Abstract
Stimulation of T cells through their antigen receptors (TCRs) causes a transient increase in the intracellular concentration of cyclic AMP (cAMP). However, sustained high levels of cAMP inhibit T-cell responses, suggesting that TCR signaling is coordinated with the activation of cyclic nucleotide phosphodiesterases (PDEs). The molecular basis of such a pathway is unknown. Here we show that TCR-dependent signaling activates PDE4B2 and that this enhances interleukin-2 production. Such an effect requires the regulatory N terminus of PDE4B2 and correlates with partitioning within lipid rafts, early targeting of this PDE to the immunological synapse, and subsequent accumulation in the antipodal pole of the T cell as activation proceeds.
One of the most robust changes following stimulation of T cells through their antigen receptors (TCRs) is a marked transient increase in intracellular cyclic AMP (cAMP) levels (23, 26, 46). The mechanism for this enhanced cAMP generation has not been elucidated, but it is likely due to the activation of adenylate cyclase by a G protein coupled to the TCR (46). However, persistent high levels of cAMP inhibit many of the biological responses of the T cell (1, 28, 54), and thus antigen receptor-mediated signaling must be coordinated with activation of cyclic nucleotide phosphodiesterases (PDEs) that degrade cAMP and/or cGMP.
PDEs comprise a superfamily of enzymes classified into at least 11 families (i.e., PDE1 to PDE11) (17, 29, 52). Members of each of these families are generated from multiple genes, by alternative splicing and/or by differential use of translation starting sites (11). All members of the PDE superfamily have a highly conserved C-terminal region that contains the catalytic domain responsible for the hydrolysis of cyclic nucleotides in a divalent-cation-dependent fashion (17, 22, 56). In contrast, the members of the PDE superfamily vary in their N-terminal regions, containing distinct domains such as calmodulin-binding sites or cGMP-binding sites, which are commonly linked to the compartmentalization and spatial distribution of a particular PDE within the cell (4, 17, 24).
The involvement of PDEs in T-cell activation has been suggested by three lines of evidence. The first line of evidence is that TCR ligation in thymocytes and primary T cells not only induces a short-lived increase in the intracellular concentrations of cAMP but also causes up-regulation of the expression of PDE RNAs and an increase in PDE activity, leading to a decrease in intracellular cAMP levels (7, 19, 28, 32). The second one is that a constitutively high level of PDE activity in some T-cell subsets, such as memory CD4+ T cells, has been related to biological responses such as infectivity by human immunodeficiency virus (48). The third line of evidence is that inhibition of PDE activity, particularly of PDE4 activity, can modulate T-cell responses either by inhibiting Th1 cytokine production, and thereby skewing the cytokine environment towards a Th2 profile, or by inducing apoptosis (5, 27, 34, 36).
The molecular basis of how PDEs can modulate T-cell activation remains unknown. Four of the 11 families of PDEs (PDE3, PDE4, PDE7, and PDE8) have so far been reported to be present in primary T cells and T-cell lines (18, 19, 28, 39, 49). At least one of them, the B2 isoform of PDE4B, selectively associates with the CD3ɛ chain of the TCR (3). Based on this finding, we hypothesized that the compartmentalization of PDE4B2 to the TCR complex observed in human peripheral blood T cells likely correlates with its involvement in TCR-mediated signaling. To test this hypothesis, we generated a doxycycline-inducible PDE4B2 expression system for a PDE4B2-green fluorescent protein (PDE4B2-GFP) fusion protein in Jurkat T cells. Jurkat T cells were particularly appropriate for these types of experiments because previous studies have shown that these cells do not express any endogenous isoform of PDE4B (16, 42). Here we report that targeted PDE4B2 has an enhancing effect on TCR-mediated T-cell activation that translates into a decreased threshold for T-cell activation and enhanced interleukin-2 (IL-2) production. Such an effect is additive to that of costimulation and correlates with a distribution of lipid raft-associated PDE4B2 proximal to the immunological synapse during early stages of TCR signaling, followed by antipodal redistribution of PDE4B2 at later stages of T-cell activation.
MATERIALS AND METHODS
Plasmids.
