Abstract
Even though endothelin is recognized as an important vasoregulatory molecule, the roles of endothelin receptors in specific cell types are not yet fully understood. Mice with a null mutation in endothelin A receptor gene (ETA) or in the gene of its ligand (endothelin 1) die neonatally due to craniofacial and cardiac abnormalities. This early lethality has in the past hindered studies on the role of endothelin in cardiovascular physiology and pathophysiology. To overcome this obstacle, we utilized the cre/loxP technology to generate mice in which the ETA gene could be deleted specifically in cardiomyocytes. The cre recombinase transgene driven by the α-myosin heavy-chain promoter deleted the floxed ETA allele specifically in the hearts of these mice, resulting in a 78% reduction in cardiac ETA mRNA level compared to wild-type controls. Cardiomyocyte-specific ETA knockout animals are viable and exhibit normal growth, cardiac anatomy, and cardiac contractility, as assessed by echocardiography. In addition, these animals exhibit hypertrophic and contractile responses to 10-day infusion of angiotensin II or isoproterenol similar to those observed in control animals. These results indicate that in adult mice cardiac ETA receptors are not necessary for either baseline cardiac function or stress-induced response to angiotensin II or isoproterenol.
Endothelin 1 (ET-1) was identified as an endothelium-derived, vasoactive 21-amino-acid peptide (34). The hormone activates two G-protein-coupled receptors, endothelin A (ETA) and endothelin B (ETB), with approximately equal affinity (2, 30). Complex physiology of the endothelin system is suggested by broad tissue distribution of the ligand and its receptors. ET-1 is secreted by many cell types, including endothelial cells and cardiomyocytes (28, 34), whereas ETA and ETB receptors are expressed by cells such as vascular and nonvascular smooth muscle cells, cardiomyocytes, neurons, and renal tubular epithelial cells (2, 9, 28, 30). The circulating levels of ET-1 in plasma are 2 orders of magnitude below the concentration needed for receptor activation, suggesting that ET-1 functions locally (4).
In the heart, ET-1 is produced by cardiomyocytes, fibroblasts, and endothelial cells (9, 28, 34). The cardiomyocytes predominantly express ETA receptor (9). The binding of ET-1 to both ETA and ETB receptors on cardiomyocytes results in activation of Gq signaling, increased intracellular calcium, and positive inotropy and chronotropy (5, 17, 19, 27; for review, see reference 8). In addition, ET-1 and its receptors mediate stress-induced remodeling in the mammalian heart (for a review, see reference 21). In vitro experiments show that ET-1-mediated activation of either ETA or ETB receptors on cardiomyocytes results in cellular hypertrophy (7, 18). In vivo studies demonstrate that levels of ET-1 and ETA are elevated in animal models of congestive heart failure (29, 35, 36). Providing the final proof of endothelins' molecular role in cardiac pathology, endothelin receptor antagonists inhibit the progression of cardiac remodeling in animal models of congestive heart failure (25, 28, 33).
Even though pharmacological effects of endothelins are well-known, detailed tissue-specific roles of ET-1 and its receptors have never been outlined in vivo. This is in part because of neonatal lethality of systemic ETA and ET-1 knockout mice due to developmental defects in cardiac and craniofacial structures (6, 23). Therefore, utilizing the cre/loxP technology (13), we have generated mice in which the ETA gene can be deleted in a selected cell population (20). In the present study, we deleted the ETA gene in cardiomyocytes by crossing mice with loxP-flanked ETA (ETAflox) alleles with transgenic mice that express cre recombinase under the control of the cardiomyocyte-specific α-myosin heavy-chain promoter (1). The mice with cardiomyocyte-specific deletion of ETA are viable and were used to study the role of ETA in cardiac physiology and stress response to angiotensin II or isoproterenol.
MATERIALS AND METHODS
Generation of cardiomyocyte-specific ETA knockout mice.
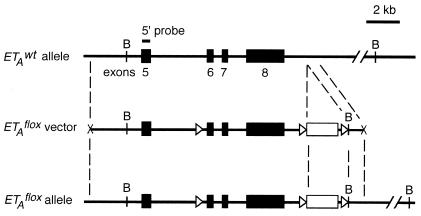
The conditional ETA knockout construct (Fig. 1) was used to generate homozygous ETAflox/flox mice as previously described (20). To target deletion of the ETA gene to cardiomyocytes, the mice were bred with animals that express cre recombinase from the transgene driven by the α-myosin heavy chain (α-MHC) promoter [cre(Tg)] (1). The specificity and efficiency of cre-mediated recombination in cardiomyocytes was evaluated by crossing cre(Tg) mice with CAG-CAT-lacZ transgenic mice, a line that expresses functional β-galactosidase only after cre-mediated recombination (3). The hearts from cre(Tg); CAG-CAT-lacZ(Tg) and cre(−); CAG-CAT-lacZ(Tg) males were harvested, whole-mount β-galactosidase stained, and sectioned as previously described (22). The genetic background of the mice used in the present study was a mixture of C57BL/6J, 129/S6, and FVB/N strains (1). To ensure an unbiased genetic background, the four experimental genotypes were generated as littermates from crosses between hemizygous cre transgenic ETAflox/wt and nontransgenic ETAflox/wt mice or from the immediate subsequent generation. The animals were housed in an animal colony with a 12-h day-night cycle on a standard Harlan Teklad diet. All procedures were approved by the Institutional Animal Care Research Advisory Committee at the University of Texas Southwestern Medical Center at Dallas.
