molecular-weight region of these blots was immunoblotted with an antibody against the Myc tag (9E10). (C) Purified His-tagged FAK (250 ng) was incubated with the indicated amount of GST or GST-N, and an in vitro kinase assay was performed in the presence of the substrate E4Y1 as described in Materials and Methods. 32P incorporated into E4Y1 was quantified by use of a phosphorimager. The 0-ng condition for each set of reactions, which contains the same volume of elution buffer as other samples, was defined as 100, and other values are expressed relative to this. (D) The quantity of fusion protein used for the 1-μg condition of these experiments was resolved by SDS-PAGE and subjected to Coomassie staining.
To further test whether the amino-terminal domain of FAK could inhibit FAK activity directly, we conducted an in vitro kinase assay using recombinant FAK purified from insect cells in the presence of increasing amounts of GST-N (Fig. 5A) or GST alone. Figure 7C shows that GST-N inhibited FAK's activity toward the peptide substrate E4Y1 in a dose-dependent manner, whereas GST alone had no significant effect on FAK activity under similar conditions. Parallel samples of GST fusion proteins used in the kinase assays were analyzed by Coomassie blue staining to show that the inhibition by GST-N was not due to the use of more protein (Fig. 7D). Together, these results provide strong support for the hypothesis that the amino-terminal domain of FAK can bind to the kinase domain of FAK and inhibit its activity.
The role of the amino-terminal domain of FAK and its interaction with the FAK kinase domain in the regulation of cell cycle progression.
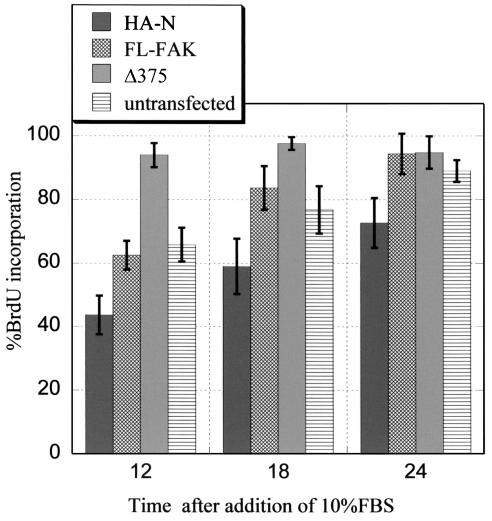
FAK and its associated signaling pathways have been shown to regulate a variety of cellular functions, including cell cycle progression (41). To determine whether the intramolecular interaction between the amino-terminal and kinase domains of FAK plays a role in regulating its cellular function, we examined the effect of truncation of the amino-terminal domain of FAK on its ability to stimulate cell cycle progression. CHO cells were transiently transfected with full-length FAK, the amino-terminal domain of FAK (HA-N), or the Δ375 FAK mutant, and BrdU incorporation assays were used to assess the effect of truncation on cell cycle progression. As shown in Fig. 8, expression of the Δ375 FAK mutant significantly accelerated cell entry into S phase upon serum stimulation. Twelve hours after serum stimulation, more than 90% of Δ375 FAK-transfected cells incorporated BrdU, compared with about 65% of nontransfected control cells under the same conditions. Eighteen and 24 h after serum stimulation, the Δ375 FAK-transfected cells maintained a high fraction of BrdU-positive cells, while the nontransfected controls showed an increase in BrdU incorporation to about 90%. In contrast to the Δ375 FAK mutant, expression of full-length FAK had little effect on cell cycle progression under these conditions. These results suggest that the amino-terminal domain of FAK is not required for cell cycle progression promoted through FAK and that the hyperphosphorylation of FAK due to its truncation increases FAK's ability to promote cell cycle progression.
FIG. 8.
