Abstract
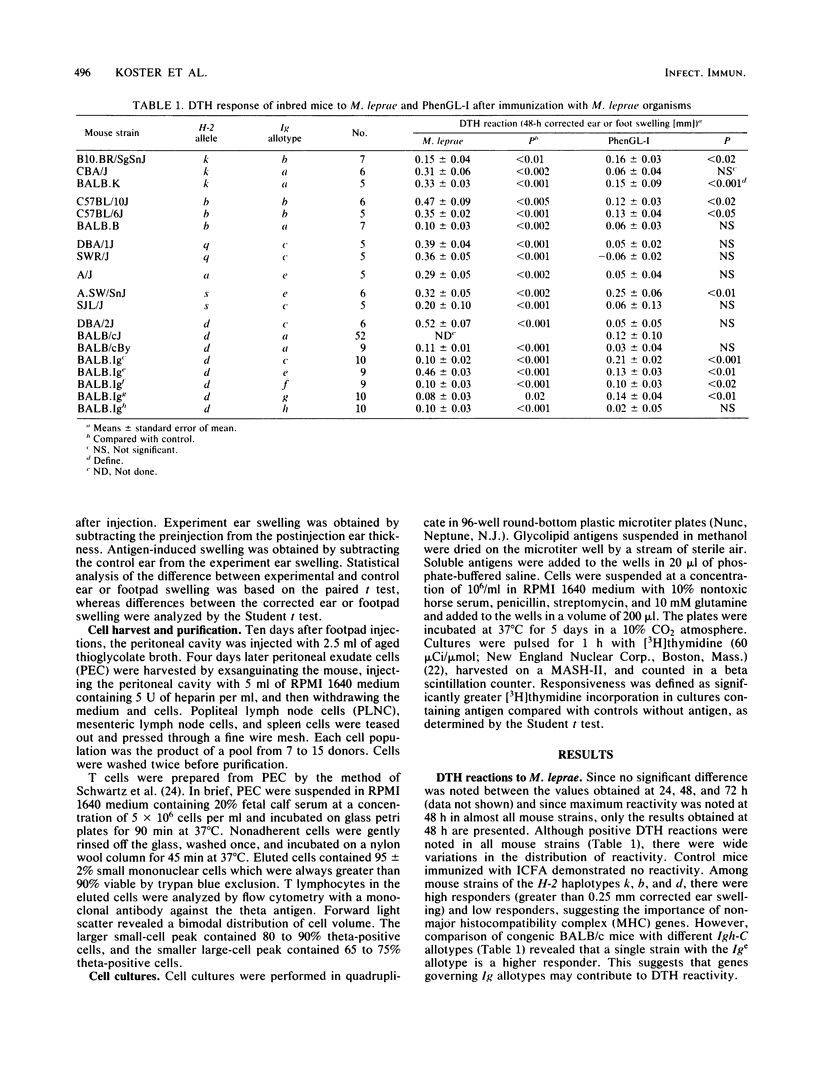
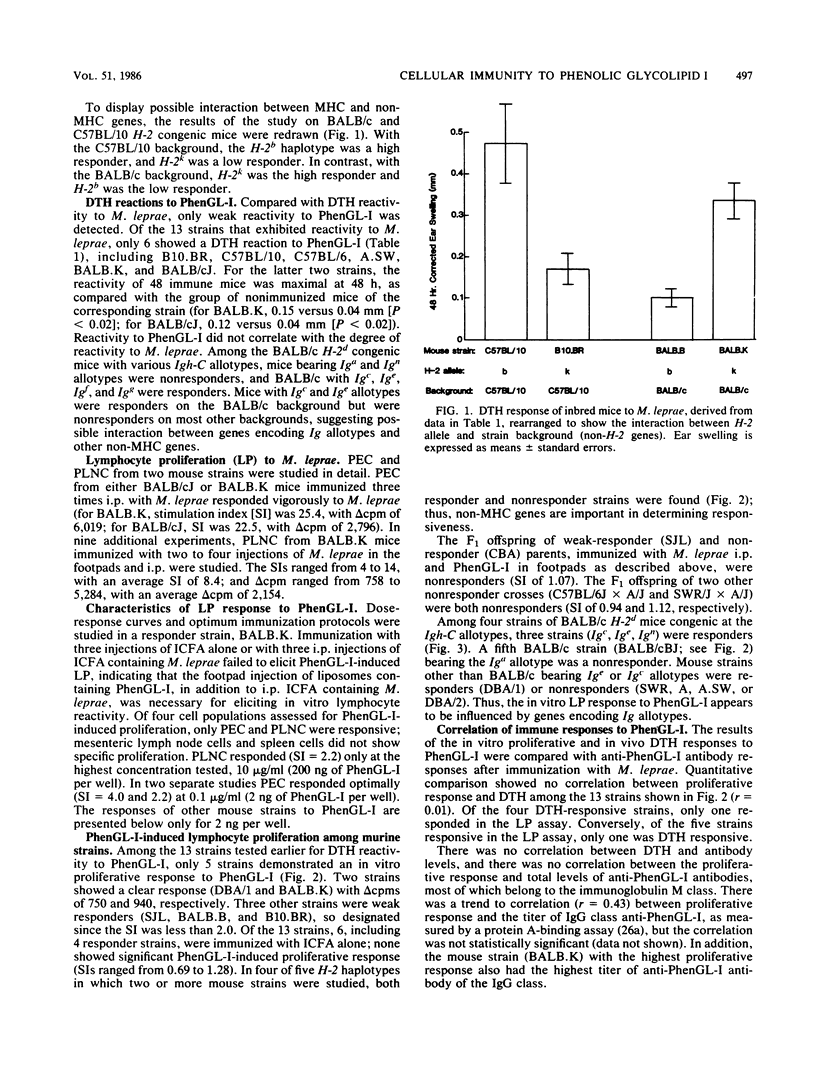
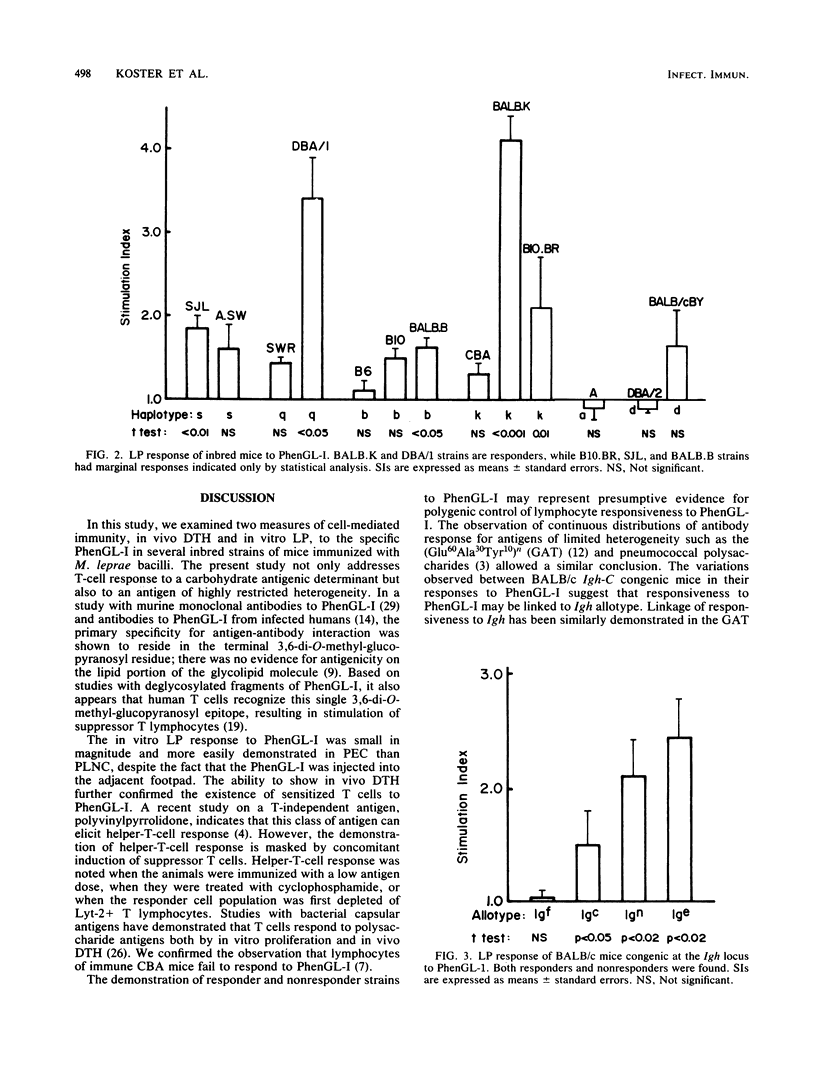
The cellular immune response to the Mycobacterium leprae-specific phenolic glycolipid I was examined in inbred mice immunized with M. leprae by in vivo delayed cutaneous hypersensitivity and in vitro lymphocyte proliferation. Whereas all mouse strains responded to M.leprae-induced delayed-type hypersensitivity and lymphocyte proliferation, only BALB.K was responsive in both assays to the glycolipid. Responsiveness was determined in part by non-H-2 genes, while the influence of H-2 genes was not apparent. Among congenic BALB/c mice differing only at Igh-C allotype loci, variations in responsiveness were found in both delayed-type hypersensitivity and lymphocytes proliferation assays, indicating a possible role for Igh-C loci-linked genes. Unresponsiveness in the lymphocyte proliferation assay to the glycolipid was inherited as a dominant trait in one set of responder X nonresponder F1 progeny. We conclude that after immunization with M. leprae organisms, the cell-mediated responses to the glycolipid, endowed with a single carbohydrate epitope, are under polygenic control, predominantly non-H-2-linked genes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adu H. O., Curtis J., Turk J. L. Role of the major histocompatibility complex in resistance and granuloma formation in response to Mycobacterium lepraemurium infection. Infect Immun. 1983 May;40(2):720–725. doi: 10.1128/iai.40.2.720-725.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B., Geckeler W. R., Weigert M. Genetics of the antibody response to dextran in mice. Science. 1972 Jul 14;177(4044):178–180. doi: 10.1126/science.177.4044.178. [DOI] [PubMed] [Google Scholar]