FIG. 1.
Diagram representing the 3′ region of wild-type ETA allele, loxP-flanked ETA vector, and recombined ETAflox allele. The exons (numbered 5 to 8) and a neomycin cassette are shown as closed and open boxes, respectively. The loxP sites are represented by open triangles, and the BamHI sites are indicated by the letter B. Also shown is the 5′ probe used to detect the ETAflox deletion in Southern blot analysis of genomic DNA digested with BamHI.
ETA genotype analysis.
The wild-type ETA (ETAwt) and ETAflox alleles were analyzed by PCR with tail DNA and primers 5′-CCTCAGGAAGGAAGTAGCAAGATTA-3′ and 5′-ACACAACCATGGTGTCGA-3′. The ETAwt and ETAflox alleles yielded 610- and 650-bp products, respectively. The PCR was run for 35 cycles of 30 s at 94°C, 30 s at 65°C, and 30 s at 72°C. The PCR genotypic analysis was confirmed by Southern blot analysis with BamHI digestion. The cre recombinase transgene was detected by PCR as previously described (1).
Detection of cre recombinase-induced ETAflox deletion.
DNA was isolated from hearts, lungs, livers, and brains of 2-month-old male mice. Approximately 20 μg of DNA was digested with BamHI, separated on an agarose gel, acid fragmented, transferred to a nylon membrane (Hybond N++), and subjected to Southern blot analysis with a 5′-internal probe (Fig. 1). The ETAwt allele, ETAflox allele, and cre recombinase-deleted ETAflox allele yielded 24-, 12-, and 3.8-kb fragments, respectively (Fig. 1).
Real-time RT-PCR.
Total RNA was isolated from hearts of 6-week-old male mice by using Trizol (Gibco-BRL) according to the manufacturer's protocols. Reverse transcription (RT) was carried out by using oligo(dT) primer and the Superscript choice system (Gibco-BRL). Real-time PCR was performed by using SYBR Green PCR Master Mix with a 400 nM concentration of each primer in a model 5700 Thermocycler (Applied Biosystems). The primers used for quantitation were as follows: 5′-CTGAAAACAATTTTTGAATTTCTTGC-3′ and 5′-TACCAAGATGTGAAGGACTGGTGG-3′ for ETA mRNA, 5′-GTAACATGCAATCGCCCGCA-3′ and 5′-GGAACCCCAATTCCTTTAA-3′ for ETB mRNA, 5′-ACAGTCCGCCTAGAAGCACT-3′ and 5′-TCCGATGCCCTGAGGCTCTT-3′ for β-actin mRNA; and 5′-TGCACCACCAACTGCTTAG-3′ and 5′-GGATGCAGGGATGATGTTC-3′ for GAPDH mRNA. The PCR was run for 2 min at 50°C, 10 min at 95°C, and 40 cycles of 15 s at 94°C, 20 s at 55°C, and 30 s at 72°C. PCR products were present only in reverse transcriptase-primed samples: 390 bp for ETA mRNA, 250 bp for ETB mRNA, 390 bp for β-actin mRNA, and 175 bp for GAPDH mRNA.
Radioligand-binding assay.
The binding assay was performed as previously described (30) by using 90 mg of cardiac protein preparation and 125I-labeled ET-1 (2,000 Ci/mmol; Amersham). The protein extracts were obtained from hearts of 6-week-old males by homogenization in 3 ml of extraction buffer (25 mM Tris-HCl [pH 7.5], 135 mM NaCl, 27 mM KCl, 1.8 mM CaCl2, 1.1 mM MgSO4) containing aprotinin (Calbiochem), 4-amidinophenylmethane sulfonyl fluoride (Sigma), and pepstatin A (Sigma). The protein concentration was then determined by Bradford assay (Bio-Rad). ETA-specific binding, ETB-specific binding, and nonspecific binding of radiolabeled ET-1 to cardiac protein preparations were determined in the presence of 1 μM ETB-specific antagonist BQ788 (American Peptide), 1 μM ETA-specific antagonist FR139317 (Fujisawa Pharmaceuticals), and 100 nM unlabeled ET-1 (American Peptide), respectively. The nonspecific binding was subtracted from the acquired data to obtain total endothelin receptor binding, ETA-specific binding, and ETB-specific binding. The cre(Tg); ETAflox/flox group consisted of four cardiac preparations, whereas the genetic controls consisted of three cardiac preparations from cre(−); ETAwt/wt and cre(Tg); ETAwt/wt mice and two cardiac preparations from cre(−); ETAflox/flox mice.
Osmotic minipump infusion.
Osmotic minipumps (model 1002; Alzet) were used to administer isoproterenol or angiotensin II at doses of 10.4 μg/kg/min and 200 ng/kg/min, respectively, as reported by another research group (10). The pumps were implanted into 10-week-old male mice subcutaneously above the scapula using ketamine and xylazine anesthesia (35 mg/kg ketamine and xylazine each). The treatment groups contained 6 male mice for the cre(Tg); ETAflox/flox group and 12 male mice for their genetic control [3 for cre(−); ETAwt/wt mice, 3 for cre(Tg); ETAwt/wt mice, and 6 for cre(−); ETAflox/flox mice].
Mouse echocardiography and necropsy.