Effects of the amino-terminal domain of FAK on cell cycle progression. CHO cells were transiently transfected with expression vectors encoding HA-tagged FAK amino-terminal domain (HA-N), full-length FAK (FL-FAK), or amino-terminal truncation mutant (Δ375). BrdU incorporation assays were performed as described, and the percentages of cells which had incorporated BrdU are represented as the averages of three experiments ± 1 standard deviation. Cells expressing the transfected plasmids were identified by immunofluorescence staining using the HA antibody Y-11.
We also asked whether expression of the amino-terminal domain of FAK could interfere with cell cycle progression in CHO cells. Figure 8 shows that at all time points examined, the percentage of cells expressing the amino-terminal domain of FAK (HA-N) which had entered S phase was significantly lower than the percentage of cells which had entered S phase in the nontransfected cell population or that of cells which expressed full-length FAK. This finding suggests that inhibition of the phosphorylation of endogenous FAK by expression of the amino-terminal domain of FAK (Fig. 7) could lead to inhibition of FAK functions in the regulation of cell cycle progression. Together, these results provide further support for a functional significance of the intramolecular interaction of FAK.
The β1 integrin's cytoplasmic domain can stimulate FAK activity in vitro.
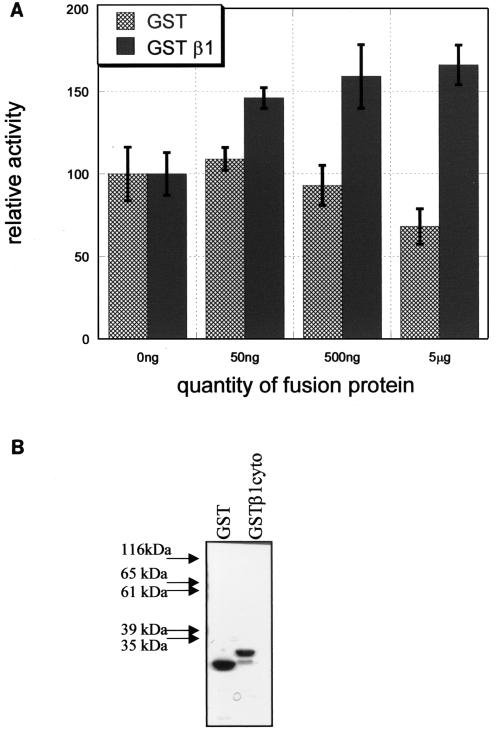
Previous studies by Schaller et al. suggested a direct interaction between the β1 integrin cytoplasmic domain and the amino-terminal domain of FAK (27). Given this and the critical importance of the β1 integrin cytoplasmic domain in inducing FAK phosphorylation (15a, 33a), our data here showing an autoinhibitory role of the amino-terminal domain of FAK raised the interesting possibility that the β1 integrin cytoplasmic domain may displace this intramolecular interaction of FAK and thus promote FAK activation. To begin to test this possibility, we examined whether the cytoplasmic tail of the β1 integrin is capable of stimulating FAK activity directly in vitro. Purified recombinant His-tagged FAK was incubated with increasing amounts of GST or a GST fusion protein containing the β1 integrin cytoplasmic domain (GST-β1cyto) and assayed for in vitro kinase activity, with E4Y1 as a substrate. Figure 9A shows that the presence of GST-β1cyto increased FAK activity in a dose-dependent manner, whereas GST alone had little effect on FAK activity (except a slight inhibitory effect at the highest dose). The samples of GST and GST-β1cyto were analyzed by SDS-PAGE followed by Coomassie blue staining to show that the stimulatory effects of GST-β1cyto were not a result of the presence of more fusion protein in the reactions (Fig. 9B). These results suggest that the β1 integrin cytoplasmic domain's interaction with the amino-terminal domain of FAK is capable of activating FAK, presumably by disrupting the interaction between the kinase and amino-terminal domains of FAK.
FIG. 9.