- Braley-Mullen H. Regulation of IgG memory responses by helper and suppressor T cells activated by the type 2 antigen, polyvinylpyrrolidone. J Exp Med. 1985 Jun 1;161(6):1357–1367. doi: 10.1084/jem.161.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braley H. C., Freeman M. J. Strain differences in the antibody plaque-forming cell responses of inbred mice to pneumococcal polysaccharide. Cell Immunol. 1971 Feb;2(1):73–81. doi: 10.1016/0008-8749(71)90026-8. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Draper P., Payne S. N., Rees R. J. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin Exp Immunol. 1983 May;52(2):271–279. [PMC free article] [PubMed] [Google Scholar]
- Brett S. J., Lowe C., Payne S. N., Draper P. Phenolic glycolipid 1 of Mycobacterium leprae causes nonspecific inflammation but has no effect on cell-mediated responses in mice. Infect Immun. 1984 Dec;46(3):802–808. doi: 10.1128/iai.46.3.802-808.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown I. N., Glynn A. A., Plant J. Inbred mouse strain resistance to Mycobacterium lepraemurium follows the Ity/Lsh pattern. Immunology. 1982 Sep;47(1):149–156. [PMC free article] [PubMed] [Google Scholar]
- Cho S. N., Yanagihara D. L., Hunter S. W., Gelber R. H., Brennan P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect Immun. 1983 Sep;41(3):1077–1083. doi: 10.1128/iai.41.3.1077-1083.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J., Adu H. O., Turk J. L. H-2 linkage control of resistance to subcutaneous infection with Mycobacterium lepraemurium. Infect Immun. 1982 Nov;38(2):434–439. doi: 10.1128/iai.38.2.434-439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J., Akuffo-Adu H., Turk J. L. H-2-linked genes which modify resistance of C57BL/10 mice to subcutaneous infection with Mycobacterium lepraemurium. Infect Immun. 1984 Dec;46(3):635–638. doi: 10.1128/iai.46.3.635-638.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorf M. E., Dunham E. K., Johnson J. P., Benacerraf B. Genetic control of the immune response: the effect of non-H-2 linked genes on antibody production. J Immunol. 1974 Apr;112(4):1329–1336. [PubMed] [Google Scholar]