A full-length human PDE4B2 cDNA (kindly supplied by T. Torphy, Centocor, Malvern, Pa.) (30) was subcloned into NheI-BamHI sites in the pEGFP-N1 expression vector (Clontech Inc., Palo Alto, Calif.) to create an in-frame translational fusion of PDE4B2 and GFP at the 3′ end. This PDE4B2 construct (referred to as wild type [wt]) lacks part of the 5′ untranslated region and spans the entire translational coding region of PDE4B2 (amino acids 1 to 564; bp 265 to 1988) (30). In addition, we generated two mutant PDE4B2-GFP constructs with deletions at the 5′ end of the cDNA resulting in the absence of the first 133 amino acids (133C PDE4B2-GFP) or the first 221 amino acids (221C PDE4B2-GFP). The mutant construct 133C PDE4B2-GFP encodes amino acids 133 to 564 and thus lacks the entire UCR2 region except 4 amino acids. The construct 221C PDE4B2-GFP encodes amino acids 221 to 564 and lacks the entire UCR2 region. The different PDE4B2-GFP cDNAs were subsequently subcloned into the NheI-NotI site of pBig2i. It is important to note that, in all three PDE4B2 chimeric constructs, expression and detection of a GFP signal are dependent on an open reading frame between PDE4B2 and GFP. As a control, the GFP fragment (806 bp) obtained from the pEGFP-N1 vector was also inserted into pBig2i (GFP). The pBig2i vector utilizes a hybrid bidirectional, tetracycline-responsive promoter element that directs expression of both the PDE4B2-GFP chimera and the tetracycline-responsive transactivator cDNAs (rtTAN) (47). All final plasmid constructs were verified by complete sequencing of their PDE4B2 cDNA inserts and accompanying vector backbones.
Cells.
Jurkat T cells (E6.1) were obtained from the American Type Culture Collection (Manassas, Va.). The B lymphoblastoid cell line LG2 (expressing high levels of HLA-DR1 and B7), used as antigen-presenting cells (APCs), was kindly provided by Eric Long (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Md.). Both cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 100 U of penicillin-streptomycin per ml, and 10 mM HEPES buffer.
Stable T-cell transfectants for the doxycycline-inducible cDNAs were generated by electroporation and selected in medium containing 400 μg of hygromycin B (Sigma, St. Louis, Mo.) per ml. PDE4B2-GFP expression in pBig2i-transfected cells was induced by overnight incubation (21 to 24 h) with doxycycline (Sigma) at various concentrations as specifically required and as indicated in the figure legends. Transient transfections were performed with the Lipofectamine PLUS system (Invitrogen, Carlsbad, Calif.), and their efficiencies were quantitated at 24 and 48 h by fluorescence microscopy and fluorescence-activated cell sorting (FACS) analysis for GFP expression. Only transient transfections with equal efficiencies of 16 to 20% were studied. For each assay described in this paper, continuous expression of the variant PDE4B2 forms by the transfected cells was ensured by induction with doxycycline for 24 h prior to the assay and their maintenance at the same described antibiotic concentration for the entire duration of the assay until the time of harvest and analysis.
Peripheral blood mononuclear cells were isolated from heparinized whole blood from normal donors by using Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden) gradients. Cells were washed in supplemented RPMI 1640 medium and resuspended at 106 cells/ml. T-cell blasts were generated by culturing peripheral blood mononuclear cells with phorbol 12-myristate 13-acetate (100 ng/ml) and ionomycin (250 ng/ml) for 72 h at 37°C with 5% CO2. T-cell blasts were rested overnight before use in any experiments. The resulting population contained >90% CD3+ cells.
RT-PCR for PDE4B2-GFP.
Total RNA was isolated from untransfected parental and PDE4B2-GFP-transfected Jurkat T cells (10 × 106) by Nucleospin RNA II column isolation (Clontech). Following quantitation and checking for integrity, each RNA sample was specifically amplified with a single-step reverse transcription-PCR (RT-PCR) kit (Clontech). cDNA synthesis and PCR were performed in a single optimized buffer in the presence of an oligo(dT) primer for cDNA synthesis and the appropriate PCR primer pairs for amplification of the specific DNA products. (5′ sense PDE4B primer as previously described [3], 3′ antisense GFP [Clontech], and 5′ and 3′ beta actin [Clontech]).
