Abstract
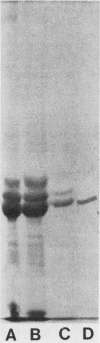
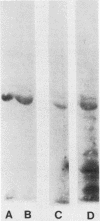
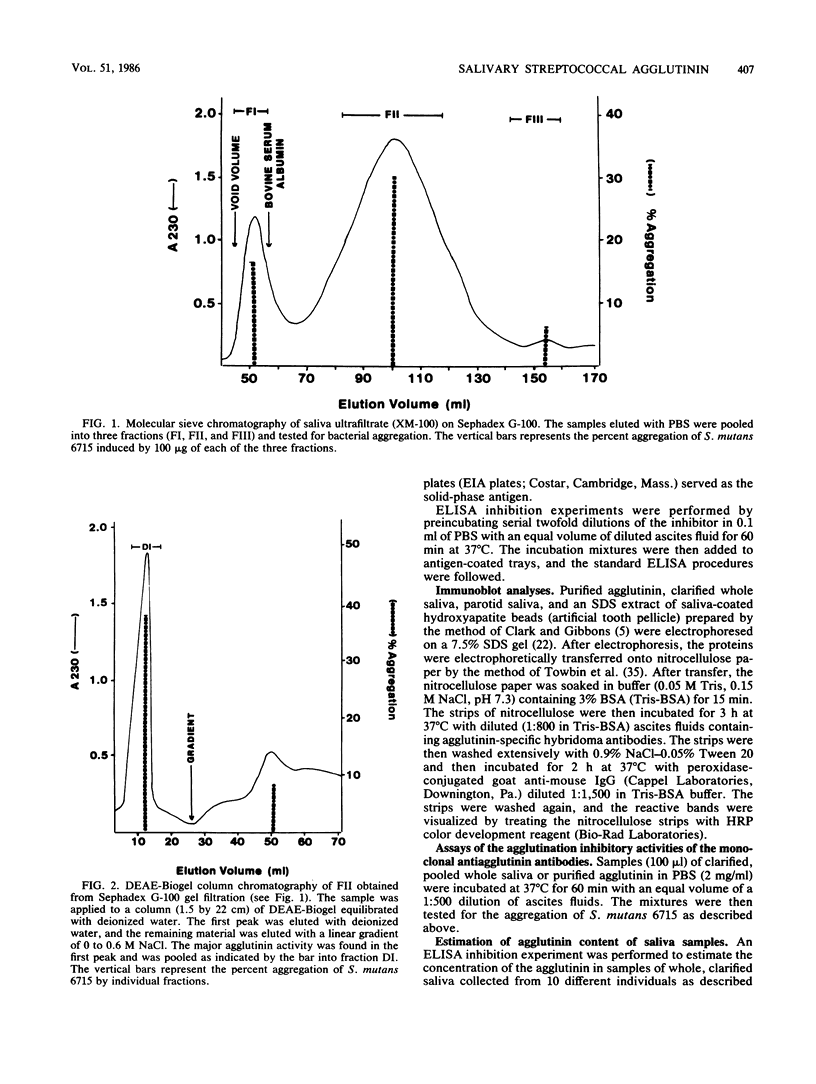
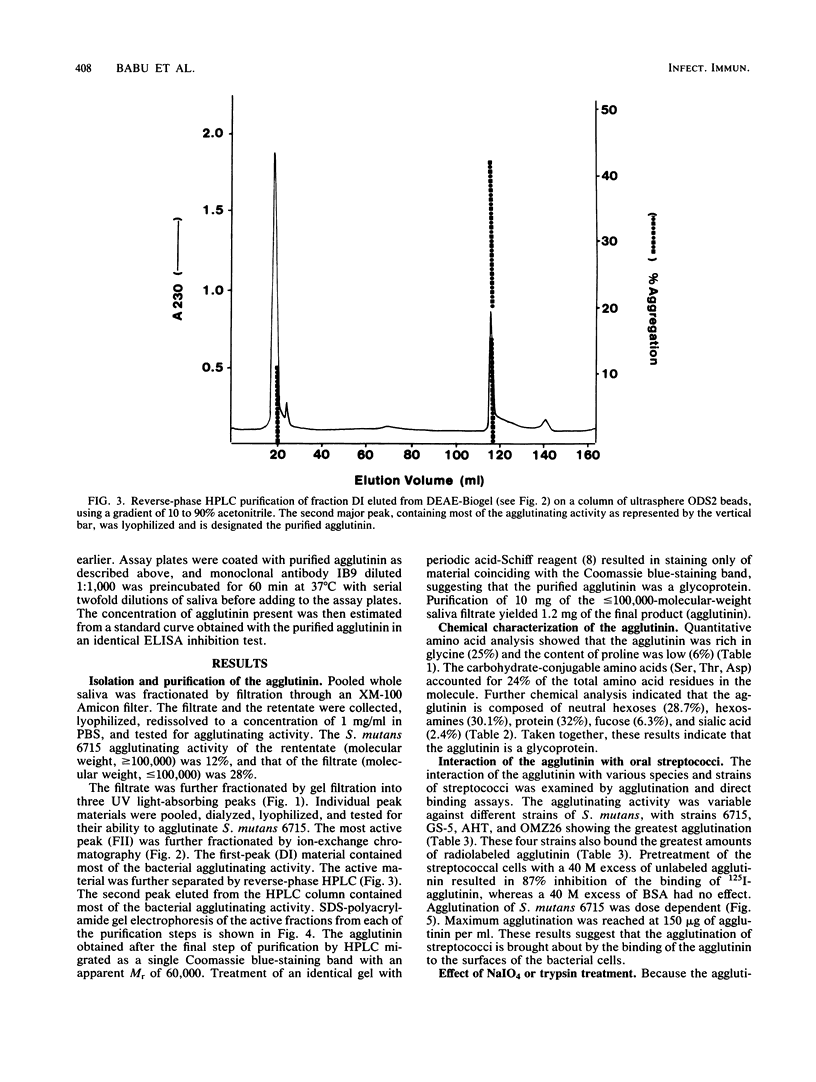
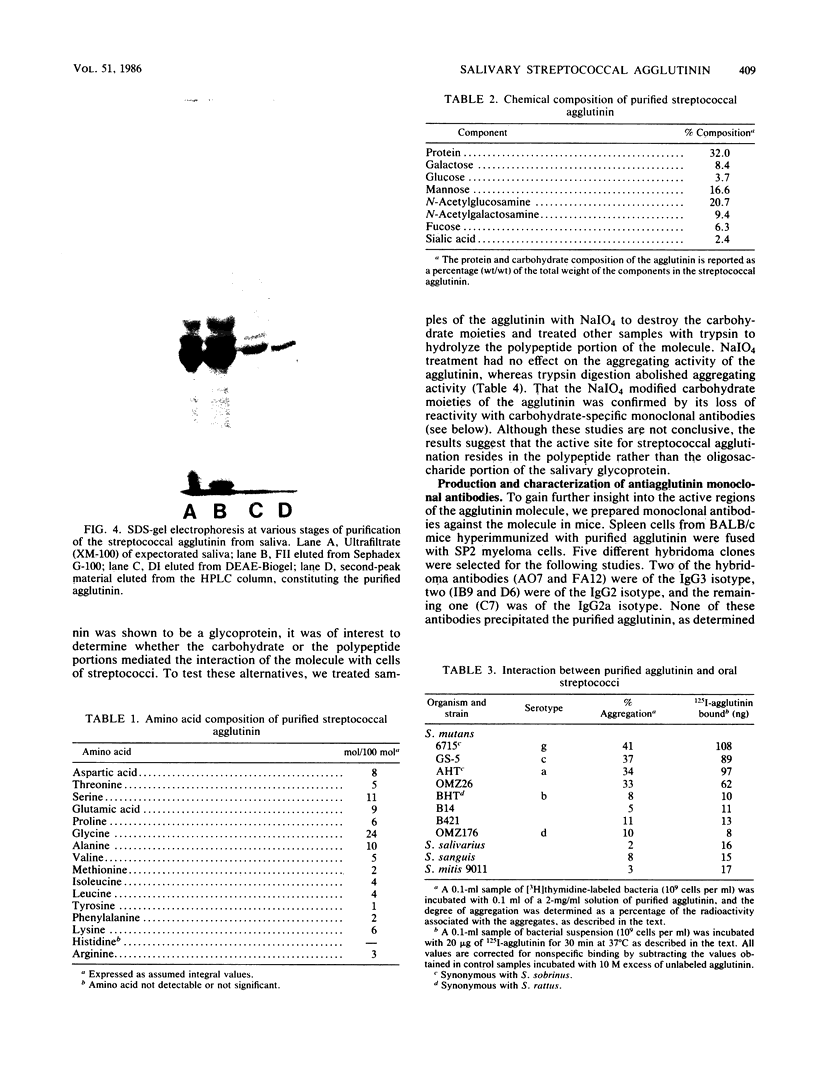
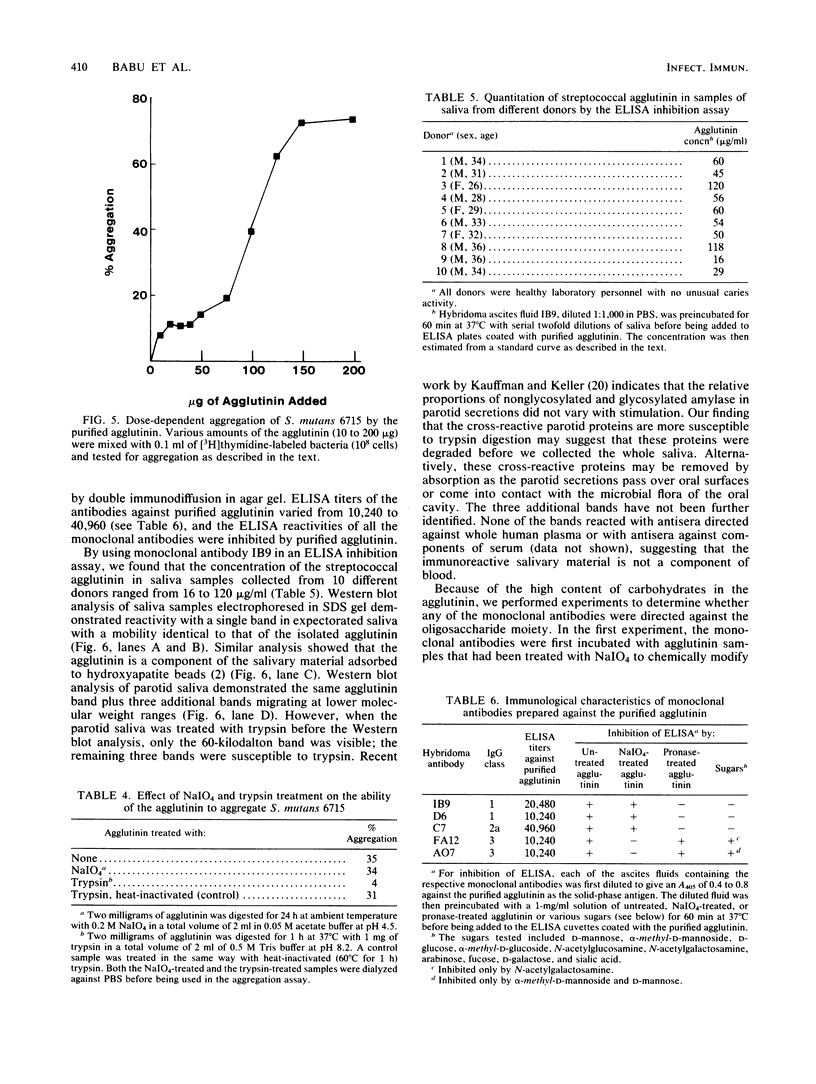
A bacterial agglutinin specific for strains of Streptococcus mutans was isolated from human saliva. Physiochemical analyses showed the agglutinin to be a glycoprotein with a molecular weight of 60,000. The agglutinin aggregated four of the eight strains of Streptococcus mutans tested but did not aggregate the strains of Streptococcus salivarius, Streptococcus sanguis, and Streptococcus mitis tested. Chemical modification of carbohydrate moieties of the agglutinin with sodium metaperiodate had no effect on aggregation, whereas modification of the polypeptide portion with trypsin abolished aggregating activity. A set of five murine hybridoma antibodies was employed to further analyze the agglutinin. Two carbohydrate-specific antibodies, directed against D-mannose and N-acetylgalactosamine moieties, respectively, failed to block agglutinin- or whole saliva-mediated aggregation of S. mutans cells. In contrast, two antibodies directed against pronase-sensitive antigenic sites blocked both agglutinin- and saliva-mediated aggregation of S. mutans cells. Western blot analysis with the agglutinin-specific hybridoma antibodies demonstrated the agglutinin in whole saliva and in artificial tooth pellicles formed on hydroxyapatite