- Draper P., Rees R. J. The nature of the electron-transparent zone that surrounds Mycobacterium lepraemurium inside host cells. J Gen Microbiol. 1973 Jul;77(1):79–87. doi: 10.1099/00221287-77-1-79. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Hunter S. W., Cho S. N., Aspinall G. O., Brennan P. J. Chemical synthesis and serology of disaccharides and trisaccharides of phenolic glycolipid antigens from the leprosy bacillus and preparation of a disaccharide protein conjugate for serodiagnosis of leprosy. Infect Immun. 1984 Jan;43(1):245–252. doi: 10.1128/iai.43.1.245-252.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal T., Myklestad B., Samuel D. R., Myrvang B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin Exp Immunol. 1971 Dec;9(6):821–831. [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Brennan P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J Bacteriol. 1981 Sep;147(3):728–735. doi: 10.1128/jb.147.3.728-735.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Fujiwara T., Brennan P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J Biol Chem. 1982 Dec 25;257(24):15072–15078. [PubMed] [Google Scholar]
- Mathew R. C., Curtis J., Turk J. L. T cell proliferation in Mycobacterium lepraemurium infection. I. Lack of correlation between antigen-specific proliferation of Lyt 1 + 23- cells and resistance in lethal infections. Immunology. 1984 Jan;51(1):185–192. [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Owen F. L. T cell alloantigens encoded by the IgT-C region of chromosome 12 in the mouse. Adv Immunol. 1983;34:1–38. doi: 10.1016/s0065-2776(08)60375-2. [DOI] [PubMed] [Google Scholar]
- Peterson L. B., Wilner G. D., Thomas D. W. Functional differentiation in the genetic control of murine T lymphocyte responses to human fibrinopeptide B. J Immunol. 1983 Feb;130(2):637–643. [PubMed] [Google Scholar]
