Abstract
Telomeres are associated with the nuclear matrix and are thought to be heterochromatic. We show here that in human cells the overexpression of green fluorescent protein-tagged heterochromatin protein 1 (GFP-HP1) or nontagged HP1 isoforms HP1Hsα or HP1Hsβ, but not HP1Hsγ, results in decreased association of a catalytic unit of telomerase (hTERT) with telomeres. However, reduction of the G overhangs and overall telomere sizes was found in cells overexpressing any of these three proteins. Cells overexpressing HP1Hsα or HP1Hsβ also display a higher frequency of chromosome end-to-end associations and spontaneous chromosomal damage than the parental cells. None of these effects were observed in cells expressing mutants of GFP-ΔHP1Hsα, GFP-ΔHP1Hsβ, or GFP-ΔHP1Hsγ that had their chromodomains deleted. An increase in the cell population doubling time and higher sensitivity to cell killing by ionizing radiation (IR) treatment was also observed for cells overexpressing HP1Hsα or HP1Hsβ. In contrast, cells expressing mutant GFP-ΔHP1Hsα or GFP-ΔHP1Hsβ showed a decrease in population doubling time and decreased sensitivity to IR compared to the parental cells. The effects on cell doubling times were paralleled by effects on tumorigenicity in mice: overexpression of HP1Hsα or HP1Hsβ suppressed tumorigenicity, whereas expression of mutant HP1Hsα or HP1Hsβ did not. Collectively, the results show that human cells are exquisitely sensitive to the amount of HP1Hsα or HP1Hsβ present, as their overexpression influences telomere stability, population doubling time, radioresistance, and tumorigenicity in a mouse xenograft model. In addition, the isoform-specific effects on telomeres reinforce the notion that telomeres are in a heterochromatinized state.
In higher eukaryotic cells, a portion of the transcriptionally inactive heterochromatin, including that of telomeres, is associated with a structure called the nuclear matrix (2, 33, 39). Conserved heterochromatin proteins (HPs), which contain a characteristic chromodomain, play a critical role in establishing and maintaining these heterochromatic domains (58). The chromodomain is a 37-amino-acid-residue region first described in two Drosophila polypeptides, HP1 and polycomb (44). Three mammalian HP1-like proteins have been identified and are known as HP1α, HP1β, and HP1γ, each containing a chromodomain and a chromoshadow domain separated by a hinge region (11, 29, 58). Their genes are localized on three different chromosomal sites (6). These proteins are relatively small, containing less than 200 amino acids, and have molecular masses of approximately 25 kDa. In mammals, chromodomain-containing proteins appear to be either structural components of large macromolecular chromatin complexes or proteins involved in remodeling the chromatin structure. In vitro binding assays have revealed that all three mouse HP1s can form hetero- and homomultimers (38). HP1α, HP1β, and HP1γ heteromers have been shown to be associated with nucleosomal core histones (63) and to reduce transcription of nearby promoters when directly tethered to DNA (7). In addition, HP1s from mice and humans interact directly with the transcriptional corepressor TIFβ (37), supporting the notion that HP1s could play a role in gene silencing. Apart from this role in regulating gene activity, HP1 has been suggested to be a conserved component of the highly compact chromatin of centromeres and telomeres in Drosophila (23). In addition, Drosophila larvae expressing reduced or mutant versions of HP1 exhibit telomeric fusions (12). Thus, HP1 proteins are nonhistone chromatin components that interact with a variety of proteins that play a role in chromatin remodeling and transcriptional silencing (30). It is thought that the proteins encoded by the HP1 class of the conserved chromobox genes are primarily involved in the packaging of chromosomal domains into a repressive heterochromatic state. However, it is not known whether the function of these genes influences telomere behavior in human cells.
Telomeres are complexes of repetitive DNA sequences and proteins constituting the ends of linear eukaryotic chromosomes. Telomeric DNA comprises variable numbers of short direct repeats in the double-stranded form and end in an overhang of the strand making up the 3′ end of the chromosome, the G-rich strand (20, 24, 59). For example, mammalian telomeres end in a single-stranded G-rich overhang (G tail) of about 100 to 200 bases (32, 34), and this G tail can invade the double-stranded portion of telomeric repeats, forming a D loop (21). The D loop structure is stabilized by various telomere-binding proteins, in particular telomere repeat binding factor 2 (TRF2) (56), and may be conserved among higher eukaryotes. The maintenance of telomeric repeat DNA is dependent on telomerase, a specialized reverse transcriptase, and recent evidence suggests this enzyme is associated with telomeric chromatin (47, 54). Other chromosome end-binding proteins, such as TRF1, bind to telomeres via the double-stranded portion of the telomeric repeats (9). There is a growing number of proteins which are found to be associated with telomeres in an undefined or indirect way (Ku, hMRE11, and certain checkpoint proteins); however, very little information is available about the precise functions of these at chromosome ends. There is good evidence that Drosophila telomeres are organized as heterochromatin, but the evidence in other organisms is less direct. Mammalian telomeres have been reported to be associated with the nuclear matrix (8, 49), and genes located near telomeres in both yeast and mammalian cells can be subject to epigenetic transcriptional position effects (16, 62). This latter observation has been taken as evidence for a heterochromatin-like state of telomeres. In mammalian interphase nuclei, the three isoforms of HP1 exhibit a punctate pattern, and on metaphase chromosomes, HP1Hsα and HP1Hsβ show a predominant centromeric staining and infrequent signals at telomeres (1, 28, 35). These studies suggest that HP1Hsα or HP1Hsβ plays a role at telomeres. Here we demonstrate that because of the overexpression of HP1Hsα or HP1Hsβ, telomere association with telomerase and telomere stability in human cells are altered. Moreover, the cells in which these proteins are overexpressed display general defects of chromosomal instability and increase in chromosomal aberrations and are growth impaired in culture as well as in a mouse xenograft tumor model. The data thus suggest that HP1-like proteins play a functional role in the particular chromatin organization at telomeres and are crucial determinants of genome stability.
MATERIALS AND METHODS
Construction of expression plasmids.
Complementary DNAs encoding HP1Hsα, HP1Hsβ, or HP1Hsγ, with green fluorescent protein (GFP) tags (GFP-HP1) or without (HP1) GFP tags, were cloned into the mammalian expression vector pIND(SP1)/Neo (Invitrogen, Carlsbad, Calif.) as described previously (22). Mutant forms of HP1Hsα, HP1Hsβ, and HP1Hsγ, referred to as ΔHP1Hsα, ΔHP1Hsβ, and ΔHP1Hsγ, had their chromodomains deleted and were created by using a PCR approach with appropriate primer pair combinations. The mutant forms tagged to GFP were cloned into the pIND(SP1)/Neo vector. Final constructs were verified by DNA sequencing.
Cell culture and derivation of cell lines.
ECR-293 cells were maintained and transfected with plasmids as described previously (22, 26). Stable lines of cells transfected with the various constructs were obtained by selection with G418 (50). Reverse transcription (RT)-PCR and Western blot analyses were employed to confirm the expression of HP1Hsα, HP1Hsβ, and HP1Hsγ proteins.
Western blot analysis.
Cell lysates were prepared according to a previously described procedure (47). Anti-HP1Hsα, anti-HP1Hsβ, and anti-HP1Hsγ antibodies were obtained from Upstate Cell Signaling, and anti-GFP antibody was obtained from Clontech. Immunoblots and detections were done according to the recommendations of the antibody suppliers.
RT-PCR.
Total RNA was isolated from cells by using the RNeasy kit (QIAGEN Inc., Valencia, Calif.). RNA was treated with RNase-free DNase (Boehringer Mannheim) (1 μg/μl) for 2 h at 37°C, followed by heat inactivation at 65°C for 10 min. The RT reaction mixture contained 1 μg of DNase-treated RNA, 50 μl of a mixture containing 1 μg of pdN6 random primers (Pharmacia) per μl, 1× first-strand buffer (GIBCO-BRL), 0.5 mM (each) deoxynucleoside triphosphates (Pharmacia), and 200 U of MMLV-RT (GIBCO-BRL) and was incubated for 1 h at 37°C. PCR was performed by using gene-specific primers along with the primers for alpha-actin. The PCR samples were resolved by electrophoresis, and the products were quantitated by ImageQuant software.
Chromatin immunoprecipitation.
Coimmunoprecipitation after formaldehyde-mediated in vivo cross-linking of DNA with proteins was performed with a human telomerase (hTERT) antibody (15, 26, 57, 61) as described previously (3, 47). Immunoprecipitated DNA was spotted onto a membrane by using a dot blotting apparatus and then hybridized to 32P-labeled DNA probes. The probes used for hybridization were telomeric repeat DNA (CCCTAA), total human genomic DNA, and a DNA fragment containing Alu repeats. The blots were stripped and successively hybridized with different probes.
Detection of telomeres and terminal restriction fragment analysis.
Detection of telomeres on metaphase chromosomes was obtained by fluorescence in situ hybridization (FISH) by using a telomere sequence-specific peptide nucleic acid (PNA) probe (10). For terminal restriction fragment analysis, DNA was isolated from exponentially growing cells by a procedure described earlier (43). This DNA was digested with the restriction enzymes RsaI and HinfI, which do not cut the terminal TTAGGG repeat sequences, and the fragments were separated by agarose gel electrophoresis and hybridized to a 32P-labeled (TTAGGG)5 probe. Detection and measurement of terminal restriction fragment lengths were performed as described previously using ImageQuant version 1.2., build 039 (Molecular Dynamics) (49, 51). Nondenaturing in-gel hybridization to determine relative amounts of telomeric single-stranded DNA (G tails) was performed as previously described (34).