Detection of PDE4B2-GFP protein expression.
Whole-cell lysates from nonstimulated, doxycycline(1 or 5 μg/ml)-induced, PDE4B2-GFP-transfected T cells were prepared by controlled agitation (10 s) of the cells in standard lysis buffer (1% Triton X-100, 1.5 M NaCl, 1 mM Tris [pH 7.5], 0.5 mM EDTA, 0.1 mM sodium orthovanadate, and a standard cocktail of protease inhibitors [13]) followed by incubation at 4°C for 30 min and centrifugation at 10, 000 × g for 10 min. To screen for PDE4B2-GFP chimera expression, the clarified lysates were immunoprecipitated overnight at 4°C with a rabbit polyclonal antiserum against GFP (final antibody concentration of 0.2 μg/ml; Clontech) and protein-A Sepharose beads (Amersham Pharmacia Biotech), electrophoresed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes, and immunoblotted with a mouse monoclonal antibody against GFP (Clontech). The methodology used for electrophoresis and immunoblotting has been previously described (9).
Flow cytometry.
Quantitative assessment of GFP-expressing cells was performed by direct flow cytometry (Becton Dickinson, San José, Calif.). Assessment of T-cell-APC conjugate formation by flow cytometry was performed as previously reported (35) with slight modifications. Briefly, parental Jurkat E6.1 T cells and doxycycline-induced (1 μg of doxycycline/ml for 48 h) wt PDE4B2-GFP-transfected T cells were stained with 0.15 μg of calcein-acetoxymethyl (Molecular Probes, Eugene, Oreg.) per ml for 30 min. Antigen-presenting B cells (LG2) were stained with 3 μg of hydroethidine (Molecular Probes) per ml for 30 min and then incubated for 1 h with or without 1 μg of staphylococcal enterotoxin E superantigen (SEE) per ml and incubated at 37°C. Next, 106 stained T cells were combined with an equal number of stained APCs with or without SEE. Cells were pelleted and incubated at 37°C for 30 min, and after being washed, they were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min on ice. At least 30,000 gated events were measured. The percentage of T-cell-APC conjugates was calculated by dividing the number of red-green events by the sum of all single-labeled green events and dual-labeled red-green events. Cell cycling was tracked by flow cytometry with an orange fluorescent 5 (and 6)-(((4-choloromethyl)benzoyl)amino)tetramethylrhodamine (CMTMR) (Molecular Probes) probe.
Confocal microscopy.
Confocal microscopy was performed with a Zeiss LSM 510 microscope. Jurkat T cells, both parental and transfected (106/ml), were incubated on polylysine (0.01%; Sigma)-coated glass-bottom microwell dishes (MatTek Corp., Ashland, Mass.) at 37°C for 10 min. To monitor the doxycycline sensitivity of the expression system and to determine differential localization of the wt PDE4B2-GFP and 133C PDE4B2-GFP, cell cultures of the stable transfected cells were first incubated with various concentrations of doxycycline (from 0 to 5 μg/ml) for 24 h. The PDE4B2-GFP distribution during immunological synapse formation was assessed by culturing T cells that were stably transfected with doxycycline-induced wt PDE4B2-GFP or 133C PDE4B2-GFP with LG2 APCs, preincubated with 100 ng of SEE per ml, for either 10 or 30 min. Following the allotted time of coincubation, the T-cell-APC conjugates were fixed with 4% paraformaldehyde for 20 min, washed with PBS-1% FCS, and stained with phycoerythrin (PE)-conjugated anti-CD3 (BD Bioscience) for 30 min on ice or stained with Hoeschst 3342 nuclear stain at 1 μg/ml for 1 h at room temperature.
An immunological synapse was defined as a flat interface between a T cell and an LG2 APC in which aggregation of CD3 was documented. To determine the PDE4B2-GFP distribution during immunological synapse formation, 100 synapses were analyzed. Proximal distribution of wt PDE4B2-GFP was defined as 50% or greater GFP expression in the half of the T cell that juxtaposes the synapse. Distal distribution of PDE4B2-GFP was defined as 50% or greater GFP expression in the half of the T cell antipolar to the synapse. Fluorescence microscope analysis of nucleus distribution during synapse formation was performed with Hoechst 33342 nuclear stain, and the distribution of stained nucleus in relation to the PE-labeled TCR was noted. Proximal distribution was defined as when the nucleus was found in the half of the T cell that juxtaposes the synapse, while distal distribution was defined as when the nucleus was found in the half of the T cell that is antipolar to the synapse. When the signals were equally divided in the poles of the T cell in relation to the immunological synapse, the distribution was scored as medial.