Echocardiography was conducted before osmotic pump implantation and repeated 10 days after the procedure. For echocardiography, the mice were anesthetized (35 mg of ketamine and xylazine each/kg) and subjected to measurements with S12 probe and SONOS 5500 (Philips Medical Imaging, Andover, Mass.) as previously described (32). Two-dimensional echocardiography in a mid-ventricular short-axis view was used to guide measurements of left ventricular (LV) end-diastolic and end-systolic dimensions, fractional shortening, and wall thickness. Apical long-axis view was used to guide Doppler measurements of LV outflow tract velocity, ejection time, and aortic velocity time integral. To determine weight of hearts, lungs, livers, and kidneys, the organs were collected from 6-week-old male mice sacrificed by using carbon dioxide. Eight animals from each genotype were used for the analysis. To examine cardiac anatomy and histology, hearts were frozen in OCT, sectioned at the plane of aortic valve to the cardiac apex, and stained with hematoxylin and eosin. The representative images are slides scanned by using Optronics charge-coupled device camera.
Statistics.
Experimental data are expressed as the mean value ± the standard error of the mean. Group data are analyzed by analysis of variance. Differences among groups are considered statistically significant for P < 0.05.
RESULTS
Generation of cardiomyocyte-specific ETA knockout mice.
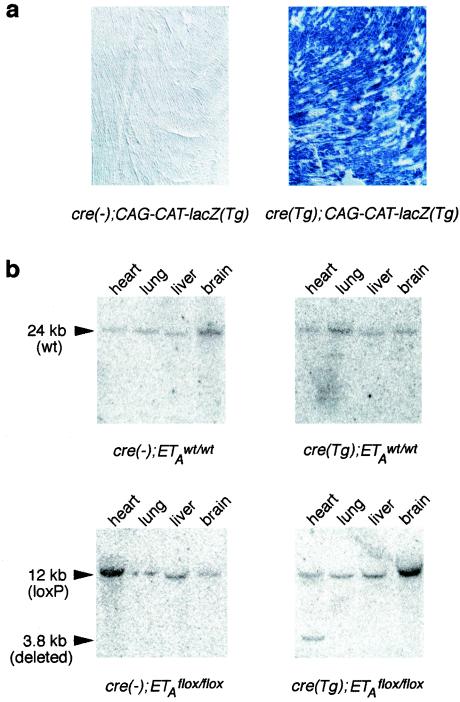
Generation of the homozygous loxP-flanked ETA (ETAflox/flox) mice has been previously described (20). These mice grow normally into adulthood without any health problems. In order to delete the ETA gene specifically in cardiomyocytes, the ETAflox/flox mice were bred with transgenic animals which express cre recombinase under the control of cardiomyocyte-specific α-MHC promoter [cre(Tg)] (1). The cre(Tg) transgenic mice are healthy when bred in hemizygous fashion in our colony. In concordance with previously published results (1), cre(Tg) mice induced functional cre recombinase expression in >90% of cardiomyocytes from cre(Tg); CAG-CAT-lacZ(Tg) mice, as determined by β-galactosidase staining (Fig. 2a). In the cre(Tg); ETAflox/wt and cre(−); ETAflox/wt crosses, the cre(Tg); ETAflox/flox mice were born at the expected Mendelian ratio with no detectable defects and grew healthy into adulthood.
FIG. 2.
Deletion of the CAG-CAT-lacZ reporter transgene and the loxP-flanked ETA allele by cre recombinase driven by the cardiac α-MHC promoter. (a) β-Galactosidase staining of heart sections from cre(Tg); CAG-CAT-lacZ(Tg) and cre(−); CAG-CAT-lacZ(Tg) mice. Staining indicating the presence of functional cre recombinase was only seen in cardiomyocytes of cre(Tg); CAG-CAT-lacZ(Tg) mice and not in any cell type of cre(−); CAG-CAT-lacZ(Tg) animals. (b) Southern blots of genomic DNA digested with BamHI were hybridized with the 5′ probe shown in Fig. 1. The expected 24-kb hybridization signal for the wild-type ETA (ETAwt) allele was only observed for all of the organ samples of cre(−); ETAwt/wt and cre(Tg); ETAwt/wt mice. The expected 12-kb hybridization signal of the ETAflox allele was only observed in the organ samples of cre(−); ETAflox/flox and cre(Tg); ETAflox/flox animals. In addition, the expected 3.8-kb band indicative of loxP-flanked ETA allele deletion was observed only in the hearts of mice with both the ETAflox/flox allele and a cardiomyocyte-specific cre recombinase transgene.
Southern blot analysis of genomic DNA digested with BamHI and hybridized with the 5′ probe (Fig. 1) produced the expected 24- and 12-kb bands in the ETAwt/wt mice and the ETAflox/flox mice, respectively (Fig. 2b). In addition, the 3.8-kb band (Fig. 2b) indicative of loxP-flanked ETA allele deletion was observed in the hearts of cre(Tg); ETAflox/flox mice. This band was not observed in the lungs, livers, and brains of the cre(Tg); ETAflox/flox mice or in any organs of the genetic controls [cre(−); ETAwt/wt mice, cre(Tg); ETAwt/wt mice, and cre(−); ETAflox/flox mice]. The intensity of the 3.8-kb band was equal to that of 12-kb band in the heart of cre(Tg); ETAflox/flox mice, indicating similar quantities of recombined and nonrecombined alleles in the cardiac tissue preparations (Fig. 2b).