The β1 integrin cytoplasmic domain can stimulate FAK in vitro. Purified His-tagged FAK (250 ng) was incubated with 30 μl of glutathione-agarose beads containing increasing amounts of GST or GSTβ1cyto fusion protein, and an in vitro kinase assay was performed in the presence of the peptide substrate E4Y1. (A) 32P incorporated into E4Y1 was quantified by use of a phosphorimager. The 0-ng condition for each set of reactions was defined as 100, and other values are expressed relative to this. The averages of four reactions ± 1 standard deviation are shown. (B) The quantity of fusion protein used for the 5-μg condition of these experiments was resolved by SDS-PAGE and subjected to Coomassie staining.
DISCUSSION
Recent studies have established FAK as a critical mediator of integrin signaling, and rapid progress has been made in delineating the signaling pathways downstream of FAK as well as their roles in the regulation of various cellular functions (9a, 20, 23, 25, 28, 40). In contrast, less is known about the molecular mechanisms by which FAK is activated by integrins or other cell surface receptors. In this paper, we have identified an intramolecular interaction between the amino-terminal and kinase domains of FAK, and we have provided evidence suggesting that this interaction serves to negatively regulate FAK phosphorylation and activity. We have also shown that disruption of this interaction might be involved in the activation of FAK by integrins.
Previous studies have focused on the role that clustering of FAK into focal adhesions plays in its activation by integrins. A region within the carboxy-terminal domain of FAK, the FAT sequence, has been shown to be necessary and sufficient for FAK's localization to focal adhesions (18). Furthermore, analysis of a series of FAK mutants in CEF cells suggested that FAT-mediated focal adhesion targeting is the critical determinant of FAK phosphorylation in cell adhesion. These data are consistent with the concept of dimerization- and/or clustering-induced tyrosine kinase activation observed in many other systems (30, 31) and proposed for FAK itself (4, 21, 34). However, they do not account for other data in the literature (e.g., integrin mutants that were able to induce FAK clustering but not its activation [37]), and they do not provide a molecular mechanism to explain how FAK might be activated once it is targeted to focal adhesions.
Although the results from Shen and Schaller (32) excluded a role for other regions of FAK in its regulation, other studies found that truncation of the amino-terminal domain of FAK resulted in an increase in phosphorylation of FAK (29) and its kinase activity (34). Our results, presented in this paper, are consistent with the latter report, and they provide several lines of evidence supporting a role for the amino-terminal domain of FAK in its regulation. First, truncation of the amino-terminal domain resulted in an increased phosphorylation of FAK as well as its substrate, paxillin, in mammalian cells (Fig. 2). Second, such truncation increased the in vitro kinase activity of FAK (Fig. 1). This suggests that the increased phosphorylation is likely a consequence of FAK activation rather than some other mechanism, such as increased accessibility of the phosphorylation sites in the truncation mutants to autophosphorylation or another kinase. Third, the amino-terminal domain of FAK was found to be capable of associating with the kinase domain of FAK, and overexpression of this domain inhibited FAK phosphorylation in vivo and its kinase activity in vitro (Fig. 5, 6, and 7). The ability of this domain to inhibit FAK activity directly in an in vitro assay with purified recombinant proteins suggests that the mechanism of inhibition seen in cells is most likely through direct binding of the amino terminus to FAK's kinase domain, rather than through indirect interference with some other proteins or signaling pathways. Lastly, truncation of the amino-terminal domain enhanced FAK's ability to stimulate cell cycle progression, whereas overexpression of this domain alone inhibited cell cycle progression (Fig. 8). Although we cannot exclude the possibility that different mechanisms are involved in the regulation of FAK in different cell types, our results strongly suggest that, in addition to the FAT sequence, the amino-terminal domain of FAK plays a critical role in FAK activation in cell adhesion.
