Abstract
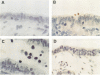
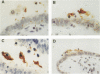
An experimental model using human fallopian tubes in organ culture was used to study the localization of purified gonococcal lipopolysaccharide (LPS). LPS was visualized by light microscopy with immunoperoxidase staining. Immediately after addition to fallopian tube organ cultures, gonococcal LPS aggregated on the tips of cilia. By 1 to 2 h after exposure, LPS could be seen distributed throughout the cytoplasm of ciliated and nonciliated cells in structures resembling vesicles. By 12 h, there were sloughed, ciliated cells present in the fallopian tube lumen, which had positive LPS stain on their surfaces as well as in their cytoplasm. By 24 h, LPS was distributed throughout the cytoplasm. Control experiments with rabbit oviduct organ cultures showed that LPS failed to attach, enter, or damage mucosal cells. These studies illustrate the initial localization of LPS on human mucosal cells and its uptake into the cells, which are coincident with toxicity for ciliated epithelial cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A., Gagliardi N. C. Antigenic heterogeneity of the non-serogroup antigen structure of Neisseria gonorrhoeae lipopolysaccharides. Infect Immun. 1979 Dec;26(3):870–874. doi: 10.1128/iai.26.3.870-874.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYDEN S. V. Fixation of bacterial products by erythrocytes in vivo and by Leucocytes. Nature. 1953 Feb 28;171(4348):402–403. doi: 10.1038/171402a0. [DOI] [PubMed] [Google Scholar]
- BUXTON A. The in vivo sensitization of avian erythrocytes with Salmonella gallinarum polysaccharide. Immunology. 1959 Jul;2:203–210. [PMC free article] [PubMed] [Google Scholar]
- Cooper K. E. Some physiological and clinical aspects of pyrogens. Ciba Found Symp. 1971:5–21. doi: 10.1002/9780470719800.ch1. [DOI] [PubMed] [Google Scholar]
- Curran R. C., Gregory J. Demonstration of immunoglobulin in cryostat and paraffin sections of human tonsil by immunofluorescence and immunoperoxidase techniques. Effects of processing on immunohistochemical performance of tissues and on the use of proteolytic enzymes to unmask antigens in sections. J Clin Pathol. 1978 Oct;31(10):974–983. doi: 10.1136/jcp.31.10.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C. R., Johnson A. P., Taylor-Robinson D., Melly M. A., McGee Z. A. Host species-specific damage to oviduct mucosa by Neisseria gonorrhoeae lipopolysaccharide. Infect Immun. 1981 Dec;34(3):1056–1058. doi: 10.1128/iai.34.3.1056-1058.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C. R., Melly M. A., Hellerqvist C. G., Coniglio J. G., McGee Z. A. Toxic activity of purified lipopolysaccharide of Neisseria gonorrhoeae for human fallopian tube mucosa. J Infect Dis. 1981 Mar;143(3):432–439. doi: 10.1093/infdis/143.3.432. [DOI] [PubMed] [Google Scholar]
- Gregg C. R., Melly M. A., McGee Z. A. Gonococcal lipopolysaccharide: a toxin for human fallopian tube mucosa. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):981–984. doi: 10.1016/0002-9378(80)91092-3. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen N. E., Sullivan R. Interaction between endotoxin and human monocytes: characteristics of the binding of 3H-labeled lipopolysaccharide and 51Cr-labeled lipid A before and after the induction of endotoxin tolerance. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3491–3495. doi: 10.1073/pnas.81.11.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Johnson A. P., Taylor-Robinson D. Human fallopian tubes in organ culture: preparation, maintenance, and quantitation of damage by pathogenic microorganisms. Infect Immun. 1976 Feb;13(2):608–618. doi: 10.1128/iai.13.2.608-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melly M. A., Gregg C. R., McGee Z. A. Studies of toxicity of Neisseria gonorrhoeae for human fallopian tube mucosa. J Infect Dis. 1981 Mar;143(3):423–431. doi: 10.1093/infdis/143.3.423. [DOI] [PubMed] [Google Scholar]
- Melly M. A., McGee Z. A., Rosenthal R. S. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J Infect Dis. 1984 Mar;149(3):378–386. doi: 10.1093/infdis/149.3.378. [DOI] [PubMed] [Google Scholar]
- Peacock W. L., Jr, Schmale J. D. Toxic constituents of Neisseria gonorrhoeae. Nature. 1969 Feb 22;221(5182):760–761. doi: 10.1038/221760a0. [DOI] [PubMed] [Google Scholar]
- SPRINGER G. F., HORTON R. E. ERYTHROCYTE SENSITIZATION BY BLOOD GROUP-SPECIFIC BACTERIAL ANTIGENS. J Gen Physiol. 1964 Jul;47:1229–1250. doi: 10.1085/jgp.47.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowter C., McGEE Z. A. Evaluation of a new technique for the demonstration of gonococci and other micro-organisms in host cells. J Clin Pathol. 1976 May;29(5):433–437. doi: 10.1136/jcp.29.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUBER H., GARSON W. Isolation of lipopolysaccharide endotoxin. J Biol Chem. 1959 Jun;234(6):1391–1393. [PubMed] [Google Scholar]
- Taylor-Robinson D., Whytock S., Green C. J., Carney F. E., Jr Effect of Neisseria gonorrhoeae on human and rabbit oviducts. Br J Vener Dis. 1974 Aug;50(4):279–288. doi: 10.1136/sti.50.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. G., Buys J., Hanstede J. G., Hagenaars A. M. Comparison of enzyme-linked immunosorbent assay and passive hemagglutination method for quantification of antibodies to lipopolysaccharide and tetanus toxoid in rats. Infect Immun. 1979 Jun;24(3):798–803. doi: 10.1128/iai.24.3.798-803.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]