Telomerase assays.
Telomerase activity was determined by using the Telomerase PCR ELISA kit (Roche) as previously described (46). Telomerase activity was determined in triplicate, and negative and positive controls were run with each experiment. An aliquot of each extract was heat inactivated for 10 min at 95°C as a negative control.
Cell growth and clonogenic survival assays.
For determination of cell growth, cells were plated in 35-mm dishes. The cell count was determined by using a Coulter counter. For clonogenic assay, cells in plateau-phase growth were plated as single cells into 60-mm dishes in 5 ml of medium, incubated for 6 h, and subsequently exposed to ionizing radiation (IR). The actual amount of cells per dish was chosen to ensure that about 50 colonies would survive a particular dose of radiation. The cells were exposed to IR in the dose range of 0 to 8 Gy at room temperature. The cells were incubated for 12 or more days and were fixed in methanol acetic acid (3:1) prior to staining with crystal violet. Only colonies containing >50 cells were counted.
Chromosome studies.
Metaphase chromosome spreads were prepared by procedures described earlier (40). Giemsa-stained chromosomes of metaphase spreads were analyzed for chromosome end-to-end associations.
Assay for chromosomal repair after IR treatment.
G1-type chromosomal aberrations were assessed as described previously (41). Briefly, cells in plateau phase were irradiated with 3 Gy, allowed to incubate for 24 h, and subcultured, and metaphases were collected. Chromosome spreads were prepared by the procedure described previously (40). The categories of G1-type asymmetrical chromosome aberrations scored included dicentrics, centric rings, interstitial deletions and acentric rings, and terminal deletions.
S-phase-specific chromosomal aberrations were analyzed at metaphase. Exponentially growing cells were irradiated with 2 Gy, and mitotic cells were collected 3 to 6 h postirradiation. Both chromosome and chromatid aberrations were scored. For G2-specific chromosomal aberrations, cells in exponential phase were irradiated with 1 Gy and metaphases were collected at 45 and 90 min following irradiation and examined for chromatid breaks and gaps per metaphase as described previously (10, 36). Fifty metaphases were scored for each postirradiation time point.
Anchorage-independent growth.
Assays for anchorage-independent growth were performed essentially as described previously (13, 31). Agar (0.5%) in Dulbecco’s minimal essential medium (DMEM) with 10% fetal calf serum was poured in each well of 12-well plates, followed by the overlaying of 1.5 ml of agar (0.3% in DMEM-10% fetal calf serum) containing a defined number of cells. The wells were overlaid with regular DMEM, which was replaced every 3 days. Fifteen days later, colonies of more than 50 cells were counted. Each experiment was repeated independently three times in duplicate, and the results are expressed as the means of the three experiments.
Tumorigenic assay.
Two-month-old NMRI nu/nu male mice were maintained in a specific-pathogen-free mouse colony for the duration of the experiments (46). The mice were randomly distributed (four per cage), and each mouse was labeled with an ear code. Two million exponentially growing ECR-293 cells with and without overexpression of wild-type or mutant GFP-HP1Hsα, GFP-HP1Hsβ, or GFP-HP1Hsγ in a volume of 200 μl were injected subcutaneously. The mice were examined daily for tumor appearance. RKO cells, which are known to produce tumors in such mice, were used as positive controls (46).
RESULTS
Overexpression of HP1Hsα or HP1Hsβ reduces the interaction of hTERT with telomeres.
Besides centric heterochromatin, HP1 is localized to telomeres of Drosophila chromosomes, and HP1 mutant Drosophila larval neuroblasts show a high frequency of telomeric associations (12). In human cells, infrequent signals of HP1Hsα and HP1Hsβ on chromosome ends have been reported (1, 28, 35). Given this functional link of HP1 localization with telomere behavior, we wished to establish whether human HP1 proteins also played a role at telomeres. We have recently demonstrated that antibodies against the catalytic subunit of hTERT can be used to immunoprecipitate telomeric DNA after in vivo cross-linking and that such hTERT-telomere interactions could be influenced by the overexpression of TRF1 (47). We reasoned that if human HP1 proteins interacted with telomeric DNA in human cells, then the overexpression of such gene products would also influence the association of hTERT with the telomeres and subsequently lead to telomere instability. To this end, we determined the RNA and protein levels of GFP-HP1Hsα, GFP-HP1Hsβ, and GFP-HP1Hsγ in ECR-293 cells and whether overexpression of such proteins could influence the interaction of hTERT with telomeres.
The RNA and protein levels of GFP-HP1 in ECR-293 were determined by RT-PCR and Western blotting. GFP-specific primers were used in RT-PCR to determine the RNA levels of GFP-HP1. RNA expression levels of GFP-HP1 were found to be identical in cells expressing wild-type or mutant human isoforms of HP1 (Fig. 1A). To determine the protein level by Western blot analysis, anti-GFP antibody was used. The levels of wild-type or mutant GFP-tagged HP1Hsα, HP1Hsβ, or HP1Hsγ in ECR-293 cells were almost identical (Fig. 1B). ECR-293 cells overexpressing various forms of human HP1 proteins were treated with formaldehyde, and isolated chromatin from these cells was immunoprecipitated by using anti-hTERT antibodies (15, 26, 57, 61). Cells overexpressing wild-type GFP-HP1Hsα or GFP-HP1Hsβ, but not those overexpressing wild-type GFP-HP1Hsγ, showed reduced interaction of hTERT with telomeres compared to that for the parental cells (Fig. 2). In cells overexpressing GFP-HP1Hsα or GFP-HP1Hsβ, hTERT protein could be cross-linked to total genomic DNA, but specific binding of hTERT to telomeres was reduced (Fig. 2). However, the expression of GFP-ΔHP1Hsα, GFP-ΔHP1Hsβ, or GFP-ΔHP1Hsγ, which lack the chromodomain of the HP1-proteins, had no effect on the interaction of hTERT with telomeres (Fig. 2). Similarly, hTERT cross-linked to total genomic DNA as well as to telomeres in cells overexpressing the wild-type HP1Hsγ protein. These observations suggest that the GFP-HP1Hsα and GFP-HP1Hsβ proteins, but not the GFP-HP1Hsγ protein, can influence the interactions of hTERT with telomeres.
FIG. 1.
Expression levels of GFP-HP1Hsα, GFP-HP1Hsβ, and GFP-HP1Hsγ. (A) RT-PCR of GFP-tagged HP1. The primers are specific to GFP. The gel displays the bands obtained after quantitative RT-PCR over 35 cycles to determine the levels of GFP-HP1 in cells. The levels of expression of wild-type and mutant GFP-HP1Hsα, GFP-HP1Hsβ, and GFP-HP1Hsγ are very similar. (B) Western blot analysis of GFP-tagged wild-type (a) and mutant (b) HP1Hsα, HP1Hsβ, and HP1Hsγ using anti-GFP antibody. Note that the levels of expression of HP1Hsα, HP1Hsβ, and HP1Hsγ are very similar.
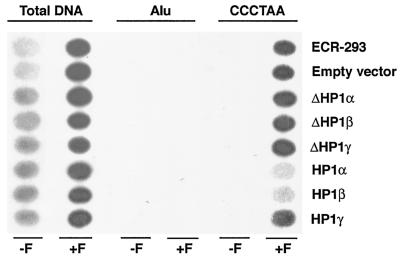
FIG. 2.
Human telomeric DNA coimmunoprecipitated by an hTERT antibody after in vivo cross-linking in cells expressing GFP-tagged HP1 proteins. ECR-293 cells overexpressing GFP-tagged wild-type (HP1Hsα, HP1Hsβ, or HP1Hsγ) or mutant (ΔHP1Hsα, ΔHP1Hsβ, or ΔHP1Hsγ) HP1 proteins were treated with formaldehyde (+F) or mock treated (−F). Chromatin was isolated and subjected to immunoprecipitation by using an anti-hTERT antibody. ECR-293 are the parental cells; empty vector cells are ECR-293 cells transfected with an empty vector. Deproteinized DNA isolated from the precipitates was denatured and spotted onto a membrane. The following probes were used for hybridization: total human genomic DNA (total DNA), a DNA fragment containing Alu repeats (Alu), or a DNA fragment containing telomeric DNA (CCCTAA). The same blot is shown after consecutive rehybridizations with the different probes. Note a decrease in the amount of telomeric DNA compared to that of the total genomic DNA in cells overexpressing GFP-HP1Hsα or GFP-HP1Hsβ. The results are representative of three independent experiments.
To determine whether GFP fusion with HP1 modifies the function of HP1 proteins, we overexpressed nontagged HP1 proteins in ECR-293 cells. First we determined the levels of HP1Hsα, HP1Hsβ, or HP1Hsγ in cells with or without overexpression of such proteins. The levels of HP1Hsα, HP1Hsβ, and HP1Hsγ were determined by Western blotting by using anti-HP1 specific antibodies. The levels of HP1Hsα, HP1Hsβ, or HP1Hsγ in cells overexpressing such proteins were about fourfold higher than those for the parental cells without overexpression of such proteins (Fig. 3A). ECR-293 cells overexpressing various forms of nontagged human HP1 proteins were examined for hTERT interactions with telomeres. Cells overexpressing nontagged HP1Hsα or HP1Hsβ, but not those overexpressing type HP1Hsγ, showed reduced interaction of hTERT with telomeres compared to that for the parental cells (Fig. 4). In cells overexpressing HP1Hsα or HP1Hsβ, hTERT protein could be cross-linked to total genomic DNA, but specific binding of hTERT to telomeres was reduced (Fig. 4). The influences of overexpression of GFP-HP1 or nontagged HP1 on hTERT interactions with telomeres are similar, suggesting that the effect of HP1 on hTERT interaction with telomeres is not due to GFP fusion with HP1. These findings are consistent with previous reports that GFP-HP1 proteins retain functions similar to those of nontagged HP1 proteins (5, 27, 38).