For confocal microscopy of primary T cells, T-cell blasts (106/ml) were incubated on polylysine (0.01%)-coated dishes at 37°C for 10 min. T cells were stained with fluorescein isothiocyanate-labeled cholera toxin, which binds to GM-1 (a lipid raft marker), for 30 min on ice. The T cells were fixed and permeabilized with 4% paraformaldehyde-0.2% Triton, washed with PBS-1% FCS, and incubated with a polyclonal anti-PDE4B2 rabbit serum for 30 min on ice. Subsequently, cells were washed and stained with a fluorescence-labeled anti-rabbit secondary antibody (Alexa Fluor 633; Molecular Probes) for 30 min on ice.
PDE activity assay.
PDE activity in noninduced and doxycycline-induced PDE4B2-GFP-transfected Jurkat T cells was determined as described by Thompson et al. (50) with some modifications. Briefly, whole-cell lysates and anti-GFP immunoprecipitates (volume corresponding to 800,000 cell equivalents, prepared exactly as described above) were assayed in a final volume of 200 μl containing 40 mM Tris-HCl (pH 8.0), 5 mM MgCl2, 15 μg of bovine serum albumin per ml, 50 μM cAMP, and 3 × 104 cpm of [3H]cAMP. The reaction mixture was incubated for 20 min at 30°C. The reaction was terminated by boiling for 1 min. Crotalus atrox snake venom (10 μg) was added and left for a further 10 min at 30°C. The assay mixture was then incubated with 1 ml of Dowex AGIX2 ion-exchange resin (three parts H2O and one part AGIX2 resin; Sigma) and microcentrifuged to sediment the resin. Supernatants (500 μl) were carefully removed and supplemented with scintillation fluid, and radioactivity was counted in a liquid scintillation counter. All assays were carried out in duplicate. Control values were obtained with lysates previously boiled for 1 min. The protein content of all lysates was quantitated with a detergent-compatible assay kit (Bio-Rad). Correspondingly, the PDE activity of lysates was normalized for protein content and reported as picomoles of activity per milligram of protein per minute, while the PDE activity of GFP immunoprecipitations was standardized and reported according to cell equivalents.
T-cell stimulation and functional assays.
The different wt or mutant stably PDE4B2-GFP-transfected Jurkat T cells and the parental Jurkat E6.1 cell line (1.5 × 105 cells/well) were stimulated in 96-well plates either with LG2 cells (7.5 × 104 cells/well) and different concentrations of SEE (Toxin Technology Inc., Sarasota, Fla.) or with combinations of anti-CD3 antibody-coated beads and soluble anti-CD28 monoclonal antibody. When used, rolipram was added at 10 μM and left for the whole duration of the culture. Anti-CD3-coated beads (Interfacial Dynamics, Portland, Oreg.) were prepared as described previously (13), with a constant amount of anti-CD3 (UCHT1; PharMingen, San Diego, Calif.) representing 20% (1 μg/107 beads) of the total protein bound to the beads and anti-HLA class I monoclonal antibody used to make up the remaining 80% (4 μg/107 beads) of protein. IL-2 in the culture supernatants was measured with an IL-2 enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences, Mississauga, Ontario, Canada).
Apoptosis assay.
T cells stably transfected with wt PDE4B2-GFP were monitored for apoptotic cell death in the presence or absence of rolipram (10 mM), following stimulation with LG2 APCs and various concentrations of SEE, by determining cytoplasmic histone-associated DNA fragments with a photometric ELISA kit (Roche). This ELISA measures mono- and oligonucleosomes in the cytoplasmic fraction of cell lysates.
Subcellular fractionation and lipid raft isolation.