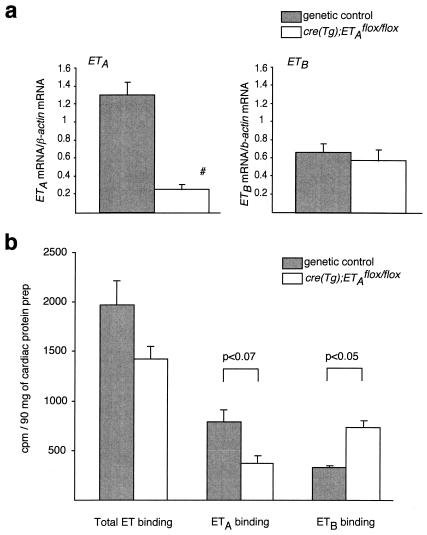
Real-time RT-PCR showed a statistically significant reduction (78%) in the ETA mRNA level and no change in the ETB mRNA level in the hearts of cre(Tg); ETAflox/flox mice compared to the genetic controls (Fig. 3a). Similar results were obtained when either β-actin mRNA or GAPDH mRNA (results not shown) was used as an internal standard.
FIG. 3.
Cardiac expression of ETA and ETB receptors in cre(Tg); ETAflox/flox mice. (a) Real-time RT-PCR on cardiac RNA showed a 78% reduction of the ETA mRNA level and no change in the ETB mRNA level in cre(Tg); ETAflox/flox mice compared to their genetic controls (n = 3 per genotype; #, P < 0.05 for cre(Tg); ETAflox/flox versus each genetic control groups). The β-actin mRNA was used as an internal standard. (b) Radioligand-binding assay for endothelin receptors on cardiac membrane preparations showed no statistically significant change in overall ET-1 binding, twofold higher levels of ETB (P < 0.05), and a trend toward lower levels of ETA (P < 0.07) in the cre(Tg); ETAflox/flox mice compared to the genetic controls [n = 4 for the cre(Tg); ETAflox/flox group; n = 8 for the genetic control group].
Due to lack of available antibodies specific to ETA receptor that can be used for immunohistochemistry, we instead used radioligand-binding assay for this purpose. The [125I]ET-1 radioligand-binding assay for endothelin receptors on heart membrane preparations showed no change in overall ET-1 binding, 2.2-fold-higher levels of ETB receptor, and a trend toward lower levels of ETA receptor in the cre(Tg); ETAflox/flox mice in comparison to the genetic controls (Fig. 3b). In the control animals, ETA receptor bound the majority of radiolabeled ET-1, as previously reported in the literature (9). In the hearts of cre(Tg); ETAflox/flox mice, however, the ETA receptor was a minor component of total endothelin binding. In conclusion, all three applied examinations—Southern blot analysis of the ETA gene, real-time RT-PCR of ETA mRNA, and the ET-1 radioligand-binding assay—demonstrated that the ETA gene is efficiently deleted in cardiomyocytes of the cre(Tg); ETAflox/flox mice.
The cardiomyocyte-specific ETA knockout animals have normal cardiac anatomy and contractility.
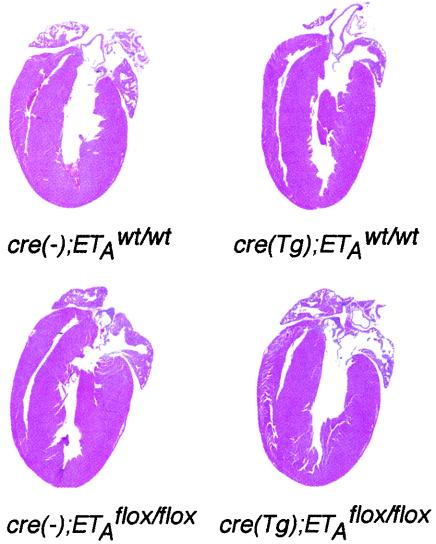
The cre(Tg); ETAflox/flox mice and their genetic controls were sacrificed at 6 weeks of age to document cardiac anatomy and weight. The hearts of cardiomyocyte-specific ETA knockout mice had normal anatomy and histology of the right ventricular and LV wall and interventricular septum (Fig. 4). The cardiac weight was 0.60% ± 0.02% of body weight, a finding similar to the cardiac weight of controls: 0.63% ± 0.01% for the cre(−); ETAwt/wt mice, 0.63% ± 0.02% for the cre(Tg); ETAwt/wt mice, and 0.64% ± 0.02% for the cre(−); ETAflox/flox mice (Table 1).
FIG. 4.
Normal cardiac anatomy of cre(Tg); ETAflox/flox mice. Hearts from 6-week-old male mice were sectioned through the plane including the aortic valve and the apex, and stained with hematoxylin and eosin. A representative sample of each genotype is shown.
TABLE 1.
Cardiac weight and function in cardiomyocyte-specific ETA knockout mice and genetic controls
| Parameter | Mean value ± SD (n)a
|
|||
|---|---|---|---|---|
| cre(−); ETAwt/wt | cre(Tg); ETAwt/wt | cre(−); ETAflox/flox | cre(Tg); ETAflox/flox | |
| Body wt (g) | 19.82 ± 0.79 (8) | 20.66 ± 0.62 (8) | 21.49 ± 0.38 (8) | 20.48 ± 0.65 (8) |
| Heart wt (% of body weight) | 0.63 ± 0.01 | 0.63 ± 0.02 | 0.64 ± 0.02 | 0.60 ± 0.02 |
| Vp (cm/s) | 70.3 ± 3.6 (16) | 64.0 ± 2.4 (16) | 75.1 ± 3.7 (8) | 71.2 ± 3.5 (8) |
| Aortic velocity time integral (cm) | 3.55 ± 0.17 | 3.03 ± 0.13b | 3.74 ± 0.21 | 3.60 ± 0.15 |
| Ejection time (ms) | 75 ± 1 | 70 ± 2 | 72 ± 2 | 76 ± 3 |
| Heart rate (bpm)c | 338 ± 13 | 353 ± 16 | 334 ± 12 | 337 ± 15 |
The number of animals in each group is in parentheses.