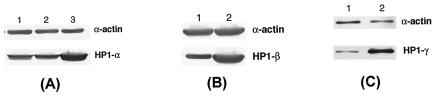
FIG. 3.
Western blot analysis of HP1Hsα, HP1Hsβ, and HP1Hsγ using protein-specific antibodies. (A) Western blot analysis of HP1Hsα. Lane 1, control cells; lane 2, control cells with empty vector; lane 3, cells with overexpression of HP1Hsα. (B) Western blot analysis of HP1Hsβ. Lane 1, control cells; lane 2, cells with overexpression of HP1Hsβ. (C) Western blot analysis of HP1Hsγ. Lane 1, control cells; lane 2, cells with overexpression of HP1Hsγ.
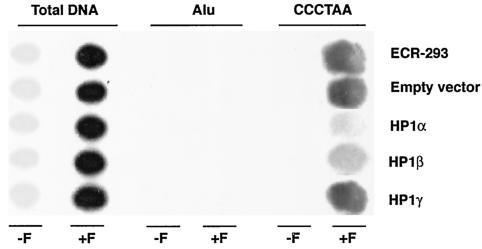
FIG. 4.
Human telomeric DNA coimmunoprecipitated by an hTERT antibody after in vivo cross-linking in cells expressing nontagged HP1 proteins. ECR-293 cells overexpressing nontagged wild-type (HP1Hsα, HP1Hsβ, or HP1Hsγ) proteins were analyzed for hTERT interactions with telomeres, as described in the legend to Fig. 2. Note a decrease in the amount of telomeric DNA compared to that of the total genomic DNA in cells overexpressing nontagged HP1Hsα or HP1Hsβ.
Given that HP1-proteins are involved in gene silencing, it was possible that the above effect was due simply to a transcriptional repression of hTERT resulting in lower levels of this protein in the cells. To investigate whether overexpression of any of the wild-type or mutant HP1 proteins influenced hTERT at the transcription level, we examined hTERT RNA levels in such cells by RT-PCR (Fig. 5A). None of the cell lines used, and in particular not the cells overexpressing HP1Hsα or HP1Hsβ, showed any significant differences in hTERT RNA compared to that for the control parental cells (Fig. 5A). Furthermore, we assayed telomerase activity in these cells and did not find any differences in overall activity of the enzyme (Fig. 5B). These results suggest that none of the HP1 proteins influences the transcription of hTERT through overexpression and that the reduced interaction of hTERT with telomeres is not due to down regulation of hTERT mRNA or inactivation of telomerase. Rather, the results suggest that the overexpression of HP1Hsα or HP1Hsβ can lead to an alteration of the telomeric chromatin, leading to decreased accessibility for telomerase.
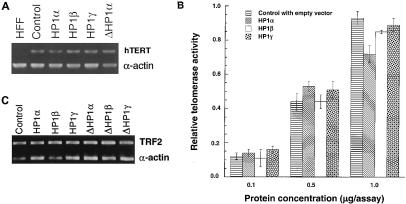
FIG. 5.
hTERT expression, telomerase activity, and TRF2 levels. (A) Levels of hTERT RNA. The gel displays the bands obtained after quantitative RT-PCR over 35 cycles to determine the levels of hTERT RNA in cells with or without overexpression of wild-type GFP-HP1Hsα, GFP-HP1Hsβ, GFP-HP1Hsγ, or GFP-ΔHP1Hsα. HFF is a negative control, RNA derived from human foreskin fibroblast cells (47). hTERT is a band specific for hTERT RNA. α-Actin is a band for alpha-actin RNA as the internal control. Note that no difference in expression of hTERT was found among cells with or without overexpression of GFP-HP1Hsα, GFP-HP1Hsβ, GFP-HP1Hsγ, or GFP-ΔHP1Hsα. (B) Levels of telomerase activity. Telomerase activity measured in extracts from cells with or without overexpression of GFP-HP1Hsα, GFP-HP1Hsβ, or GFP-HP1Hsγ by TRAP enzyme-linked immunosorbent assay. Note that no difference in telomerase activity was observed in cells with or without HP1Hsα, HP1Hsβ, or HP1Hsγ. (C) Levels of TRF2 RNA. The gel displays the bands obtained after quantitative RT-PCR over 35 cycles to determine the levels of TRF2 RNA in cells with or without overexpression of wild-type GFP-HP1Hsα, GFP-HP1Hsβ, GFP-HP1Hsγ, GFP-ΔHP1Hsα, GFP-ΔHP1Hsβ, or GFP-ΔHP1Hsγ. TRF2, band specific for TRF2 RNA; α-actin, band for alpha-actin RNA as the internal control. Note that no difference in levels of TRF2 was found among cells with or without overexpression of wild-type or mutant HP1Hsα, HP1Hsβ, or HP1Hsγ.
Since TRF2 plays a critical role in telomere stabilization, we determined whether overexpression of any of the wild-type or mutant HP1 proteins influenced TRF2 at the transcription level. TRF2 RNA levels were examined in such cells by RT-PCR (Fig. 5C). None of the cell lines used, and in particular not the cells overexpressing HP1Hsα or HP1Hsβ, showed any significant difference in TRF2 RNA levels from those of the control parental cells (Fig. 5C).
Telomere instability is induced by overexpression of HP1Hsα or HP1Hsβ.
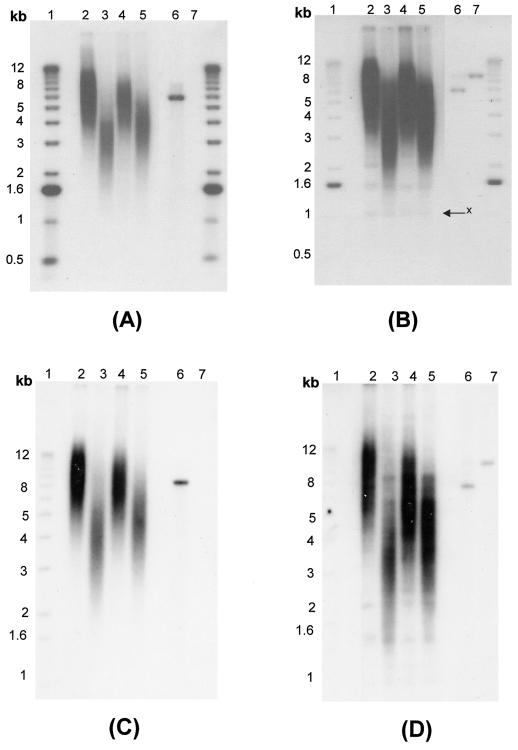
The occurrence of telomere fusions in Drosophila with HP1 mutations suggests that HP1 might function in protecting telomeres from fusions (12). In addition, HP1/ORC-associated protein is required for telomere capping in Drosophila (4). In mammals, HP1 might affect the functioning of other telomere-binding proteins, such as Ku, TRF1, or TRF2, which are responsible for telomere stabilization (9). For example, TRF2 plays a critical role in telomere stabilization, specifically in the G overhangs of human cells (53, 56). Furthermore, overexpression of TRF1 influenced the ability of hTERT to bind to telomeric DNA (47), a situation similar to what we found when HP1Hsα or HP1Hsβ was overexpressed. Overexpression of TRF1 also leads to a decrease in overall telomere length, and it was suggested that this effect could be due to an inhibition of telomerase, either by preventing access to the substrate or by inhibiting the enzyme (55). Therefore, we examined telomere length in cells with or without overexpression of the various HP1 proteins. DNA isolated from ECR-293 cells overexpressing GFP-tagged or nontagged HP1Hsα, HP1Hsβ, or HP1Hsγ was analyzed by using an in-gel hybridization technique for detection of the terminal restriction fragments (Fig. 6). DNA isolated from cells overexpressing HP1Hsα, HP1Hsβ, or HP1Hsγ harbor shorter terminal restriction fragments than those of parental cells or cells expressing mutant forms of any of the HP1 proteins (Fig. 6B and D and data not shown). This result is consistent with the hypothesis that, when overexpressed, mammalian HP1 proteins may bind to telomeric regions and have effects that are similar to the overexpression of TRF1, namely inhibition of the interaction of telomerase with telomeres and the induction of telomere shortening.
FIG. 6.
