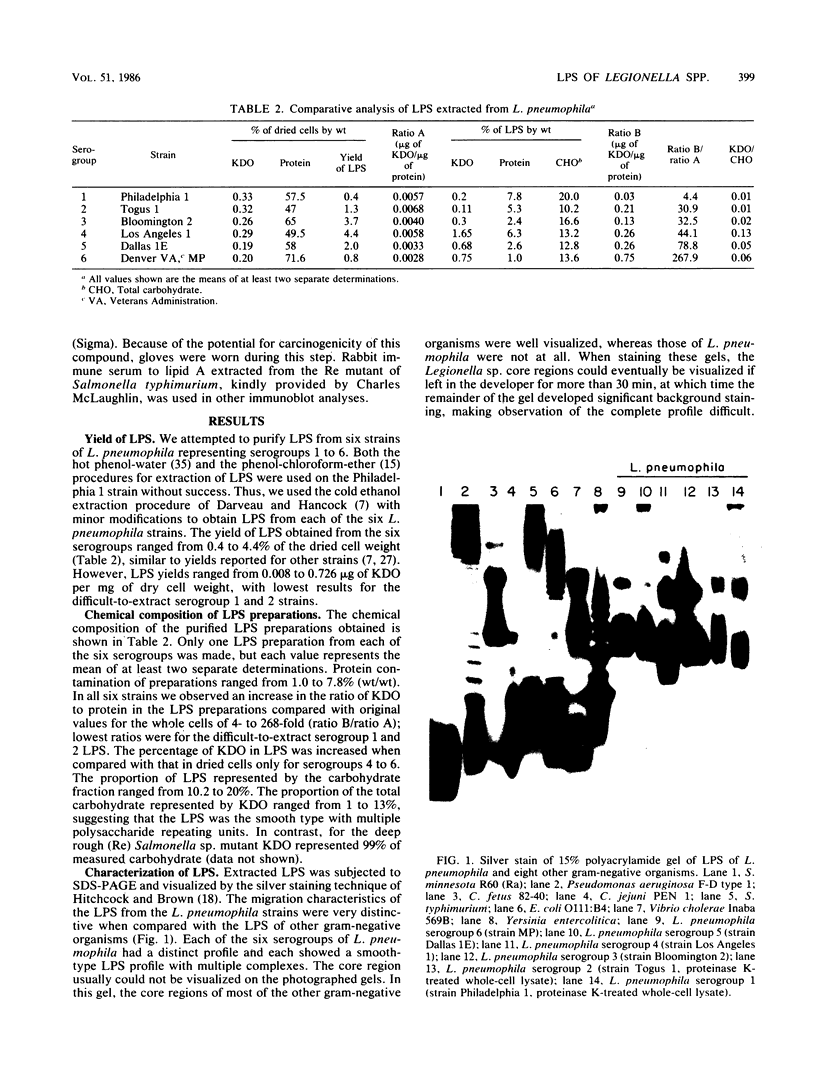
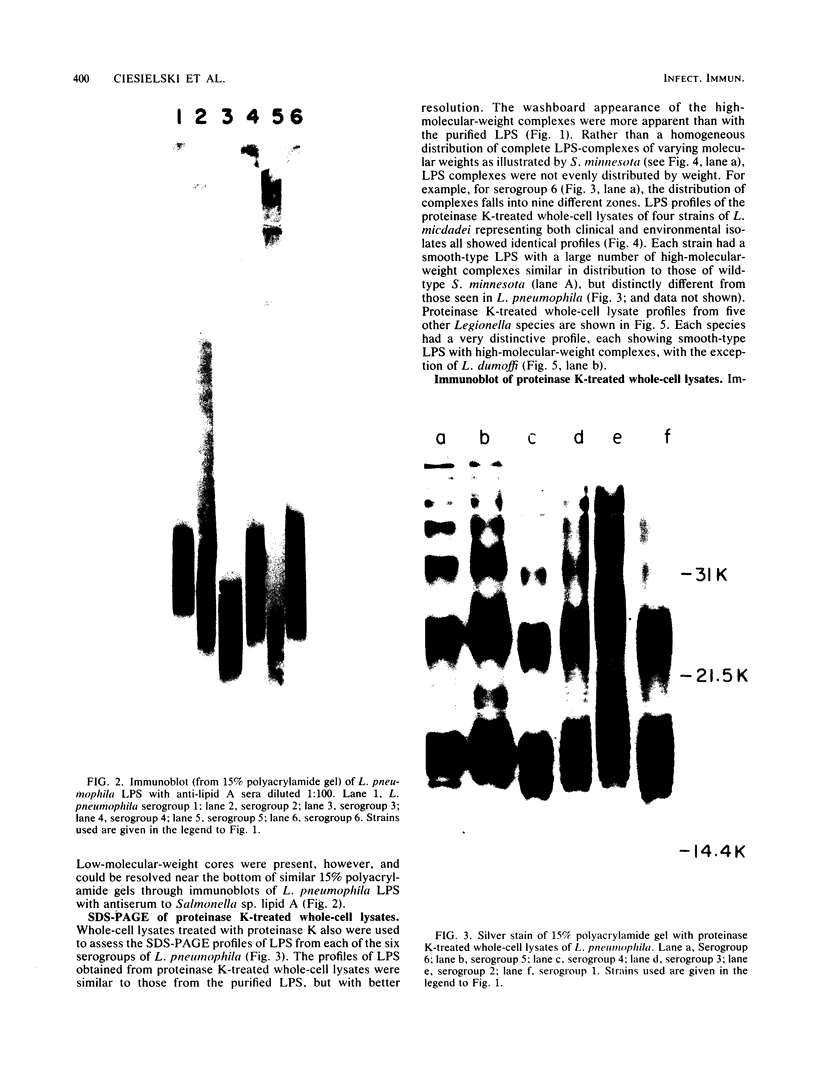
Abstract
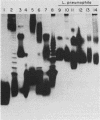
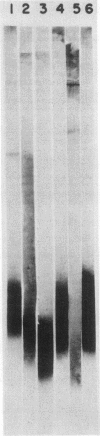
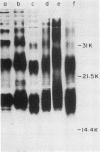
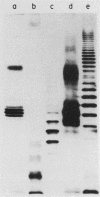
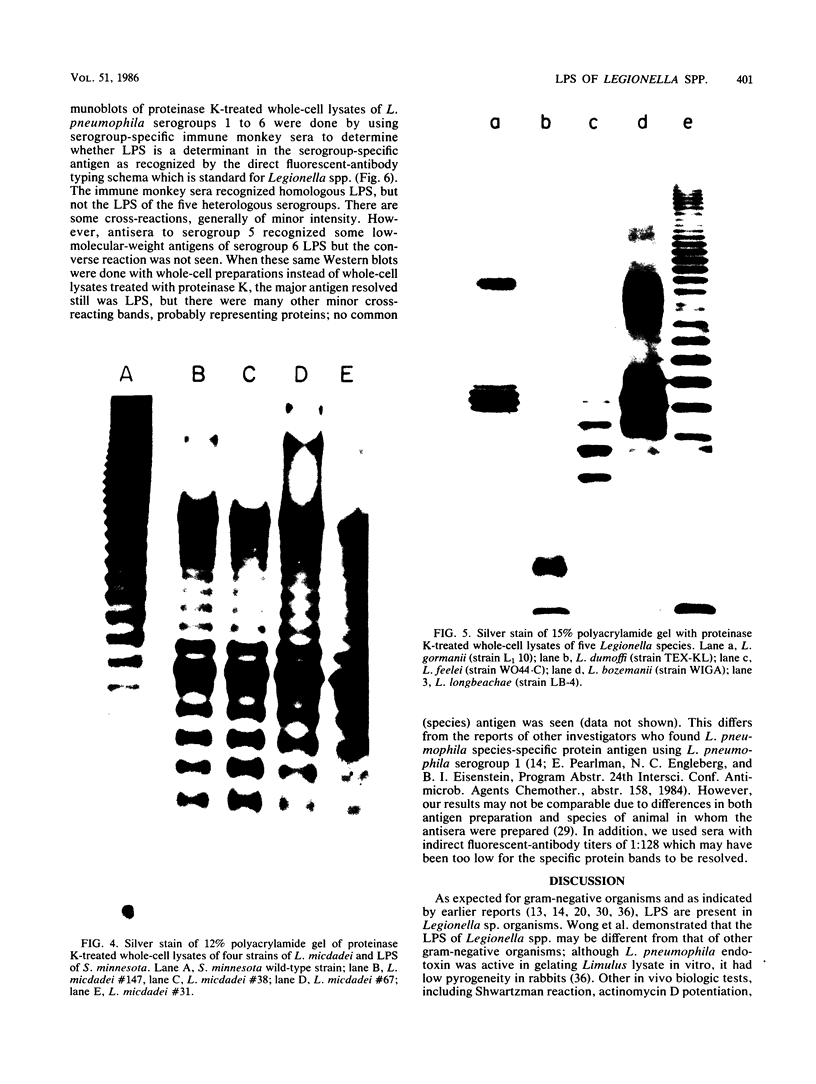
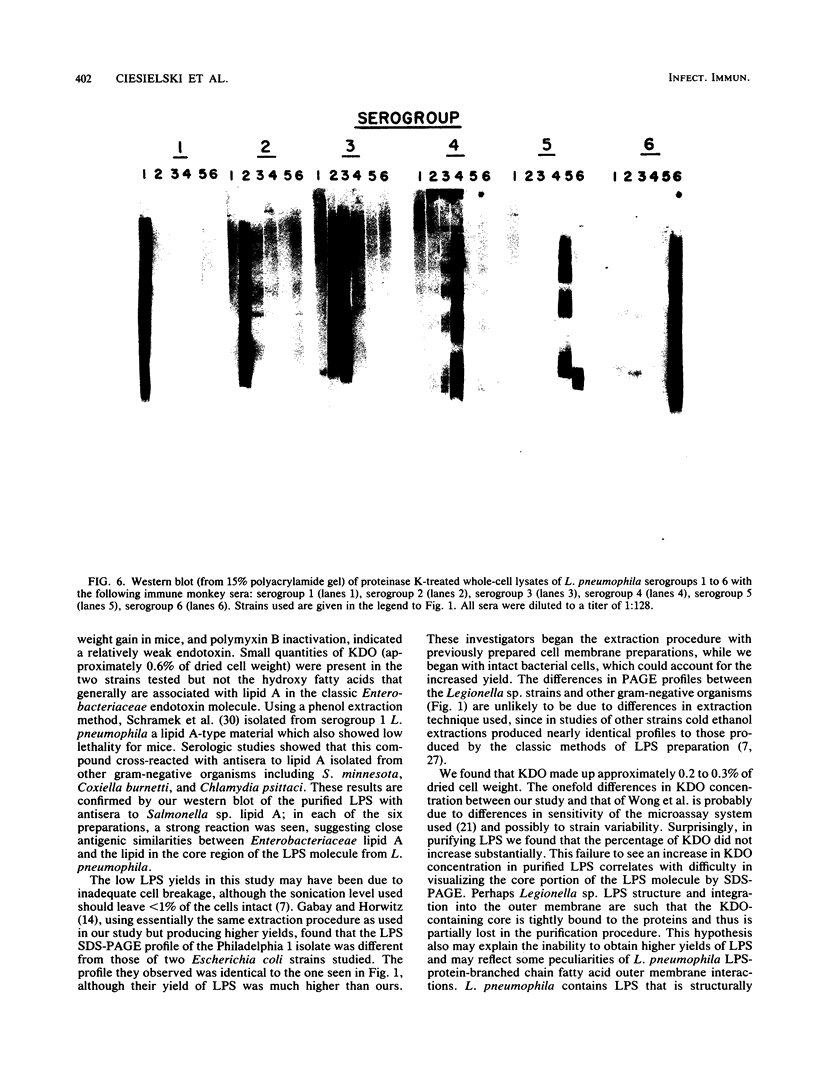
We studied the lipopolysaccharide (LPS) of Legionella pneumophila and six other Legionella species to determine whether strain differences were apparent. The LPS was purified by a cold ethanol extraction procedure, and total carbohydrates represented 10 to 20% of LPS weight. 2-keto-3-deoxyoctonate represented 1 to 13% of the total carbohydrate present in the LPS. By sodium dodecyl sulfate-polyacrylamide gel electrophoresis, all strains except L. dumoffi showed smooth-type LPS with multiple high-molecular-weight complexes. Proteinase K-treated, whole-cell lysates showed profiles similar to those of purified LPS. Each serogroup of L. pneumophila and each Legionella species had a distinct sodium dodecyl sulfate-polyacrylamide gel electrophoresis profile. L. pneumophila lipid A is antigenically related to the lipid A of Enterobacteriaceae. In immunoblot assays with the LPS of L. pneumophila serogroups 1 to 6 as antigens, serogroup-specific immune monkey sera recognized homologous purified LPS, but not the LPS of the five heterologous serogroups. These studies indicate that LPS composition may be a determinant of serogroup specificity as defined by the immunofluorescence-based serogrouping schema for L. pneumophila and other Legionella species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Blaser M. J., Hopkins J. A., Berka R. M., Vasil M. L., Wang W. L. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect Immun. 