It has been shown previously that the FAK amino-terminal domain can bind to peptides corresponding to β integrin cytoplasmic domains in vitro (27). Based on these data, it was proposed that integrins could activate FAK by this direct binding mechanism. However, this proposal is challenged by others based on the lack of evidence of a stable association of FAK with integrins in vivo or by other methods in vitro. It should be pointed out that a number of more recent studies did find integrins and FAK coexisting in the same immune complexes (11, 33), although the role of such a direct interaction between integrins and FAK in FAK activation remains controversial. Interestingly, we found that incubation of a GST fusion protein containing the β1 integrin cytoplasmic domain with recombinant FAK stimulated its kinase activity in vitro (Fig. 9). This is consistent with the previous finding that the β1 integrin cytoplasmic domain can bind to the FAK amino-terminal domain and suggests that such binding may displace the inhibitory intramolecular interaction of FAK, thus leading to its activation. Nevertheless, in view of our observation of a lack of stable binding of GST-β1cyto with FAK in vitro (6; unpublished observation), we suggest that the interaction between FAK and the β1 integrin cytoplasmic domain is transient and/or of low affinity. Given that integrins and FAK are both clustered into focal adhesions in adherent cells, such a low-affinity interaction may be sufficient for activation of FAK by integrins. This interpretation would also explain the critical importance of the FAT sequence and focal adhesion localization in the activation of FAK, as proposed previously (32).
The above considerations lead us to propose the following two-step working model for FAK activation by integrins in cell adhesion (Fig. 10). In suspended cells, the amino terminus of FAK interacts with the kinase domain through an intramolecular interaction. This intramolecular interaction maintains FAK in an underphosphorylated state that might correspond to FAK's activity in suspended cells or when FAK is localized in the cytosol of the cell. Upon integrin-mediated cell adhesion, FAK becomes localized to focal adhesions through its carboxy-terminal FAT sequence and its interactions with proteins such as talin and paxillin (6, 19, 35), which in turn interact with integrins. Once present in these structures, the cytoplasmic domain of integrins could interact with the amino terminus of FAK, which releases autoinhibition within FAK and leads to its activation. As discussed above, this model would explain various data suggesting the involvement of both the FAT sequence and the amino-terminal domain of FAK in the regulation of its activation. In addition, this model also suggests that two separate sets of residues within the β integrin subunit may be necessary for full activation of FAK, one for interaction with molecules such as talin or paxillin that mediate FAK's localization and the other for direct FAK interaction. This could potentially provide an explanation for current data showing β integrin mutants that allow FAK localization but do not activate FAK (37). Additional mutational analysis of the β integrin cytoplasmic domains will be necessary to further evaluate this working model. The crystal structure of the kinase domain of FAK has been solved recently (22). Determination of the structure of the amino-terminal FERM-like domain of FAK will help to define the molecular features of this intramolecular interaction and to provide further insights into the regulatory mechanisms.
FIG. 10.
Working hypothesis of two-step model of FAK activation by integrins in cell adhesion. T/P/X denotes cytoskeletal talin (T), paxillin (P), and potentially other proteins (X) that function to mediate FAK localization to focal contacts and coclustering with integrins.
The amino-terminal domain of FAK has also been shown to interact with receptor tyrosine kinases like the platelet-derived growth factor receptor and epidermal growth factor (EGF) receptor as well as the cytoplasmic tyrosine kinase Etk (8). Indeed, the amino-terminal domain of FAK is required for platelet-derived growth factor- and EGF-stimulated cell migration, as measured in the reconstitution system of FAK−/− cells (33). Therefore, it will be interesting to determine whether such an interaction is involved in the activation of FAK by growth factor receptors. The interaction of the FERM domain of FAK with Etk was proposed to be involved in the activation of Etk by FAK in cell adhesion (8). It is not clear, however, whether this interaction also results in FAK's activation by Etk.