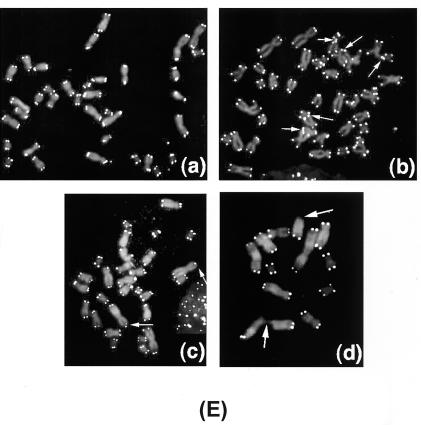
Single-strand extensions (G tails), terminal restriction fragment sizes, and telomere FISH. (A and B) G tail and terminal restriction fragment sizes in cells overexpressing GFP-fused wild-type (GFP-HP1Hsα, GFP-HP1Hsβ, or GFP-HP1Hsγ) HP1 proteins. (C and D) G tail and terminal restriction fragment sizes in cells overexpressing nontagged wild-type (HP1Hsα, HP1Hsβ, or HP1Hsγ) HP1 proteins. In panels A and C, nondenaturing in-gel hybridizations to genomic DNA digested with restriction enzymes HinfI and RsaI and using a telomeric repeat probe of the C-rich strand are shown. This method allows visualizing G-strand overhangs on telomeres. Signals were quantified by PhosphorImager analysis and corrected for DNA loading by using the rehybridized gel shown in panels B and D. Lane 1, molecular mass standards; lane 2, DNA from parental ECR-293 cells; lanes 3 to 5, DNA from ECR-293 cells overexpressing HP1Hsα, HP1Hsβ, or HP1Hsγ, respectively; lane 6, denatured plasmid single-stranded DNA containing telomeric repeats (positive control); lane 7, double-stranded plasmid DNA used as a negative control (detected only once the DNA is denatured, as seen in panels B and D). Panels B and D show the same gel as in panels A and C after denaturing of the DNA in the gel and rehybridization with the same probe. The arrow in panel B indicates an internal restriction fragment carrying telomeric repeats that was used to correct for DNA loading. Note that cells with overexpression of GFP-fused HP1 shown in panels A and B have effects on G overhangs and telomere size similar to those seen in the cells with overexpression of nontagged HP1 proteins shown in panels C and D. (E) Telomere FISH analysis showing sections of metaphase chromosomal spreads derived from parental ECR-293 cells (a), ECR-293 cells overexpressing HP1Hsα (b and c), or ECR-293 cells overexpressing HP1Hsβ (d). Note the chromosome end associations in panels b and d and an absence of telomeric signals in panels c and d (indicated by arrows). Telomeric signals are present on some telomere fusion sites (indicated by arrows in panel b).
Another possibility is that the binding of HP1 to telomeres may interfere with the functioning of some of the normal telomere-binding proteins. Displacing TRF2 from telomeres by a dominant-negative allele has two main consequences for chromosomal ends: loss of the single-stranded G strand overhang and induction of telomeric fusions (56). Given this observation, we assessed these two phenotypes in cells overexpressing the various HP1 proteins (Fig. 6A and C). Signals for G strand overhangs (G tails) were examined on terminal repeat fragments derived from such cells by the nondenaturing in-gel hybridization method (32, 34). The advantage of this method is that terminal fragments of any size can be analyzed by using an end-labeled d(CCCTAA)3 probe and that DNA loading errors can be corrected after rehybridization of the gels. As shown in Fig. 6A (GFP-tagged) and Fig. 6C (nontagged), for DNA isolated from cells overexpressing GFP-tagged and nontagged HP1Hsα, HP1Hsβ, or HP1Hsγ, the signals for G tails were significantly and reproducibly reduced by about 30 to 50% compared to those for DNA isolated from parental cells. Southern analysis of telomeric DNA yields an appraisal of the population of terminal repeat fragments generated and does not monitor ends of individual chromosomes. We therefore performed FISH for telomeric repeats on metaphase spreads by using a telomere-specific Cy3-labeled (CCCTAA)3 peptide nucleic acid probe. Fifty metaphase chromosome spreads from cells overexpressing GFP-tagged or nontagged HP1Hsα, HP1Hsβ, or HP1Hsγ as well as from the parental cells were included and analyzed (see representative examples in Fig. 6E). While no significant overall changes in signal intensities could be detected in cells overexpressing HP1Hsα or HP1Hsβ, there was a slightly higher proportion of chromatid ends (about 9% of telomeres per metaphase) that had fewer telomere-specific fluorescent signals than those for the parental cells (about 2% of telomeres per metaphase).
In order to determine the influence of overexpression of HP1Hsα, HP1Hsβ, or HP1Hsγ on the frequency of chromosome end-to-end associations, 200 metaphases were examined for each case and the frequencies of abnormalities were established and compared to those for the parental cells. Cells overexpressing HP1Hsα or HP1Hsβ had about 0.45 chromosome end-to-end associations per metaphase, whereas the parental cells displayed 0.12 chromosome end-to-end associations per metaphase (Table 1). None of the other cells examined showed any increase in the frequency of chromosome end associations (Table 1). Since chromosome end-to-end associations may lead to anaphase bridge formation, the same cells were analyzed for anaphase bridges by omitting the Colcemid treatment. For each case, 300 cells at anaphase were examined for bridges. Cells overexpressing HP1Hsα or HP1Hsβ displayed a threefold higher frequency of anaphase bridges than that for parental cells (Table 1). Furthermore, we determined whether the chromosome end-to-end fusions observed in cells overexpressing HP1Hsα or HP1Hsβ were associated with losses of telomeric repeats at the fusion sites. Telomeric signals were seen in about 8% of the fusion sites, indicating that total loss of telomeres is not required for the formation of chromosome end-to-end associations in these cells (Fig. 6E).
TABLE 1.
Comparison of the frequencies of chromosome end-to-end associations at metaphase and bridges at anaphase in cells overexpressing various HP1 proteinsa
| Cell type | Chromosome end associations/200 metaphases | Bridges/300 anaphases |
|---|---|---|
| Control | 24 | 16 |
| Control with empty vector | 21 | 13 |
| GFP-HP1Hsα | 94b | 49b |
| GFP-HP1Hsβ | 88b | 43b |
| GFP-HP1Hsγ | 32 | 15 |
| HP1Hsα | 86b | 54b |
| HP1Hsβ | 92b | 51b |
| HP1Hsγ | 24 | 10 |
| GFP-ΔHP1Hsα | 20 | 17 |
| GFP-ΔHP1Hsβ | 22 | 11 |
| GFP-ΔHP1Hsγ | 28 | 18 |
GFP fusion did not influence the function of HP1 proteins, as GFP-HP1 cells have an effect on chromosome aberrations similar to that seen in cells with overexpression of nontagged HP1.
The frequency is significantly different from that for the controls (ECR-293 cells) as assessed by chi-square analysis (P < 0.01).
To determine whether spontaneous chromosome aberrations were also increased in cells with enhanced telomere instability, we examined cells for chromosome as well as chromatid aberrations. Again, cells overexpressing HP1Hsα or HP1Hsβ displayed a higher frequency of chromatid and chromosomal aberrations than the parental cells (Table 2). No significant increases in these frequencies were observed in cells overexpressing HP1Hsγ or any of the mutant forms of the HP1-like proteins (Table 2).
TABLE 2.
Comparison of the frequencies of chromatid and chromosome aberrations in cells overexpressing various HP1 proteinsa
| Cell type | Chromosome gaps + breaks | Chromatid gaps + breaks |
|---|---|---|
| Control | 2 | 3 |
| Control with empty vector | 1 | 3 |
| GFP-HP1Hsα | 10b | 5 |
| GFP-HP1Hsβ | 11b | 9b |
| GFP-HP1Hsγ | 3 | 2 |
| HP1Hsα | 12b | 4 |
| HP1Hsβ | 10b | 11b |
| HP1Hsγ | 2 | 4 |
| GFP-ΔHP1Hsα | 1 | 4 |
| GFP-ΔHP1Hsβ | 2 | 1 |
| GFP-ΔHP1Hsγ | 2 | 3 |
GFP fusion did not influence the function of HP1 proteins, as GFP-HP1 cells have an effect similar to that seen in cells with overexpression of nontagged HP1. Two hundred metaphases were examined for each cell type, and the number of breaks per 100 metaphases is reported for each case.
Frequencies for chromosomal or chromatid types of aberrations in cells overexpressing HP1Hsα or HP1Hsβ are significantly higher than those for the control cells, as assessed by chi-square analysis (P < 0.05).
Overexpression of HP1Hsα or HP1Hsβ influences population doubling time.
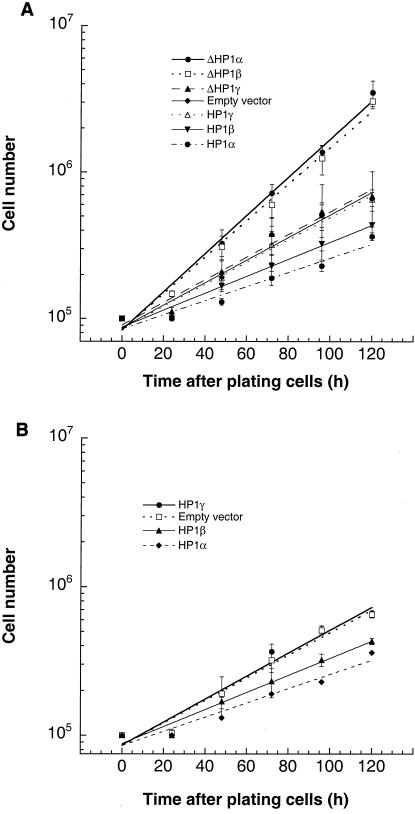
Since we had shown that overexpression of HP1Hsα or HP1Hsβ proteins induced chromosomal instabilities, we were interested in examining if such cells displayed altered growth rates. We tested whether overexpression of wild-type or mutant HP1Hsα, HP1Hsβ, or HP1Hsγ influenced cell population doubling times by performing standard growth curve assays. The population doubling times of cells overexpressing wild-type HP1Hsα or HP1Hsβ were increased by about 10 h compared to that for the control cells (Fig. 7). However, no such effect was observed in cells overexpressing HP1Hsγ (Fig. 7). Curiously, the population doubling times of cells expressing mutant GFP-ΔHP1Hsα or GFP-ΔHP1Hsβ, but not those expressing mutant GFP-ΔHP1Hsγ, were reduced by approximately 9 h compared to that for parental ECR-293 cells.