beads incubated with saliva. These results suggest that a 60-kilodalton glycoprotein of human saliva is a bacterial agglutinin with specificity for certain strains of S. mutans. They further suggest that aggregation is mediated by polypeptide rather than carbohydrate determinants of the glycoprotein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arneberg P. Partial characterization of five glycoprotein fractions secreted by the human parotid glands. Arch Oral Biol. 1974 Oct;19(10):921–928. doi: 10.1016/0003-9969(74)90055-7. [DOI] [PubMed] [Google Scholar]
- Babu J. P., Simpson W. A., Courtney H. S., Beachey E. H. Interaction of human plasma fibronectin with cariogenic and non-cariogenic oral streptococci. Infect Immun. 1983 Jul;41(1):162–168. doi: 10.1128/iai.41.1.162-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A., Connell G. E. Purification and partial characterization of four proteins from human parotid saliva. Biochem J. 1971 Jul;123(3):455–464. doi: 10.1042/bj1230455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challacombe S. J., Russell M. W., Hawkes J. E., Bergmeier L. A., Lehner T. Passage of immunoglobulins from plasma to the oral cavity in rhesus monkeys. Immunology. 1978 Dec;35(6):923–931. [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaola C., Herrera M. S., Mandel I. D. Host proteins in dental plaques of caries-resistant versus caries-susceptible human groups. Arch Oral Biol. 1984;29(5):353–355. doi: 10.1016/0003-9969(84)90159-6. [DOI] [PubMed] [Google Scholar]