P < 0.05 for the difference in value versus other groups.
bpm, beats per minute.
To document inotropy and chronotropy, anesthetized 10-week-old male mice were subjected to echocardiography. The LV internal dimensions, wall thickness, fractional shortening, and LV mass of cre(Tg); ETAflox/flox animals did not differ from the genetic controls (data not shown). In addition, cardiomyocyte-specific ETA knockout animals' peak velocity of left outflow tract (Vp; 71.2 ± 3.5 cm/s), aortic velocity time integral (3.60 ± 0.15 cm), ejection time (76 ± 3 ms), and heart rate (337 ± 15 bpm) did not differ from the genetic controls (Table 1). The only statistically significant difference observed was the reduction of the aortic velocity time integral in the cre(Tg); ETAwt/wt mice (3.03 ± 0.13 cm) compared to the cre(−); ETAwt/wt mice (3.55 ± 0.17 cm), cre(−); ETAflox/flox mice (3.74 ± 0.21 cm), and cre(Tg); ETAflox/flox mice (3.60 ± 0.15 cm). All of the measured contractile parameters corresponded to the values reported in the murine literature (32), with the exception of aortic velocity time integral, which, to our knowledge, has not been previously reported.
The cardiomyocyte-specific ETA knockout mice exhibit similar hypertrophic response to infusion of angiotensin II or isoproterenol as the control animals. Since cre(Tg); ETAflox/flox mice have normal cardiac anatomy and basal contractility, we extended our studies to find possible differences among the genotypes in response to cardiac stress induced by continuous infusion of angiotensin II or isoproterenol. The cardiomyocyte-specific ETA knockout male mice and their genetic controls were subjected to baseline echocardiography and then were infused via osmotic pump for 10 days with either saline, angiotensin II, or isoproterenol. The animals were again examined by echocardiography after the infusion period. Continuous infusion of saline resulted in no changes in Vp and ejection time in the cre(Tg); ETAflox/flox mice and the genetic controls (Table 2). Compared to the saline treatment, infusion of angiotensin II led to similar increases (ca. 8%) of the heart weight of all genotypes (Table 2). Angiotensin II treatment did not change peak velocity and ejection time in either the cardiomyocyte-specific ETA knockout mice or the genetic controls. Compared to the saline treatment, infusion of isoproterenol led to similar increases (ca. 30%) in the heart weight in all genotypes (Table 2). Compared to the baseline measurements, isoproterenol treatment increased peak velocity and decreased ejection time equally in the cardiomyocyte-specific ETA knockout animals and the genetic controls. Similar degrees of cardiac hypertrophy have been reported in response to angiotensin II or isoproterenol infusion in wild-type mice (10).
TABLE 2.
Cardiac weight and function in cardiomyocyte-specific ETA knockout mice and genetic controls subjected to 10-day infusion of saline, angiotensin II, or isoproterenol by using osmotic minipumps
| Parameter | Mean value ± SDa
|
|||||
|---|---|---|---|---|---|---|
| Saline
|
Angiotensin II
|
Isoproterenol
|
||||
| Control (6) | KO (12) | Control (6) | KO (12) | Control (6) | KO (12) | |
| Body wt (g) | 27.3 ± 0.9 | 25.3 ± 1.2 | 27.3 ± 0.8 | 27.2 ± 0.8 | 25.7 ± 0.6 | 25.6 ± 1.2 |
| Heart wt (% body wt) | 0.59 ± 0.01 | 0.63 ± 0.01 | 0.64 ± 0.01* | 0.68 ± 0.01* | 0.84 ± 0.03* | 0.81 ± 0.02* |
| Vp before (cm/s) | 78.9 ± 4.1 | 69.6 ± 8.7 | 69.1 ± 4.0 | 77.8 ± 6.4 | 66.5 ± 4.9 | 71.4 ± 3.4 |
| Vp after (cm/s) | 78.8 ± 3.7 | 77.1 ± 4.9 | 82.1 ± 5.6 | 83.9 ± 3.7 | 135 ± 10.0*† | 132 ± 20.1*† |
| TE (ms) beforeb | 72 ± 2 | 76 ± 1 | 75 ± 2 | 71 ± 3 | 69 ± 3 | 77 ± 8 |
| TE (ms) after | 74 ± 2 | 80 ± 2 | 75 ± 3 | 78 ± 2 | 44 ± 2*† | 49 ± 2*† |
The numbers of animals used in experiments are indicated in parentheses.
TE, ejection time. *, P < 0.05 versus saline-treated mice with the same genotype; †, P < 0.05 versus baseline measurement before drug administration.