FIG. 7.
Influence of overexpression of wild-type or mutant HP1Hsα, HP1Hsβ, or HP1Hsg on cell growth. Cells overexpressing the indicated forms of HP1 proteins were seeded in plates, and cell counts were determined at regular intervals. The actual numbers of cells are plotted against the hours of growth in a semilog diagram. The values shown are the means of the results from three experiments. (A) The effects of wild-type and mutant GFP-HP1Hsα, GFP-HP1Hsβ, and GFP-HP1Hsγ on cell growth. (B) Effects of wild-type nontagged HP1Hsα, HP1Hsβ, and HP1Hsγ on cell growth. Note that the influence of GFP-tagged HP1 proteins on cell growth is similar to the influence of nontagged HP1 proteins.
Effects of IR on cell survival and chromosomal repair.
The detected differences in doubling times could indicate that the affected cell lines were also altered in their ability to repair DNA damage. Notably, cells with telomere dysfunction in late-generation Terc−/− mice displayed a radiosensitivity syndrome associated with accelerated mortality (60). The radiosensitivity of telomere dysfunctional cells is also correlated with defective DNA repair (60). Furthermore, it has been suggested that short telomeres in mammals result in organismal hypersensitivity to IR (17). Since overexpression of HP1Hsα or HP1Hsβ influences telomere stability and cell doubling times, we determined whether overexpression of wild-type or mutant HP1 proteins influences cell survival and/or chromosomal repair after exposure to IR.
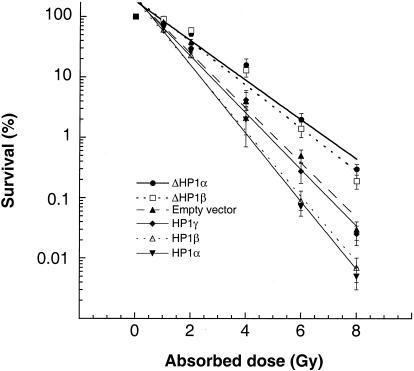
Cell survival after treatment with IR was determined by using a colony formation assay described previously (10). Consistent with the differences in population doubling times, cells overexpressing GFP-HP1Hsα or GFP-HP1Hsβ exhibited enhanced sensitivity to IR treatment compared to that for parental cells (Fig. 8). Furthermore, and again consistent with the doubling-time data, cells expressing GFP-ΔHP1Hsα or GFP-ΔHP1Hsβ exhibited decreased IR sensitivity for reproductive cell death and no changes in IR response for cell death were observed in cells overexpressing GFP-HP1Hsγ or GFP-ΔHP1Hsγ (Fig. 8 and data not shown). Thus, cells overexpressing wild-type HP1Hsα or HP1Hsβ have defects in telomere metabolism and display karyotypic instability and prolonged population doubling times as well as decreased cell survival after IR treatment. All of these cellular phenotypes could be linked with defective chromosomal repair.
FIG. 8.
Comparison of cell survival after IR treatment. Dose response curves for cells overexpressing the indicated GFP-tagged HP1 proteins are shown. Cells were treated with ionizing radiation while growing exponentially and asynchronously. Cells overexpressing GFP-ΔHP1Hsα or GFP-ΔHP1Hsβ are more resistant to damage induced by gamma rays than cells overexpressing GFP-HP1Hsα or GFP-HP1Hsβ. The values shown are the means of the results from three to four experiments.
One way to address whether DNA repair is affected in these cells is to compare cell cycle stage-specific chromosomal aberrations in cells with and without overexpression of the HP1 proteins. We first set out to determine frequencies of chromosome aberrations induced by IR in G1-, S-, and G2-phase cells. Cell cycle phase-specific chromosome aberrations were ascertained based on the frequency of chromosomal and chromatid aberrations observed at metaphase. G1-specific aberrations detected at metaphase are mostly of the chromosomal type and display a high frequency of dicentrics (41). S-phase-type aberrations detected at metaphase are of the chromosomal and chromatid types. G2-type aberrations detected at metaphase are predominantly of the chromatid type with the least number of dicentrics. To determine G1-type chromosomal damage, plateau-phase cells were treated with 3 Gy and replated 24 h after irradiation and aberrations were scored at metaphase as previously described (10). Compared to those for parental cells, there is a significant increase in residual IR-induced G1 chromosomal aberrations seen at metaphase in cells overexpressing GFP-HP1Hsα or GFP-HP1Hsβ (Fig. 9A). In contrast, cells expressing GFP-ΔHP1Hsα, GFP-ΔHP1Hsβ, or GFP-ΔHP1Hsγ did not show any differences in the G1-phase type of chromosomal aberrations from those of parental cells after treatment with IR (data not shown). To determine whether defective repair can be documented in cells overexpressing GFP-HP1Hsα or GFP-HP1Hsβ in phases of the cell cycle other than G1, we evaluated S-phase-specific chromosomal aberrations in such cells. We first determined the time needed for S-phase cells to reach metaphase after IR treatment. Exponentially growing cells were labeled with BrdU for 30 min as previously described (40) and then irradiated with 2 Gy. Anti-BrdU immunostaining was performed to determine when metaphase chromosomes contain BrdU. In these experiments, BrdU-labeled metaphases appeared approximately 3 h postirradiation (data not shown). Thus, cells overexpressing HP1 proteins were treated with 2 Gy of IR, and metaphases were collected after 3 to 5 h of treatment. Cells overexpressing GFP-HP1Hsα or GFP-HP1Hsβ, collected 3 h postirradiation, displayed higher frequencies of metaphases with chromatid and chromosome aberrations than those for parental cells (Fig. 9B). These observations established that overexpression of GFP-HP1Hsα or GFP-HP1Hsβ, but not GFP-HP1Hsγ, influences G1-phase- and S-phase-specific chromosomal repair. Similar results were obtained when G2-phase-specific chromosomal repair was evaluated (Fig. 9C). These observations reinforce the idea that overexpression of HP1Hsα or HP1Hsβ can influence the capacity for global DNA repair, as a propensity for a higher frequency of chromosomal aberrations was observed irrespective of which cell cycle phase was analyzed by the assays.
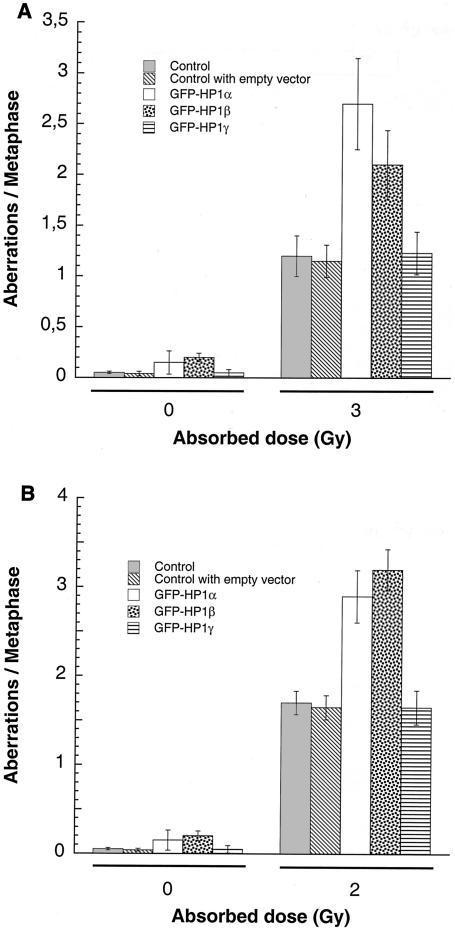
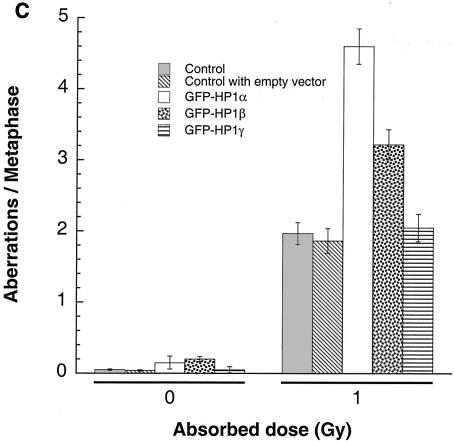
FIG. 9.
G1, S, and G2 chromosomal aberrations after IR treatment. (A) Cells in plateau phase were irradiated with 3 Gy, incubated for 24 h postirradiation, and then subcultured, and metaphases were collected. G1-type aberrations were examined at metaphase. Categories of asymmetric chromosomal aberrations scored included dicentrics, centric rings, interstitial deletions and acentric rings, and terminal deletions. The frequency of chromosomal aberrations was higher in EC-293 cells overexpressing wild-type GFP-HP1Hsα and GFP-HP1Hsβ but not in cells overexpressing GFP-HP1Hsγ. (B) Cells in exponential phase were irradiated with 2 Gy. Metaphases were harvested 6 h following irradiation and examined for chromosomal aberrations. The frequencies of chromatid and chromosomal aberrations were higher in EC-293 cells overexpressing wild-type GFP-HP1Hsα or GFP-HP1Hsβ than in those expressing GFP-HP1Hsγ. (C) Cells in exponential phase were irradiated with 1 Gy. Metaphases were harvested 1 h following irradiation and examined for chromosomal aberrations. The frequency of chromatid aberrations was higher in EC-293 cells overexpressing wild-type GFP-HP1Hsα or GFP-HP1Hsβ but not in those expressing GFP-HP1Hsγ. Note that cells overexpressing GFP-HP1Hsα or GFP-HP1Hsβ have higher frequencies of chromosomal aberrations than those of the parental control cells in all phases of the cell cycle, suggesting a global defective DNA repair.