- Douglas C. W., Russell R. R. The adsorption of human salivary components to strains of the bacterium Streptococcus mutans. Arch Oral Biol. 1984;29(10):751–757. doi: 10.1016/0003-9969(84)90002-5. [DOI] [PubMed] [Google Scholar]
- Ericson T., Rundegren J. Characterization of a salivary agglutinin reacting with a serotype c strain of Streptococcus mutans. Eur J Biochem. 1983 Jun 15;133(2):255–261. doi: 10.1111/j.1432-1033.1983.tb07456.x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Dental caries. Annu Rev Med. 1975;26:121–136. doi: 10.1146/annurev.me.26.020175.001005. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Golub E. E., Thaler M., Davis C., Malamud D. Bacterial aggregating activity in human saliva: simultaneous determination of free and bound cells. Infect Immun. 1979 Dec;26(3):1028–1034. doi: 10.1128/iai.26.3.1028-1034.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty D. L., Beachey E. H., Simpson W. A., Dale J. B. Hybridoma antibodies against protective and nonprotective antigenic determinants of a structurally defined polypeptide fragment of streptococcal M protein. J Exp Med. 1982 Apr 1;155(4):1010–1018. doi: 10.1084/jem.155.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. I., Gibbons R. J., Spinell D. M. Characteristics of some high molecular weight constituents with bacterial aggregating activity from whole saliva and dental plaque. Caries Res. 1971;5(2):111–123. doi: 10.1159/000259739. [DOI] [PubMed] [Google Scholar]