DISCUSSION
ET-1 acts as a local factor involved in cardiac and craniofacial development, as well as in the regulation of cardiac contractility and hypertrophy (21). However, our knowledge of endothelin physiology is still fragmentary due to several factors, including broad expression of ET-1 and its receptors and neonatal lethality of both systemic ET-1 and ETA knockouts (6, 23). In order to broaden our current knowledge of endothelin biology, we generated mice with loxP-flanked ETA allele. In these mice, the ETA gene can be deleted in a selected cell population by cell-specific expression of cre recombinase transgene. We have already successfully deleted the ETA gene selectively in smooth muscle cells by using cre recombinase driven by smooth muscle MHC promoter (20). The deletion of ETA from smooth muscle cells has resulted in hypotensive phenotype in these mice.
The goal of current study was to define the cardiac role of ETA receptors. By using mice expressing cre recombinase under the control of α-MHC promoter, we targeted ETA gene deletion to cardiomyocytes. The success of ETA gene deletion was confirmed by Southern blot analysis, real-time RT-PCR, and ET-1 radioligand-binding assay. Southern blot analysis showed the presence of deleted ETA allele in the heart but not in other organs of cre(Tg); ETAflox/flox mice. The similar intensities of deleted and nondeleted ETA alleles in Fig. 2b demonstrated that the gene was deleted in nearly 50% of genomes in the cardiac tissues of cre(Tg); ETAflox/flox mice. Since cardiomyocytes contribute ca. 50% of tissue DNA in the adult mouse heart (31), this indicated that the majority of cardiomyocytes contained deleted loxP-flanked ETA alleles. Real-time RT-PCR documented a significant reduction in ETA mRNA levels in the hearts of cre(Tg); ETAflox/flox mice compared to the genetic controls. Radioligand-binding assay showed a trend toward lower ETA protein levels in cardiac membrane preparations from the cre(Tg); ETAflox/flox mice. Residual levels of ETA mRNA and protein in the cardiac samples from cre(Tg); ETAflox/flox mice are likely due to intact expression of ETA receptor by cardiac fibroblasts and vascular smooth muscle cells (9, 21). Because of the trend toward lower levels of ETA and twofold-increased levels of ETB receptor in the hearts of cre(Tg); ETAflox/flox mice, ETB receptor becomes the dominant endothelin receptor in cardiac tissue of these mice. With the previous findings that the majority of cardiomyocytes express functional cre recombinase in the α-MHC-driven cre recombinase transgenic mice (1), these results demonstrate that cre(Tg); ETAflox/flox mice lack ETA specifically in the cardiomyocytes.
The cardiomyocyte-specific ETA knockout mice are viable and have no detectable abnormalities in cardiac anatomy and weight. The ETA knockout mice also have similar cardiac contractility (as measured by Vp), aortic velocity time integral, ejection time, and heart rate) as the genetic controls. Although ET-1 has been reported to have a positive inotropic and chronotropic effect via the ETA receptor (5, 17, 19, 27), our results suggest that ETA receptor on murine cardiomyocytes is dispensable for the maintenance of basal cardiac anatomy, inotropy, and chronotropy.
The cardiomyocyte-specific ETA knockout mice also exhibited similar hypertrophic response to 10-day infusion of angiotensin II or isoproterenol compared to the control animals. This is a surprising result since multiple studies suggested a role of ETA receptor in these pharmacologically induced hypertrophies. First, angiotensin II and isoproterenol have been shown to increase levels of ET-1 in cultured rat cardiomyocytes (18, 24). Second, ETA receptor antagonists reduced angiotensin II-mediated cardiomyocyte hypertrophy in vitro (18). Third, the combined ETA/ETB receptor antagonist bosentan inhibited angiotensin II-induced cardiac hypertrophy and arterial hypertension in rats (16). In contrast to these results, our findings demonstrate that angiotensin II- and isoproterenol-induced hypertrophies do not require the presence of ETA receptors on cardiomyocytes. However, since we found that the protein levels of ETB receptor is twofold higher in the hearts of ETA knockout mice compared to the genetic controls, ETB may be compensating for the lack of ETA receptors on cardiomyocytes. Another possibility is that ET-1 produced within the heart acts primarily on the ETA receptors on cardiac fibroblasts in order to induce cardiac remodeling. In several studies, endothelin signaling through cardiac fibroblasts has been shown to play an active role in the induction of cardiac remodeling (9, 11; for review, see reference 26). Furthermore, the observed elevated levels of ETB receptors in the hearts of ETA knockout mice also demonstrate the presence of complex mechanisms that control levels of ETA and ETB receptors in cardiac tissue.
Similar results, i.e., the lack of protection from pressure-induced myocardial hypertrophy, were reported in mice lacking angiotensin II receptor type 1A (AT1A) (14, 15). This was unexpected since pharmacological antagonism of AT1 angiotensin receptor is widely used in prevention of cardiac remodeling in both animal models and patients (for review, see reference 12). Thus, both the cardiomyocyte-specific knockout of ETA receptor and the systemic knockout of AT1A angiotensin II receptor do not recapitulate the pharmacological benefits of ETA and AT1 antagonism in cardiac hypertrophy (25, 28, 33). These discrepancies await further studies.
Acknowledgments
We thank Kristine E. Kamm for helpful discussions and Mark Valasek for editorial suggestions. We also thank Sahar Seyedkalal and Shelley Dixon for technical help.
M.Y. is an Investigator of the Howard Hughes Medical Institute. R.M.K. is a trainee in Medical Scientist Training Program at the University of Texas Southwestern Medical Center at Dallas. This study is supported in part by research funds from the W. M. Keck Foundation, the Perot Family Foundation, and the ERATO project of Japan Science and Technology Corp.