Jurkat T cells (100 × 106) stably transfected with wt PDE4B2-GFP or 133C PDE4B2-GFP were induced for 48 h with 2 μg of doxycycline per ml. Lipid raft isolation was done as reported previously (13). Briefly, transfected Jurkat T cells (100 × 106) or primary T-cell blasts (250 × 106) were lysed in buffer containing 0.5% Triton X-100, 25 mM MES (morpholineethanesulfonic acid), 150 mM NaCl, 1 mM Na3VO4, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 1 μg of aprotinin per ml. The lysates were then homogenized, mixed with an equal volume of 85% (wt/vol) sucrose, and put under a step gradient consisting of 35 and 5% (wt/vol) layers of sucrose in MBS (25 mM MES, 150 ml NaCl [pH 6.5]) supplemented with 1 mM Na3VO4 and 2 mM EDTA. Samples were then ultracentrifuged for 18 h at 200,000 × g and 4°C. Twelve 1-ml fractions were taken, starting at the top of the step sucrose gradient, with fraction 5 containing the cloudy band indicative of lipid rafts and fraction 12 taken as the soluble fraction. Fraction aliquots were mixed with 4× sample buffer (8% 2-mercaptoethanol, 250 mM Tris, 40% glycerol, 2% bromophenol blue) to a final dilution of 1×. Sedimented lipid raft fractions (fractions 5) were pelleted by adding an equal volume of MBS and centrifuging for 1 h at 14,000 rpm and 4°C on a tabletop microcentrifuge, and 4× sample buffer was added for resolution by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting as described above. The pelleted rafts and/or various gradient fractions were screened for GFP in the transfected Jurkat T cells or for PDE4B2 in primary T cells by using a rabbit polyclonal antiserum raised against the PDE4B2 peptide KEHGGTFSSTGISGGSGD (Dalton Chemical Laboratories Inc., Toronto, Ontario, Canada). To identify the fractions containing the soluble cellular proteins, membranes were also screened for total cytoplasmic ERK by using a rabbit antiserum from StressGen (Victoria, British Columbia, Canada). GM-1 was detected in the gradient fractions by blotting with cholera toxin B-peroxidase conjugate (Sigma).
Statistical analysis.
Statistical analysis of experimental data was performed by analysis of variance and subsequent Student's t test for individual group comparisons. A statistical difference was considered significant when the P value was <0.05.
RESULTS
Expression of functional PDE4B2-GFP in Jurkat T cells.
The contribution of PDEs to the activation of T lymphocytes through the TCR remains unknown. To establish the involvement of these enzymes in T-cell activation, and since we had previously reported that PDE4B2 selectively associates with the TCR complex (3), we developed a regulated expression system for wt or mutant PDE4B2 tagged with GFP at the C terminus that uses Jurkat T cells, which do not express any endogenous PDE4B isoform (16, 42). Briefly, open reading frame fusions between the cDNA coding for wt PDE4B2 or mutant, N-terminally deleted PDE4B2 (133C PDE4B2 or 221C PDE4B2) and the cDNA encoding GFP were generated and introduced into a doxycycline-inducible gene expression vector (pBig2i) (Fig. 1A). The resulting plasmids were transfected into Jurkat T cells, and stable transfected T-cell clones were selected for each construct.
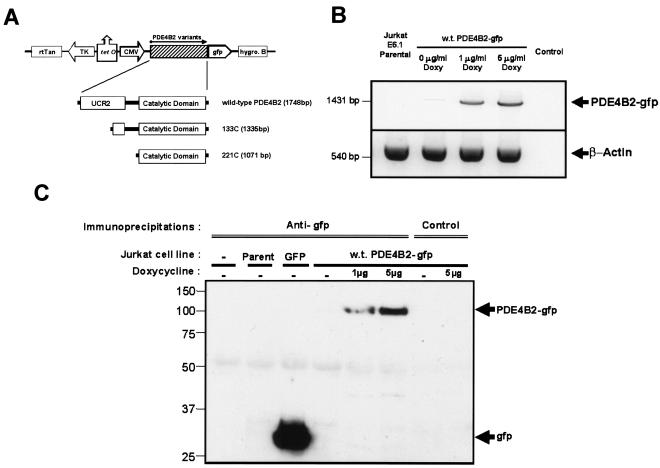
FIG. 1.