- Pollack A., Bagwell C. B., Irvin G. L., 3rd Radiation from tritiated thymidine perturbs the cell cycle progression of stimulated lymphocytes. Science. 1979 Mar 9;203(4384):1025–1027. doi: 10.1126/science.424727. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., Jackson L., Paul W. E. T lymphocyte-enriched murine peritoneal exudate cells. I. A reliable assay for antigen-induced T lymphocyte proliferation. J Immunol. 1975 Nov;115(5):1330–1338. [PubMed] [Google Scholar]
- Serjeantson S. W. HLA and susceptibility to leprosy. Immunol Rev. 1983;70:89–112. doi: 10.1111/j.1600-065x.1983.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Shapiro M. E., Onderdonk A. B., Kasper D. L., Finberg R. W. Cellular immunity to Bacteroides fragilis capsular polysaccharide. J Exp Med. 1982 Apr 1;155(4):1188–1197. doi: 10.1084/jem.155.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher C., Yanagihara D., Brennan P. J., Koster F. T., Tung K. S. Antibody response to phenolic glycolipid I in inbred mice immunized with Mycobacterium leprae. Infect Immun. 1985 May;48(2):474–479. doi: 10.1128/iai.48.2.474-479.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R. Influence of route of Mycobacterium lepraemurium injection on susceptibility to mouse leprosy and on lymphoblastic transformation. Infect Immun. 1980 Jun;28(3):660–668. doi: 10.1128/iai.28.3.660-668.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Khanolkar S. R., Barg L. L., Buchanan T. M. Generation and characterization of monoclonal antibodies to the phenolic glycolipid of Mycobacterium leprae. Infect Immun. 1984 Jan;43(1):183–188. doi: 10.1128/iai.43.1.183-188.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Gonzalez N. M., de Vries R. R., Convit J., van Rood J. J. HLA-linked control of predisposition to lepromatous leprosy. J Infect Dis. 1985 Jan;151(1):9–14. doi: 10.1093/infdis/151.1.9. [DOI] [PubMed] [Google Scholar]