- Hay D. I. The adsorption of salivary proteins by hydroxyapatite and enamel. Arch Oral Biol. 1967 Aug;12(8):937–946. doi: 10.1016/0003-9969(67)90088-x. [DOI] [PubMed] [Google Scholar]
- Hogg S. D., Embery G. The isolation and partial characterization of a sulphated glycoprotein from human whole saliva which aggregates strains of Streptococcus sanguis but not Streptococcus mutans. Arch Oral Biol. 1979;24(10-11):791–797. doi: 10.1016/0003-9969(79)90040-2. [DOI] [PubMed] [Google Scholar]
- Kang A. H. Studies on the location of intermolecular cross-links in collagen. Isolation of a CNBr peptide containing -hydroxylysinonorleucine. Biochemistry. 1972 May 9;11(10):1828–1835. doi: 10.1021/bi00760a015. [DOI] [PubMed] [Google Scholar]
- Kashket S., Donaldson C. G. Saliva-induced aggregation of oral streptococci. J Bacteriol. 1972 Dec;112(3):1127–1133. doi: 10.1128/jb.112.3.1127-1133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman D. L., Keller P. J. Relationship of the basic glycoprotein to the basic proline-rich proteins in human parotid saliva. Arch Oral Biol. 1983;28(1):61–67. doi: 10.1016/0003-9969(83)90027-4. [DOI] [PubMed] [Google Scholar]
- Köhler B., Pettersson B. M., Bratthall D. Streptococcus mutans in plaque and saliva and the development of caries. Scand J Dent Res. 1981 Feb;89(1):19–25. doi: 10.1111/j.1600-0722.1981.tb01273.