1983 Oct;42(1):276–284. doi: 10.1128/iai.42.1.276-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry W. B., Pittman B., Harris P. P., Hebert G. A., Thomason B. M., Thacker L., Weaver R. E. Detection of Legionnaires disease bacteria by direct immunofluorescent staining. J Clin Microbiol. 1978 Sep;8(3):329–338. doi: 10.1128/jcm.8.3.329-338.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester I. R., Meadow P. M., Pitt T. L. The relationship between the O-antigenic lipopolysaccharides and serological specificity in strains of Pseudomonas aeruginosa of different O-serotypes. J Gen Microbiol. 1973 Oct;78(2):305–318. doi: 10.1099/00221287-78-2-305. [DOI] [PubMed] [Google Scholar]
- Collins M. T., Cho S. N., Høiby N., Espersen F., Baek L., Reif J. S. Crossed immunoelectrophoretic analysis of Legionella pneumophila serogroup 1 antigens. Infect Immun. 1983 Mar;39(3):1428–1440. doi: 10.1128/iai.39.3.1428-1440.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein P. H., Bibb W. F., Gorman G. W., Thacker W. L., Brenner D. J., Wilkinson H. W., Moss C. W., Buddington R. S., Dunn C. J., Roos P. J. Legionella pneumophila serogroup 9: a cause of human pneumonia. Ann Intern Med. 1984 Aug;101(2):196–198. doi: 10.7326/0003-4819-101-2-196. [DOI] [PubMed] [Google Scholar]
- Elliott J. A., Johnson W., Helms C. M. Ultrastructural localization and protective activity of a high-molecular-weight antigen isolated from Legionella pneumophila. Infect Immun. 1981 Feb;31(2):822–824. doi: 10.1128/iai.31.2.822-824.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesher A. R., Ito S., Mansheim B. J., Kasper D. L. The cell envelope of the Legionnaires' disease bacterium. Morphologic and biochemical characteristics. Ann Intern Med. 1979 Apr;90(4):628–630. doi: 10.7326/0003-4819-90-4-628. [DOI] [PubMed] [Google Scholar]