The effect on tumorigenicity of overexpressing HP1 proteins.
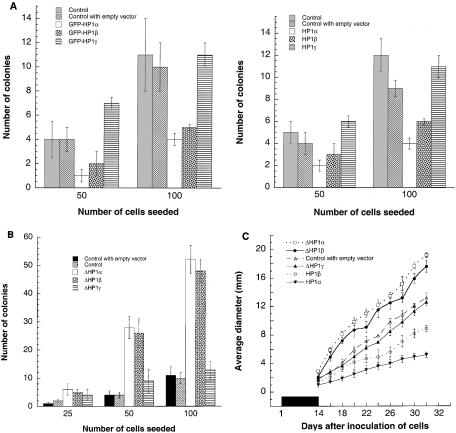
Defective DNA repair has been linked with oncogenic malignant transformation (14, 42). In addition, HP1Hsα has been shown to be down regulated in invasive metastatic breast cancer cells, and it has been proposed that down regulation of this gene is associated with tumor cell invasion and metastasis (27). As described above, overexpression of HP1Hsα or HP1Hsβ influences telomere metabolism, spontaneous formation of chromosomal aberrations, population doubling time, IR response for cell survival, and repair of chromosomal damage. All of these cellular effects have been linked with the oncogenic transformation and metastatic potential of a cell. To determine if the observed phenotypes of cells overexpressing HP1Hsα, HP1Hsβ, or HP1Hsγ also had consequences for tumorigenicity, we performed both in vitro and in vivo assays. In the in vitro assay, the various cell lines were assessed for their potential for anchorage-independent growth, one of the hallmarks of the tumorigenic state (13). Parental ECR-293 cells express the E1A gene derived from adenovirus 5 (18) and have a relatively low capacity for colony formation in this assay (Fig. 10A). Various numbers of cells overexpressing wild-type or mutant forms of HP1 were seeded and cultured in soft agar for 15 days prior to the colony count. Again consistent with our previous results, cells overexpressing wild-type HP1Hsα or HP1Hsβ had significantly reduced capacity for colony formation compared to that for parental ECR-293 cells (Fig. 10A). The correlation with the previous results also held for the cells expressing GFP-ΔHP1Hsα or GFP-ΔHP1Hsβ, as a significantly greater number of colonies could be counted for these cells (Fig. 10B).
FIG. 10.
Influence of HP1Hsα, HP1Hsβ, and HP1Hsγ on oncogenic transformation in vitro (A and B) and in vivo (C) and determination of colony formation by the agarose-independent anchorage assay. (A) Cells overexpressing GFP-HP1Hsα or GFP-HP1Hsβ (A-1) or nontagged HP1Hsα or HP1Hsβ (A-2) have a lower number of colonies than the parental cells. Cells overexpressing HP1Hsγ did not show any change in colony formation. (B) Cells with expression of mutant GFP-HP1Hsα or GFP-HP1Hsβ form a higher number of colonies than the parental cells. Cells expressing mutant GFP-HP1Hsγ did not show any influence on colony formation. (C) Cells with or without overexpression of wild-type and mutant GFP-HP1Hsα, GFP-HP1Hsβ, or GFP-HP1Hsγ were injected subcutaneously into mice. Tumor growth was measured starting at day 14 after inoculation. Note that tumors derived from cells overexpressing GFP-HP1Hsα or GFP-HP1Hsβ grow more slowly than those of the control, while those derived from cells expressing mutant GFP-HP1Hsα or GFP-HP1Hsβ grow faster. Tumors derived from cells overexpressing wild-type or mutant GFP-HP1Hsγ did not show any change in growth compared to that for the control.
Tumorigenicity of the various cells was determined by injecting cells overexpressing the HP1 proteins into nude mice. Compared to results obtained with the parental cells, tumor formation was reduced by approximately 50% for cells overexpressing GFP-ΔHP1Hsα or GFP-ΔHP1Hsβ and the resulting tumor growth rates were lower (Fig. 10C and data not shown). In contrast, cells expressing mutant GFP-ΔHP1Hsα or GFP-ΔHP1Hsβ did not show any decrease in tumor formation and the tumor growth rates were significantly faster with these cells (Fig. 10C). The results obtained with the in vivo tumorigenicity assay show a remarkable correlation with the effects observed in the cells cultured in vitro. We concluded that the overexpression of HP1Hsα or HP1Hsβ has a major impact on the cells' ability to grow in culture as well as to form tumors in mice. Curiously, overexpression of the mutant forms of GFP-ΔHP1Hsα or GFP-ΔHP1Hsβ, which lack the conserved chromodomain, appears to have an opposite effect.
DISCUSSION
HP1 is generally believed to act as a structural adaptor by mediating stable macromolecular complexes between nucleosomes, possibly organizing higher-order chromatin structures. HP1 is now known to be a highly interactive protein that is capable of interacting with a host of proteins with a range of nuclear activities (25). Thus, it can play a major role in maintaining the transcriptionally repressed state of heterochromatin. In Drosophila, heterozygous loss of HP1 results in loss of gene silencing, whereas overexpression of HP1 generally results in increased gene silencing (11). In human cells, overexpression of HP1Hsα or HP1Hsβ leads to an alteration in the transcriptional activity of certain genes (22). Such alterations could have profound effects on cell growth, but they have not yet been investigated in detail. Both in vitro as well as in vivo results presented here support the notion that human cells are very sensitive to the levels and activity of GFP-tagged as well as nontagged HP1Hsα and HP1Hsβ. For example, cells overexpressing HP1Hsα and HP1Hsβ have longer population doubling times (grow more slowly) than the parental control cells. The effects of overexpression of HP1Hsα or HP1Hsβ on chromatin is further evident from the fact that cells with such expression have higher residual chromosomal damage and display higher IR-induced chromosomal aberrations in G1, S, or G2 than the parental cells. The significant differences in the frequencies of aberrations per metaphase between cells with and without overexpression of HP1Hsα and HP1Hsβ are consistent with survival studies, suggesting that HP1Hsα and HP1Hsβ also have the capacity to modulate response to the IR. Finally, overexpression of these two proteins also negatively affects the growth capacity of these cells in soft agar and in mice. The most straightforward interpretation of these results is that overexpression of HP1Hsα or HP1Hsβ enhances heterochromatization, thereby leading to altered transcriptional profiles for a number of genes in the affected areas of chromatin. Alternatively or in addition to this effect, repair of chromosomal damage may be reduced in areas with increased binding of HP1 proteins. There is increasing evidence that access to the underlying chromatin for DNA repair machineries is important for efficient repair (19, 48). Thus, overexpressing HP1 proteins could interfere with the local chromatin remodeling necessary for DNA repair, thereby causing increased IR sensitivity and chromosomal aberrations, as observed here.
Interestingly, cells expressing GFP-ΔHP1Hsα and GFP-ΔHP1Hsβ that lack their chromodomains displayed opposite effects for a number of the phenotypes assayed: shortened population-doubling times, higher rates of survival after IR treatment, and increased growth capacities in both soft agar and mice. Note that highly invasive metastatic breast carcinoma cells appear not to express HP1Hsα, suggesting that elimination of the function of the HP1Hsα gene may lead to an enhancement in cell growth (27). Therefore, we speculate that the mutant forms of GFP-ΔHP1Hsα and GFP-ΔHP1Hsβ expressed here may function as dominant-negative alleles of these proteins. Although we do not show direct evidence for such dominant-negative activity, the observed effects on the growth characteristics of the cells are consistent with this possibility and would further underscore the sensitivity of human cells to the levels of HP1Hsα and HP1Hsβ.
Apart from these overall effects on cell growth properties, we also assessed the effects of overexpression of the HP1 proteins on one specific chromosomal domain, the telomere. These investigations were motivated by the observation that HP1 mutations in Drosophila lead to telomere fusions, suggesting that HP1 might function in protecting telomeres (12). Furthermore, it has been shown recently that in Drosophila the HP1/ORC-associated protein is also required for telomere capping (4). There is also circumstantial evidence linking HP1 with telomeres in human cells, since Ku70, one of the telomere-associated proteins, may interact with HP1 (52). Moreover, either human HP1Hsα or HP1Hsβ has been reported to be found occasionally at telomeres (1, 28, 35); thus, their expression levels might affect the functioning of telomere-binding proteins such as Ku, TRF1, or TRF2, which are responsible for telomere stabilization (9). Indeed, we show that cells overexpressing HP1Hsα or HP1Hsβ display increased levels of chromosomal aberrations, including telomere-telomere associations. In addition, while the lengths of telomeric repeat tracts vary in such cells, the signals for G overhangs at telomeres are significantly reduced. Such phenotypes have also been reported to occur in cells overexpressing a dominant-negative allele of TRF2, albeit in a much more pronounced fashion (56). Given these similarities, we speculate that the overexpression of the HP1 proteins reported here may partially inhibit TRF2 function. Recently, we have shown that hTERT, the catalytic subunit of telomerase, is constitutively associated with telomeres (47). Consistent with the proposal that HP1 proteins can interfere with the binding of telomere-specific proteins, our results also demonstrate that this telomere association of telomerase is reduced when HP1Hsα or HP1Hsβ is overexpressed. These results suggest that HP1Hsα and HP1Hsβ can associate with telomeric chromatin, thereby exerting a negative effect on the binding of other proteins.