The amino-terminal region of FAK contains a FERM-like domain (13). FERM domains have been shown to be mediators of both protein-protein and lipid interactions. Note that in many cases FERM domain-containing proteins are regulated by intramolecular interactions involving binding of the FERM domain with another region of the molecule. Such interactions can be inhibitory, such as the case for ezrin, in which the amino-terminal FERM domain interacts with the carboxy terminus, leading to inhibition of the actin binding activity of ezrin (12). On the other hand, the interaction of the FERM-like domain with the kinase domain of JAK is necessary for maintaining JAK in an active state (42). It will be interesting to determine the structural basis for the differences between the FERM-like domains of FAK and JAK in their inhibitory and activating roles, respectively.
Acknowledgments
Lee Ann Cooper and Tang-Long Shen contributed equally to the work presented in this paper.
We are grateful to F. Matsumura of Rutgers University for recombinant baculovirus encoding His-tagged FAK and to C. E. Turner of SUNY Health Science Center in Syracuse for pEGFP-Paxillin. We thank our colleagues Luis Rodriguez, Daniel Rhoades, Xiaoyang Wu, Boyi Gan, Tamas Nagy, Xu Peng, and Zara Melkoumian for their critical reading of the manuscript and helpful comments.
This research was supported by NIH grants GM48050 and GM52890 to J.-L.G.
REFERENCES
- 1.Akiyama, S. K., S. S. Yamada, K. M. Yamada, and S. E. LaFlamme. 1994. Transmembrane signal transduction by integrin cytoplasmic domains expressed in single-subunit chimeras. J. Biol. Chem. 269:15961-15964. [PubMed] [Google Scholar]
- 2.Calalb, M. B., T. R. Polte, and S. K. Hanks. 1995. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol. Cell. Biol. 15:954-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cary, L. A., J. F. Chang, and J. L. Guan. 1996. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J. Cell Sci. 109:1787-1794. [DOI] [PubMed] [Google Scholar]
- 4.Chan, P. Y., S. B. Kanner, G. Whitney, and A. Aruffo. 1994. A transmembrane-anchored chimeric focal adhesion kinase is constitutively activated and phosphorylated at tyrosine residues identical to pp125FAK. J. Biol. Chem. 269:20567-20574. [PubMed] [Google Scholar]
- 5.Chen, H. C., P. A. Appeddu, H. Isoda, and J. L. Guan. 1996. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J. Biol. Chem. 271:26329-26334. [DOI] [PubMed] [Google Scholar]
- 6.Chen, H. C., P. A. Appeddu, J. T. Parsons, J. D. Hildebrand, M. D. Schaller, and J. L. Guan. 1995. Interaction of focal adhesion kinase with cytoskeletal protein talin. J. Biol. Chem. 270:16995-16999. [DOI] [PubMed] [Google Scholar]
- 7.Chen, H. C., and J. L. Guan. 1996. The association of focal adhesion kinase with a 200-kDa protein that is tyrosine phosphorylated in response to platelet-derived growth factor. Eur. J. Biochem. 235:495-500. [DOI] [PubMed] [Google Scholar]
- 8.Chen, R., O. Kim, M. Li, X. Xiong, J. L. Guan, H. J. Kung, H. Chen, Y. Shimizu, and Y. Qiu. 2001. Regulation of the PH-domain-containing tyrosine kinase Etk by focal adhesion kinase through the FERM domain. Nat. Cell. Biol. 3:439-444. [DOI] [PubMed] [Google Scholar]
- 9.Cooley, M. A., J. M. Broome, C. Ohngemach, L. H. Romer, and M. D. Schaller. 2000. Paxillin binding is not the sole determinant of focal adhesion localization or dominant-negative activity of focal adhesion kinase/focal adhesion kinase-related nonkinase. Mol. Biol. Cell 11:3247-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Damsky, C. H., and D. Ilic. 2002. Integrin signaling: it’s where the action is. Curr. Opin. Cell Biol. 14:594-602. [DOI] [PubMed] [Google Scholar]