- Fumarola D. Legionella pneumophila and limulus endotoxin assay: recent findings. Infection. 1979;7(4):198–199. doi: 10.1007/BF01640945. [DOI] [PubMed] [Google Scholar]
- Gabay J. E., Horwitz M. A. Isolation and characterization of the cytoplasmic and outer membranes of the Legionnaires' disease bacterium (Legionella pneumophila). J Exp Med. 1985 Feb 1;161(2):409–422. doi: 10.1084/jem.161.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J. Aberrant migration of lipopolysaccharide in sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Eur J Biochem. 1983 Jul 1;133(3):685–688. doi: 10.1111/j.1432-1033.1983.tb07517.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Interaction of the Legionnaires' disease bacterium (Legionella pneumophila) with human phagocytes. I. L. pneumophila resists killing by polymorphonuclear leukocytes, antibody, and complement. J Exp Med. 1981 Feb 1;153(2):386–397. doi: 10.1084/jem.153.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W., Elliott J. A., Helms C. M., Renner E. D. A high molecular weight antigen in Legionnaires' disease bacterium: isolation and partial characterization. Ann Intern Med. 1979 Apr;90(4):638–641. doi: 10.7326/0003-4819-90-4-638. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McKinney R. M., Thomason B. M., Harris P. P., Thacker L., Lewallen K. R., Wilkinson H. W., Hebert G. A., Moss C. W. Recognition of a new serogroup of Legionnaires disease bacterium. J Clin Microbiol. 1979 Jan;9(1):103–107. doi: 10.1128/jcm.9.1.103-107.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr T. R., Jr, Bryan L. E. Lipopolysaccharide banding patterns of Neisseria meningitidis and Neisseria gonorrhoeae. J Clin Microbiol. 