Intriguingly, some of the phenotypes we observed appear to be HP1 isoform specific. For example, while the overexpression of HP1Hsγ does induce a loss of G overhangs at telomeres, this loss has no effect on telomerase binding to telomeres or any of the cellular growth phenotypes. We speculate that while this isoform may still partially interfere with some of the telomere-binding proteins, such as TRF1 or TRF2, it is not able to influence the binding of other proteins such as hTERT or to affect cellular repair activities.
There is recent evidence that telomerase may have functions other than the synthesis of telomeric repeats of the G-strand (47). It has been suggested that telomere dysfunction in mTerc-null mice impairs overall DNA repair, which may subsequently lead to cell growth arrest (60), and late generation telomerase knockout mice have been reported to display increased radiosensitivity (17). Interestingly, the results presented here suggest a correlation between the negative effects of HP1Hsα or HP1Hsβ overexpression on telomerase association with telomeres and reduced growth potential as well as increased radiosensitivity. While it is likely that these different effects are the result of HP1 binding to different chromosomal domains and therefore of independent origin, it remains possible that the interference with telomere functions could contribute to the overall growth defects. First, the chromosomal end-to-end associations with telomeric sequences at the fusion points may reflect an inhibition of the TRF2 protein, and the resulting dicentric chromosomes may induce cell cycle arrests and genomic instability. Second, our recent findings have established an intimate relationship between hTERT-telomere interactions and an alteration in transcription of a subset of genes in primary human fibroblasts (47). Thus, eliminating telomerase binding by HP1Hsα or HP1Hsβ overexpression may also influence the transcriptional activity of a number of genes independently of the effects on transcription exerted directly by overexpression of these proteins. Therefore, we suggest that the overall growth phenotypes and radiosensitivity observed in these cells may be the results of a combination of effects.
Overall, our results show that overexpression of HP1Hsα and HP1Hsβ influences telomere stability, chromosome repair, and cell growth, as well as cell survival after IR treatment in human cells. These observations are consistent with a model that predicts that telomere function in mammalian cells is exquisitely sensitive to the amount of heterochromatin proteins. Recent studies have indeed shown that overexpression of HP1Hsα or HP1Hsβ alters transcription of a subset of genes (22). Alteration in transcription and changes in growth pattern are consistent with increased oncogenic potential of cells expressing mutant HP1Hsα and HP1Hsβ, which may function as dominant-negative alleles. Interestingly, Piacentini et al. (45) have also shown failure of HP1 mutants lacking the chromodomain to associate with puffs in Drosophila euchromatin. We therefore propose that the expression levels of HP1Hsα or HP1Hsβ can play a critical role during the process of tumorigenesis. Consistent with this idea, HP1Hsα has been shown to be down regulated in metastatic human breast tumors (27). Further experiments are required to determine the specific contributions of telomeric effects and the alterations in gene expression profiles to the potential oncogenic malignant transformation or metastasis by HP1Hsα or HP1Hsβ function.
Acknowledgments
This investigation was supported by grant NS34746 from NIH; by the Department of the Army; by the A-T Children's Society; and by funds from the Department of Radiation Oncology, Washington University School of Medicine, to T.K.P. H.J.W was supported on this project by a grant from the National Institutes of Health (grant RO1-CA66974). R.J.W. is a Chercheur National of the Fonds de la Recherche en Santé du Québec (FRSQ) and acknowledges support by the Canadian NCI (grant 010049).
REFERENCES
- 1.Aagaard, L., M. Schmid, P. Warburton, and T. Jenuwein. 2000. Mitotic phosphorylation of SUV39H1, a novel component of active centromeres, coincides with transient accumulation at mammalian centromeres. J. Cell Sci. 113:817-829. [DOI] [PubMed] [Google Scholar]
- 2.Blobel, G. 1985. Gene gating: a hypothesis. Proc. Natl. Acad. Sci. USA 82:8527-8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braunstein, M., A. B. Rose, S. G. Holmes, C. D. Allis, and J. R. Broach. 1993. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 7:592-604. [DOI] [PubMed] [Google Scholar]
- 4.Cenci, G., G. Siriaco, G. D. Raffa, R. Kellum, and M. Gatti. 2003. The Drosophila HOAP protein is required for telomere capping. Nat. Cell Biol. 5:82-84. [DOI] [PubMed] [Google Scholar]
- 5.Cheutin, T., A. J. McNairn, T. Jenuwein, D. M. Gilbert, P. B. Singh, and T. Misteli. 2003. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science 299:721-725. [DOI] [PubMed] [Google Scholar]
- 6.Chevillard, C., W. Reik, M. McDermott, M. Fontes, M. G. Mattei, and P. B. Singh. 1993. Chromosomal localization of human homologs of the Drosophila heterochromatin protein 1 (HP1) gene. Mamm. Genome 4:124-126. [DOI] [PubMed] [Google Scholar]
- 7.Cryderman, D. E., H. Tang, C. Bell, D. S. Gilmour, and L. L. Wallrath. 1999. Heterochromatic silencing of Drosophila heat shock genes acts at the level of promoter potentiation. Nucleic Acids Res. 27:3364-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lange, T. 1992. Human telomeres are attached to the nuclear matrix. EMBO J. 11:717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lange, T. 2002. Protection of mammalian telomeres. Oncogene 21:532-540. [DOI] [PubMed] [Google Scholar]
- 10.Dhar, S., J. A. Squire, M. P. Hande, R. J. Wellinger, and T. K. Pandita. 2000. Inactivation of 14-3-3σ influences telomere behavior and ionizing radiation-induced chromosomal instability. Mol. Cell. Biol. 20:7764-7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eissenberg, J. C., and S. C. Elgin. 2000. The HP1 protein family: getting a grip on chromatin. Curr. Opin. Genet. Dev. 10:204-210. [DOI] [PubMed] [Google Scholar]
- 12.Fanti, L., G. Giovinazzo, M. Berloco, and S. Pimpinelli. 1998. The heterochromatin protein 1 prevents telomere fusions in Drosophila. Mol. Cell 2:527-538. [DOI] [PubMed] [Google Scholar]
- 13.Freedman, V. H., and S. I. Shin. 1974. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell 3:355-359. [DOI] [PubMed] [Google Scholar]
- 14.Freyer, G. A., D. A. Palmer, Y. Yu, R. C. Miller, and T. K. Pandita. 1996. Neoplastic transformation of mouse C3H10T1/2 cells following exposure to neutrons does not involve mutation of ras gene as analyzed by SSCP and cycle sequencing. Mutat. Res. 357:237-244. [DOI] [PubMed] [Google Scholar]
- 15.Fu, W., M. Killen, C. Culmsee, S. Dhar, T. K. Pandita, and M. P. Mattson. 2000. The catalytic subunit of telomerase is expressed in developing brain neurons and serves a cell survival-promoting function. J. Mol. Neurosci. 14:3-15. [DOI] [PubMed] [Google Scholar]
- 16.Gottschling, D. E., O. M. Aparicio, B. L. Billington, and V. A. Zakian. 1990. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell 63:751-762. [DOI] [PubMed] [Google Scholar]
- 17.Goytisolo, F. A., E. Samper, J. Martin-Caballero, P. Finnon, E. Herrera, J. M. Flores, S. D. Bouffler, and M. A. Blasco. 2000. Short telomeres result in organismal hypersensitivity to ionizing radiation in mammals. J. Exp. Med. 192:1625-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 19.Green, C. M., and G. Almouzni. 2002. When repair meets chromatin. First in series on chromatin dynamics. EMBO Rep. 3:28-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greider, C. W. 1999. Telomeres do D-loop-T-loop. Cell 97:419-422. [DOI] [PubMed] [Google Scholar]
- 21.Griffith, J. D., L. Comeau, S. Rosenfield, R. M. Stansel, A. Bianchi, H. Moss, and T. de Lange. 1999. Mammalian telomeres end in a large duplex loop. Cell 97:503-514. [DOI] [PubMed] [Google Scholar]
- 22.Hwang, K. K., and H. J. Worman. 2002. Gene regulation by human orthologs of Drosophila heterochromatin protein 1. Biochem. Biophys. Res. Commun. 293:1217-1222. [DOI] [PubMed] [Google Scholar]
- 23.James, T. C., J. C. Eissenberg, C. Craig, V. Dietrich, A. Hobson, and S. C. Elgin. 1989. Distribution patterns of HP1, a heterochromatin-associated nonhistone chromosomal protein of Drosophila. Eur. J. Cell Biol. 50:170-180. [PubMed] [Google Scholar]
- 24.Kelleher, C., M. T. Teixeira, K. Forstemann, and J. Lingner. 2002. Telomerase: biochemical considerations for enzyme and substrate. Trends Biochem. Sci. 27:572-579. [DOI] [PubMed] [Google Scholar]