- 10.Dunty, J. M., and M. D. Schaller. 2002. The N termini of focal adhesion kinase family members regulate substrate phosphorylation, localization, and cell morphology. J. Biol. Chem. 277:45644-45654. [DOI] [PubMed] [Google Scholar]
- 11.Eliceiri, B. P., X. S. Puente, J. D. Hood, D. G. Stupack, D. D. Schlaepfer, X. Z. Huang, D. Sheppard, and D. A. Cheresh. 2002. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J. Cell Biol. 157:149-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gary, R., and A. Bretscher. 1995. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol. Biol. Cell 6:1061-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girault, J. A., G. Labesse, J. P. Mornon, and I. Callebaut. 1998. Janus kinases and focal adhesion kinases play in the 4.1 band: a superfamily of band 4.1 domains important for cell structure and signal transduction. Mol. Med. 4:751-769. [PMC free article] [PubMed] [Google Scholar]
- 14.Girault, J. A., G. Labesse, J. P. Mornon, and I. Callebaut. 1999. The N-termini of FAK and JAKs contain divergent band 4.1 domains. Trends Biochem. Sci. 24:54-57. [DOI] [PubMed] [Google Scholar]
- 15.Guan, J. L., and D. Shalloway. 1992. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature 358:690-692. [DOI] [PubMed] [Google Scholar]
- 15a.Guan, J. L., J. E. Trevithick, and R. O. Hynes. 1991. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 2:951-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han, D. C., and J. L. Guan. 1999. Association of focal adhesion kinase with Grb7 and its role in cell migration. J. Biol. Chem. 274:24425-24430. [DOI] [PubMed] [Google Scholar]
- 17.Han, D. C., T. L. Shen, H. Miao, B. Wang, and J. L. Guan. 2002. EphB1 associates with Grb7 and regulates cell migration. J. Biol. Chem. 277:45655-45661. [DOI] [PubMed] [Google Scholar]
- 18.Hildebrand, J. D., M. D. Schaller, and J. T. Parsons. 1993. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J. Cell Biol. 123:993-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildebrand, J. D., M. D. Schaller, and J. T. Parsons. 1995. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol. Biol. Cell 6:637-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilic, D., C. H. Damsky, and T. Yamamoto. 1997. Focal adhesion kinase: at the crossroads of signal transduction. J. Cell Sci. 110:401-407. [DOI] [PubMed] [Google Scholar]
- 21.Katz, B., S. Miyamoto, H. Teramoto, M. Zohar, D. Krylov, C. Vinson, J. Gutkind, and K. Yamada. 2002. Direct transmembrane clustering and cytoplasmic dimerization of focal adhesion kinase initiates its tyrosine phosphorylation. Biochim. Biophys. Acta 1592:141. [DOI] [PubMed] [Google Scholar]
- 22.Nowakowski, J., C. N. Cronin, D. E. McRee, M. W. Knuth, C. G. Nelson, N. P. Pavletich, J. Rogers, B. C. Sang, D. N. Scheibe, R. V. Swanson, and D. A. Thompson. 2002. Structures of the cancer-related Aurora-A, FAK, and EphA2 protein kinases from nanovolume crystallography. Structure (Cambridge) 10:1659-1667. [DOI] [PubMed] [Google Scholar]
- 23.Parsons, J. T., K. H. Martin, J. K. Slack, J. M. Taylor, and S. A. Weed. 2000. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene 19:5606-5613. [DOI] [PubMed] [Google Scholar]
- 24.Reddy, M. A., C. A. Wass, K. S. Kim, D. D. Schlaepfer, and N. V. Prasadarao. 2000. Involvement of focal adhesion kinase in Escherichia coli invasion of human brain microvascular endothelial cells. Infect. Immun. 68:6423-6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaller, M. D. 2001. Paxillin: a focal adhesion-associated adaptor protein. Oncogene 20:6459-6472. [DOI] [PubMed] [Google Scholar]