REFERENCES
- 1.Agah, R., P. A. Frenkel, B. A. French, L. H. Michael, P. A. Overbeek, and M. D. Schneider. 1997. Gene recombination in postmitotic cells: targeted expression of cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J. Clin. Investig. 100:169-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai, H., S. Hori, I. Aramori, H. Okhuba, and S. Nakanishi. 1990. Cloning and expression of a cDNA encoding an endothelin receptor. Nature 348:730-732. [DOI] [PubMed] [Google Scholar]
- 3.Araki, K., M. Araki, J. Miyazaki, and P. Vassalli. 1995. Site-specific recombination of a transgene in fertilized eggs by transient expression of cre recombinase. Proc. Natl. Acad. Sci. USA 92:160-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battistini, B., P. D'Orleans-Juste, and P. Sirois. 1993. Endothelins: circulatory plasma levels and presence in other biologic fluids. Lab. Investig. 68:600-628. [PubMed] [Google Scholar]
- 5.Beyer, M. E., G. Slesak, H. M. Hoffmeister. 1995. In vivo hemodynamic and inotropic effects of the endothelinB agonist IRL 1620. J. Cardiovasc. Physiol. 26S3:S190-S192. [PubMed] [Google Scholar]
- 6.Clouthier, D. E., K. Hosoda, J. A. Richardson, S. C. Williams, H. Yanagisawa, T. Kuwaki, M. Kumada, R. E. Hammer, and M. Yanagisawa. 1998. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development 125:813-824. [DOI] [PubMed] [Google Scholar]
- 7.Cullen, J. P., D. Bell, E. J. Kelso, B. J. McDermott. 2001. Use of A-192621 to provide evidence for involvement of endothelin ETB receptors in endothelin-1-mediated cardiomyocyte hypertrophy. Eur. J. Pharmacol. 417:157-168. [DOI] [PubMed] [Google Scholar]
- 8.Douglas, S. A., and E. H. Ohlstein. 1997. Signal transduction mechanisms mediating vascular actions of endothelin. J. Vasc. Res. 34:152-164. [DOI] [PubMed] [Google Scholar]
- 9.Fareh, J., R. M. Touyz, E. L. Schiffrin, and G. Thibault. 1996. Endothelin-1 and angiotensin II receptors in cells from rat hypertrophied heart. Circ. Res. 78:302-311. [DOI] [PubMed] [Google Scholar]
- 10.Friddle, C. J., T. Koga, E. M. Rubin, and J. Bristow. 2000. Expression profiling reveals distinct sets of genes altered during induction and regression of cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 97:6745-6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray, M. O., C. S. Long, J. E. Kalinyak, H. T. Li, and J. S. Karliner. 1998. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-B and endothelin-1 from fibroblasts. Cardiovasc. Res. 40:352-363. [DOI] [PubMed] [Google Scholar]
- 12.Griendling, K. K., B. Lassegue, and R. W. Alexander. 1996. Angiotensin receptors and their therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 36:281-306. [DOI] [PubMed] [Google Scholar]
- 13.Gu, H., J. D. Marth, P. C. Orban, H. Mossman, and K. Rajewsky. 1994. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science 265:103-106. [DOI] [PubMed] [Google Scholar]
- 14.Hamawaki, M., T. M. Coffman, A. Lashus, M. Koide, M. R. Zile, M. I. Oliverio, G. DeFreyte, G. Cooper IV, and B. A. Carabello. 1998. Pressure-overload hypertrophy is unabated in mice devoid of AT1A receptors. Am. J. Physiol. 274:H868-H873. [DOI] [PubMed] [Google Scholar]
- 15.Harada, K., I. Komuro, I. Shiojima, D. Hayashi, S. Kudoh, T. Mizuno, K. Kijima, H. Matsubara, T. Sugaya, K. Murakami, and Y. Yazaki. 1998. Pressure overload induces hypertrophy in angiotensin II type 1A receptor knockout mice. Circulation 97:1952-1959. [DOI] [PubMed] [Google Scholar]
- 16.Herizi, A., B. Jover, N. Bouriquet, and A. Mimran. 1998. Prevention of cardiovascular and renal effects of angiotensin II by endothelin blockade. Hypertension 31:10-14. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa, T., M. Yanagisawa, S. Kimura, K. Goto, and T. Masaki. 1988. Positive chronotropic effects of endothelin, a novel endothelium-derived vasoconstrictor peptide. Pfluegers Arch. 413:108-110. [DOI] [PubMed] [Google Scholar]
- 18.Ito, H., Y. Hirata, S. Adachi, M. Tanaka, M. Tsujino, A. Koike, A. Nogami, F. Murumo, and M. Hiroe. 1993. Endothelin-1 is an autocrine/paracrine factor in the mechanism of angiotensin II-induced hypertrophy in cultured rat cardiomyocytes. J. Clin. Investig. 92:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasai, H., M. Takanashi, C. Takasaki, and M. Endoh. 1994. Pharmacological properties of endothelin receptor subtypes mediating positive inotropic effects in rabbit heart. Am. J. Physiol. 266:H2220-H2228. [DOI] [PubMed] [Google Scholar]
- 20.Kedzierski, R. M., T. Ohuchi, E. Isotani, Y. Y. Kisanuki, S. C. Williams, R. E. Hammer, J. A. Richardson, C. P. Reagan, G. K. Owens, K. E. Kamm, and M. Yanagisawa. Endothelin-A receptor gene knockout in smooth muscle causes hypotension and reveals an endothelin-B-mediated compensation in mice. In press.