- 25.Kellum, R. 2003. Is HP1 an RNA detector that functions both in repression and activation? J. Cell Biol. 161:671-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kharbanda, S., V. Kumar, S. Dhar, P. Pandey, C. Chen, P. Majumder, Z. M. Yuan, Y. Whang, W. Strauss, T. K. Pandita, D. Weaver, and D. Kufe. 2000. Regulation of the hTERT telomerase catalytic subunit by the c-Abl tyrosine kinase. Curr. Biol. 10:568-575. [DOI] [PubMed] [Google Scholar]
- 27.Kirschmann, D. A., R. A. Lininger, L. M. Gardner, E. A. Seftor, V. A. Odero, A. M. Ainsztein, W. C. Earnshaw, L. L. Wallrath, and M. J. Hendrix. 2000. Down-regulation of HP1Hsα expression is associated with the metastatic phenotype in breast cancer. Cancer Res. 60:3359-3363. [PubMed] [Google Scholar]
- 28.Koering, C. E., A. Pollice, M. P. Zibella, S. Bauwens, A. Puisieux, M. Brunori, C. Brun, L. Martins, L. Sabatier, J. F. Pulitzer, and E. Gilson. 2002. Human telomeric position effect is determined by chromosomal context and telomeric chromatin integrity. EMBO Rep. 3:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorentz, A., K. Ostermann, O. Fleck, and H. Schmidt. 1994. Switching gene swi6, involved in repression of silent mating-type loci in fission yeast, encodes a homologue of chromatin-associated proteins from Drosophila and mammals. Gene 143:139-143. [DOI] [PubMed] [Google Scholar]
- 30.Ma, J., K. K. Hwang, H. J. Worman, J. C. Courvalin, and J. C. Eissenberg. 2001. Expression and functional analysis of three isoforms of human heterochromatin-associated protein HP1 in Drosophila. Chromosoma 109:536-544. [DOI] [PubMed] [Google Scholar]
- 31.MacAuley, A., and T. Pawson. 1988. Cooperative transforming activities of ras, myc, and src viral oncogenes in nonestablished rat adrenocortical cells. J. Virol. 62:4712-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makarov, V. L., Y. Hirose, and J. P. Langmore. 1997. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell 88:657-666. [DOI] [PubMed] [Google Scholar]
- 33.Mathog, D., M. Hochstrasser, Y. Gruenbaum, H. Saumweber, and J. Sedat. 1984. Characteristic folding pattern of polytene chromosomes in Drosophila salivary gland nuclei. Nature 308:414-421. [DOI] [PubMed] [Google Scholar]
- 34.McElligott, R., and R. J. Wellinger. 1997. The terminal DNA structure of mammalian chromosomes. EMBO J. 16:3705-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minc, E., Y. Allory, H. J. Worman, J. C. Courvalin, and B. Buendia. 1999. Localization and phosphorylation of HP1 proteins during the cell cycle in mammalian cells. Chromosoma 108:220-234. [DOI] [PubMed] [Google Scholar]
- 36.Morgan, S. E., C. Lovly, T. K. Pandita, Y. Shiloh, and M. B. Kastan. 1997. Fragments of ATM which have dominant-negative or complementing activity. Mol. Cell. Biol. 17:2020-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen, A. L., J. A. Ortiz, J. You, M. Oulad-Abdelghani, R. Khechumian, A. Gansmuller, P. Chambon, and R. Losson. 1999. Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J. 18:6385-6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen, A. L., M. Oulad-Abdelghani, J. A. Ortiz, E. Remboutsika, P. Chambon, and R. Losson. 2001. Heterochromatin formation in mammalian cells: interaction between histones and HP1 proteins. Mol. Cell 7:729-739. [DOI] [PubMed] [Google Scholar]
- 39.Pandita, T. K. 2002. ATM function and telomere stability. Oncogene 21:611-618. [DOI] [PubMed] [Google Scholar]
- 40.Pandita, T. K. 1983. Effect of temperature variation on sister chromatid exchange frequency in cultured human lymphocytes. Hum. Genet. 63:189-190. [DOI] [PubMed] [Google Scholar]
- 41.Pandita, T. K., and C. R. Geard. 1996. Chromosome aberrations in human fibroblasts induced by monoenergetic neutrons. I. Relative biological effectiveness. Radiat. Res. 145:730-739. [PubMed] [Google Scholar]
- 42.Pandita, T. K., E. J. Hall, T. K. Hei, M. A. Piatyszek, W. E. Wright, C. Q. Piao, R. K. Pandita, J. C. Willey, C. R. Geard, M. B. Kastan, and J. W. Shay. 1996. Chromosome end-to-end associations and telomerase activity during cancer progression in human cells after treatment with alpha-particles simulating radon progeny. Oncogene 13:1423-1430. [PubMed] [Google Scholar]
- 43.Pandita, T. K., S. Pathak, and C. R. Geard. 1995. Chromosome end associations, telomeres and telomerase activity in ataxia telangiectasia cells. Cytogenet. Cell Genet. 71:86-93. [DOI] [PubMed] [Google Scholar]
- 44.Paro, R., and D. S. Hogness. 1991. The polycomb protein shares a homologous domain with a heterochromatin-associated protein of Drosophila. Proc. Natl. Acad. Sci. USA 88:263-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piacentini, L., L. Fanti, M. Berloco, B. Perrini, and S. Pimpinelli. 2003. Heterochromatin protein 1 (HP1) is associated with induced gene expression in Drosophila euchromatin. J. Cell Biol. 161:707-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawant, S. G., V. Gregoire, S. Dhar, C. B. Umbricht, S. Cvilic, S. Sukumar, and T. K. Pandita. 1999. Telomerase activity as a measure for monitoring radiocurability of tumor cells. FASEB J. 13:1047-1054. [DOI] [PubMed] [Google Scholar]
- 47.Sharma, G. G., A. Gupta, H. Wang, H. Scherthan, S. Dhar, V. Gandhi, G. Iliakis, J. W. Shay, C. S. Young, and T. K. Pandita. 2003. hTERT associates with human telomeres and enhances genomic stability and DNA repair. Oncogene 22:131-146. [DOI] [PubMed] [Google Scholar]
- 48.Smerdon, M. J., and A. Conconi. 1999. Modulation of DNA damage and DNA repair in chromatin. Prog. Nucleic Acid Res. Mol. Biol. 62:227-255. [DOI] [PubMed] [Google Scholar]
- 49.Smilenov, L. B., S. Dhar, and T. K. Pandita. 1999. Altered telomere nuclear matrix interactions and nucleosomal periodicity in ataxia telangiectasia cells before and after ionizing radiation treatment. Mol. Cell. Biol. 19:6963-6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smilenov, L. B., W. Mellado, P. H. Rao, S. G. Sawant, C. B. Umbricht, S. Sukumar, and T. K. Pandita. 1998. Molecular cloning and chromosomal localization of Chinese hamster telomeric protein chTRF1. Its potential role in chromosomal instability. Oncogene 17:2137-2142. [DOI] [PubMed] [Google Scholar]
- 51.Smilenov, L. B., S. E. Morgan, W. Mellado, S. G. Sawant, M. B. Kastan, and T. K. Pandita. 1997. Influence of ATM function on telomere metabolism. Oncogene 15:2659-2665. [DOI] [PubMed] [Google Scholar]
- 52.Song, K., Y. Jung, D. Jung, and I. Lee. 2001. Human Ku70 interacts with heterochromatin protein 1α. J. Biol. Chem. 276:8321-8327. [DOI] [PubMed] [Google Scholar]
- 53.Stansel, R. M., T. de Lange, and J. D. Griffith. 2001. T-loop assembly in vitro involves binding of TRF2 near the 3′ telomeric overhang. EMBO J. 20:5532-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taggart, A. K., S. C. Teng, and V. A. Zakian. 2002. Est1p as a cell cycle-regulated activator of telomere-bound telomerase. Science 297:1023-1026. [DOI] [PubMed] [Google Scholar]
- 55.van Steensel, B., and T. de Lange. 1997. Control of telomere length by the human telomeric protein TRF1. Nature 385:740-743. [DOI] [PubMed] [Google Scholar]
- 56.van Steensel, B., A. Smogorzewska, and T. de Lange. 1998. TRF2 protects human telomeres from end-to-end fusions. Cell 92:401-413. [DOI] [PubMed] [Google Scholar]
- 57.Vaziri, H., J. A. Squire, T. K. Pandita, G. Bradley, R. M. Kuba, H. Zhang, S. Gulyas, R. P. Hill, G. P. Nolan, and S. Benchimol. 1999. Analysis of genomic integrity and p53-dependent G1 checkpoint in telomerase-induced extended-life-span human fibroblasts. Mol. Cell. Biol. 19:2373-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, G., A. Ma, C. M. Chow, D. Horsley, N. R. Brown, I. G. Cowell, and P. B. Singh. 2000. Conservation of heterochromatin protein 1 function. Mol. Cell. Biol. 20:6970-6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wellinger, R. J., and D. Sen. 1997. The DNA structures at the ends of eukaryotic chromosomes. Eur. J. Cancer 33:735-749. [DOI] [PubMed] [Google Scholar]
- 60.Wong, K. K., S. Chang, S. R. Weiler, S. Ganesan, J. Chaudhuri, C. Zhu, S. E. Artandi, K. L. Rudolph, G. J. Gottlieb, L. Chin, F. W. Alt, and R. A. DePinho. 2000. Telomere dysfunction impairs DNA repair and enhances sensitivity to ionizing radiation. Nat. Genet. 26:85-88. [DOI] [PubMed] [Google Scholar]
- 61.Wood, L. D., T. L. Halvorsen, S. Dhar, J. A. Baur, R. K. Pandita, W. E. Wright, M. P. Hande, G. Calaf, T. K. Hei, F. Levine, J. W. Shay, J. J. Wang, and T. K. Pandita. 2001. Characterization of ataxia telangiectasia fibroblasts with extended life-span through telomerase expression. Oncogene 20:278-288. [DOI] [PubMed] [Google Scholar]
- 62.Wright, W. E., and J. W. Shay. 1992. Telomere positional effects and the regulation of cellular senescence. Trends Genet. 8:193-197. [DOI] [PubMed] [Google Scholar]
- 63.Zhao, T., T. Heyduk, C. D. Allis, and J. C. Eissenberg. 2000. Heterochromatin protein 1 binds to nucleosomes and DNA in vitro. J. Biol. Chem. 275:28332-28338. [DOI] [PubMed] [Google Scholar]