- 26.Schaller, M. D., J. D. Hildebrand, J. D. Shannon, J. W. Fox, R. R. Vines, and J. T. Parsons. 1994. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 14:1680-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaller, M. D., C. A. Otey, J. D. Hildebrand, and J. T. Parsons. 1995. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J. Cell Biol. 130:1181-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlaepfer, D. D., C. R. Hauck, and D. J. Sieg. 1999. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 71:435-478. [DOI] [PubMed] [Google Scholar]
- 29.Schlaepfer, D. D., and T. Hunter. 1996. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol. Cell. Biol. 16:5623-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103:211-225. [DOI] [PubMed] [Google Scholar]
- 31.Schlessinger, J. 2002. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 110:669-672. [DOI] [PubMed] [Google Scholar]
- 32.Shen, Y., and M. D. Schaller. 1999. Focal adhesion targeting: the critical determinant of FAK regulation and substrate phosphorylation. Mol. Biol. Cell 10:2507-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sieg, D. J., C. R. Hauck, D. Ilic, C. K. Klingbeil, E. Schaefer, C. H. Damsky, and D. D. Schlaepfer. 2000. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell. Biol. 2:249-256. [DOI] [PubMed] [Google Scholar]
- 33a.Tahiliani, P. D., L. Singh, K. L. Auer, and S. E. LaFlamme. 1997. The role of conserved amino acid motifs within the integrin beta3 cytoplasmic domain in triggering focal adhesion kinase phosphorylation. J. Biol. Chem. 272:7892-7898. [DOI] [PubMed] [Google Scholar]
- 34.Toutant, M., A. Costa, J. M. Studler, G. Kadare, M. Carnaud, and J. A. Girault. 2002. Alternative splicing controls the mechanisms of FAK autophosphorylation. Mol. Cell. Biol. 22:7731-7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner, C. E., and J. T. Miller. 1994. Primary sequence of paxillin contains putative SH2 and SH3 domain binding motifs and multiple LIM domains: identification of a vinculin and pp125Fak-binding region. J. Cell Sci. 107:1583-1591. [DOI] [PubMed] [Google Scholar]
- 36.Ueda, H., S. Abbi, C. Zheng, and J. L. Guan. 2000. Suppression of Pyk2 kinase and cellular activities by FIP200. J. Cell Biol. 149:423-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wennerberg, K., A. Armulik, T. Sakai, M. Karlsson, R. Fassler, E. M. Schaefer, D. F. Mosher, and S. Johansson. 2000. The cytoplasmic tyrosines of integrin subunit beta1 are involved in focal adhesion kinase activation. Mol. Cell. Biol. 20:5758-5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Withers, B. E., P. R. Keller, and D. W. Fry. 1996. Expression, purification and characterization of focal adhesion kinase using a baculovirus system. Protein Exp. Purif. 7:12-18. [DOI] [PubMed] [Google Scholar]
- 39.Xing, Z., H. C. Chen, J. K. Nowlen, S. J. Taylor, D. Shalloway, and J. L. Guan. 1994. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol. Biol. Cell 5:413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao, J. H., and J. L. Guan. 2000. Role of focal adhesion kinase in signaling by the extracellular matrix. Prog. Mol. Subcell. Biol. 25:37-55. [DOI] [PubMed] [Google Scholar]
- 41.Zhao, J. H., H. Reiske, and J. L. Guan. 1998. Regulation of the cell cycle by focal adhesion kinase. J. Cell Biol. 143:1997-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou, Y. J., M. Chen, N. A. Cusack, L. H. Kimmel, K. S. Magnuson, J. G. Boyd, W. Lin, J. L. Roberts, A. Lengi, R. H. Buckley, R. L. Geahlen, F. Candotti, M. Gadina, P. S. Changelian, and J. J. O'Shea. 2001. Unexpected effects of FERM domain mutations on catalytic activity of Jak3: structural implication for Janus kinases. Mol. Cell 8:959-969. [DOI] [PubMed] [Google Scholar]