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Ellison S. A., Bahl O. P. The isolation from human parotid saliva and partial characterization of the protein core of a major parotid glycoprotein. Arch Oral Biol. 1973 Jul;18(7):827–837. doi: 10.1016/0003-9969(73)90053-8. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Rowan J., Straffon L. H., Loos P. J. Association of Streptococcus mutants with human dental decay. Infect Immun. 1975 Jun;11(6):1252–1260. doi: 10.1128/iai.11.6.1252-1260.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyachi Y., Vaitukaitis J. L., Nieschlag E., Lipsett M. B. Enzymatic radioiodination of gonadotropins. J Clin Endocrinol Metab. 1972 Jan;34(1):23–28. doi: 10.1210/jcem-34-1-23. [DOI] [PubMed] [Google Scholar]
- Oemrawsingh I., Roukema P. A. Isolation, purification and chemical characterization of mucins from human submandibular glands. Arch Oral Biol. 1974 Aug;19(8):615–626. doi: 10.1016/0003-9969(74)90129-0. [DOI] [PubMed] [Google Scholar]
- Oppenheim F. G., Hay D. I., Franzblau C. Proline-rich proteins from human parotid saliva. I. Isolation and partial characterization. Biochemistry. 1971 Nov;10(23):4233–4238. doi: 10.1021/bi00799a013. [DOI] [PubMed] [Google Scholar]
- Scherp H. W. Dental caries: prospects for prevention. Science. 1971 Sep 24;173(4003):1199–1205. doi: 10.1126/science.173.4003.1199. [DOI] [PubMed] [Google Scholar]
- Tabak L. A., Levine M. J., Mandel I. D., Ellison S. A. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol. 1982 Feb;11(1):1–17. doi: 10.1111/j.1600-0714.1982.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]