1984 Apr;19(4):558–560. doi: 10.1128/jcm.19.4.558-560.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Perez G. I., Blaser M. J. Lipopolysaccharide characteristics of pathogenic campylobacters. Infect Immun. 1985 Feb;47(2):353–359. doi: 10.1128/iai.47.2.353-359.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez G. I., Hopkins J. A., Blaser M. J. Antigenic heterogeneity of lipopolysaccharides from Campylobacter jejuni and Campylobacter fetus. Infect Immun. 1985 May;48(2):528–533. doi: 10.1128/iai.48.2.528-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson J. S., Plikaytis B. B., Wilkinson H. W. Immunologic response of patients with legionellosis against major protein-containing antigens of Legionella pneumophila serogroup 1 as shown by immunoblot analysis. J Clin Microbiol. 1986 Jan;23(1):92–99. doi: 10.1128/jcm.23.1.92-99.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramek S., Kazár J., Bazovská S. Lipid A in Legionella pneumophila. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Jul;252(3):401–404. [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]
- Wong K. H., Moss C. W., Hochstein D. H., Arko R. J., Schalla W. O. "Endotoxicity" of the Legionnaires' disease bacterium. Ann Intern Med. 1979 Apr;90(4):624–627. doi: 10.7326/0003-4819-90-4-624. [DOI] [PubMed] [Google Scholar]
- Wong K. H., Schalla W. O., Arko R. J., Bullard J. C., Feeley J. C. Immunochemical, serologic, and immunologic properties of major antigens isolated from the Legionnaires' disease bacterium. Observations bearing on the feasibility of a vaccine. Ann Intern Med. 1979 Apr;90(4):634–638. doi: 10.7326/0003-4819-90-4-634. [DOI] [PubMed] [Google Scholar]