- 21.Kedzierski, R. M., and M. Yanagisawa. 2001. Endothelin system: the double-edged sword in health and disease. Annu. Rev. Pharmacol. Toxicol. 41:851-876. [DOI] [PubMed] [Google Scholar]
- 22.Kisanuki, Y. Y., R. E. Hammer, J. Miyazaki, S. C. Williams., J. A. Richardson, and M. Yanagisawa. 2001. Tie2-cre transgenic mice: a new model for endothelial cell lineage analysis in vivo. Dev. Biol. 230:230-242. [DOI] [PubMed] [Google Scholar]
- 23.Kurihara, Y., H. Kurihara, H. Suzuki, T. Kodama, K. Maemura, R. Nagai, H. Oda, T. Kuwaki, W. H. Cao, N. Kamada, et al. 1994. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature 368:703-710. [DOI] [PubMed] [Google Scholar]
- 24.Morimoto, T., K. Hasegawa, H. Wada, T. Kakita, and S. Kaburagi. 2001. Calcineurin-GATA4 pathway is involved in beta-adrenergic agonist-responsive endothelin-1 transcription in cardiac myocytes. J. Biol. Chem. 276:34983-34989. [DOI] [PubMed] [Google Scholar]
- 25.Mulder, P., V. Richard, G. Derumeaux, M. Hogie, J. P. Henry, F. Lallemand, P. Compagnon, B. Mace, E. Comoy, B. Letac, and C. Thuillez. 1997. Role of endogenous endothelin in chronic heart failure: effects of long-term treatment with an endothelin antagonist on survival, haemodynamics, and cardiac remodeling. Circulation 96:1976-1982. [DOI] [PubMed] [Google Scholar]
- 26.Nicoletti, A., and J.-B. Michel. 1999. Cardiac fibrosis and inflammation: interaction with hemodynamic and hormonal factors. Cardiovasc. Res. 41:532-543. [DOI] [PubMed] [Google Scholar]
- 27.Saetrum Opgaard, O., S. Moller, R. de Vries, L. Edvinsson, and P. R. Saxena. 2000. Positive inotropic responses mediated by endothelin ETA and ETB receptors in human myocardial trabeculae. Clin. Sci. 99:161-168. [DOI] [PubMed] [Google Scholar]
- 28.Sakai, S., T. Miyauchi, M. Kobayashi, I. Yamaguchi, K. Goto, and Y. Sugishita. 1996. Inhibition of myocardial endothelin pathway improves long-term survival in heart failure. Nature 384:353-355. [DOI] [PubMed] [Google Scholar]
- 29.Sakai, S., T. Miyauchi, T. Sakurai, Y. Kasuya, M. Ihara, I. Yamaguchi, K. Goto, and Y. Sugishita. 1996. Endogenous endothelin-1 participates in the maintenance of cardiac function in rats with congestive heart failure. Marked increase in endothelin-1 production in the failing heart. Circulation 93:1214-1222. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai, T., M. Yanagisawa, Y. Takuwa, H. Miyazaki, S. Kimura, K. Goto, and T. Masaki. 1990. Cloning of a cDNA encoding a non-isopeptide-selective subtype of an endothelin receptor. Nature 348:732-735. [DOI] [PubMed] [Google Scholar]
- 31.Soonpaa, M. H., K. K. Kim, L. Pajak, M. Franklin, and L. J. Field. 1996. Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. 271:H2183-H2189. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka, N., N. Dalton, L. Mao, H. A. Rockman, K. L. Peterson, K. R. Gottshall, J. J. Hunter, K. R. Chien, and J. Ross, Jr. 1996. Transthoracic echocardiography in models of cardiac disease in the mouse. Circulation 94:1109-1170. [DOI] [PubMed] [Google Scholar]
- 33.Yamauchi-Kohno, R., T. Miyauchi, T. Hoshino, T. Kobayashi, H. Aihara, S. Sakai, H. Yabana, K. Goto, Y. Sugishita, and S. Murata. 1999. Role of endothelin in deterioration of heart failure due to cardiomyopathy in hamsters: increase in endothelin-1 production in the heart and the beneficial effects of endothelin-A receptor antagonist on survival and cardiac function. Circulation 99:2171-2176. [DOI] [PubMed] [Google Scholar]
- 34.Yanagisawa, M., H. Kurihara, S. Kimura, Y. Tomobe, M. Kobayashi, Y. Mitsui, Y. Yazaki, K. Goto, and T. Masaki. 1988. A novel vasoconstrictor peptide produced by vascular endothelial cells. Nature 332:411-415. [DOI] [PubMed] [Google Scholar]
- 35.Yorikane, R., S. Sakai, T. Miyauchi, T. Sakurai, Y. Sugishita, and K. Goto. 1993. Increased production of endothelin-1 in the hypertrophied rat heart due to pressure overload. FEBS Lett. 332:31-34. [DOI] [PubMed] [Google Scholar]
- 36.Zolk, O., J. Quattek, G. Sitzler, T. Schrader, G. Nickenig, P. Schnabel, K. Shimada, M. Takahashi, and M. Bohm. 1999. Expression of endothelin-1, endothelin-converting enzyme, and endothelin receptors in chronic heart failure. Circulation 99:2118-2123. [DOI] [PubMed] [Google Scholar]