Characterization of a doxycycline-inducible PDE4B2-GFP expression system in Jurkat T cells. (A) wt PDE4B2-GFP cDNA or the 5′ deletion mutant 133C PDE4B2-GFP or 221C PDE4B2-GFP cDNA was subcloned into the doxycycline-inducible pBig2i vector. In this vector, expression of the PDE4B2-GFP cDNA is driven by the cytomegalovirus promoter, which is turned on by the rtTAN transactivator driven by the doxycycline-inducible thymidine kinase promoter. The selection agent for transfected clones is hygromycin B. (B) Expression of wt PDE4B2-GFP RNA in noninduced or induced T cells stably transfected with wt PDE4B2-GFP as detected by RT-PCR. With the combination of a PDE4B2 sense primer and a GFP antisense primer, a single amplification product of the expected 1,431 bp was detected only in transfected, induced T cells. Amplification of β-actin confirmed RNA presence in all of the samples. (C) Expression of PDE4B2-GFP protein in stably transfected Jurkat T cells. Anti-GFP or isotype-matched control immunoprecipitates from nontransfected Jurkat cells or from Jurkat cells transfected with GFP or with PDE4B2-GFP were immunoblotted for GFP. wt PDE4B2-GFP expression was induced with doxycycline at 1 and 5 μg/ml. The time of doxycycline induction was 24 h prior to cell harvest for the experiments described for panels B and C.
Next, we confirmed that the PDE4B2-GFP cDNAs were transcribed and translated into a full-length PDE4B2-GFP protein in the stable T-cell clones. As shown in Fig. 1B, RT-PCR with an antisense primer for GFP and a sense PDE4B2 primer amplified a fragment of the appropriate size only in doxycycline-induced, transfected Jurkat cells, while no amplification was observed from total RNA of nontransfected Jurkat T cells or uninduced Jurkat T cells. This result was corroborated at the protein level by performing GFP immunoblotting for GFP immunoprecipitations (Fig. 1C): only doxycycline-induced samples showed expression of PDE4B2-GFP fusion proteins, and only a full-length PDE4B2-GFP signal was detected in significant amounts.
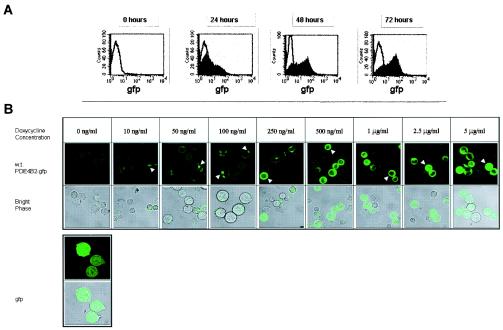
Expression of wt PDE4B2-GFP progressively increased over a 72-h period following addition of doxycycline to the culture, as shown by the increase in fluorescence detected by FACS (Fig. 2A). Expression of the PDE4B2-GFP also was dependent on the magnitude of doxycycline induction (Figs. 1C and 2B). Confocal microscopy allowed us to correlate the intracellular distribution of PDE4B2 with the amount of this enzyme being expressed. We observed that at low doxycycline concentrations, i.e., low levels of PDE4B2-GFP expression, this enzyme was localized in focal points randomly in the periphery of the cell (Fig. 2B). In contrast, at higher levels of expression (>500 ng/ml), PDE4B2-GFP became more ubiquitously distributed throughout the cytoplasm but was absolutely excluded from the nucleus. GFP-transfected T cells demonstrated a homogeneous distribution of signal throughout the entire nucleus and cytoplasm independent of doxycycline concentration.
FIG. 2.
wt PDE4B2-GFP expression upon doxycycline induction. (A) Time course of wt PDE4B2-GFP expression in stably transfected Jurkat T cells. Stably transfected Jurkat T cells were cultured in the presence of doxycycline (2 μg/ml) for 24, 48, or 72 h, and green fluorescence signal was analyzed by flow cytometry. The experiments were repeated three times. (B) Localization of wt PDE4B2-GFP in Jurkat T cells by confocal microscopy. Confocal microscopy of wt PDE4B2-GFP expression in stably transfected Jurkat cells incubated in the presence of various doxycycline concentrations, ranging from 10 ng/ml to 5 μg/ml, for 24 h was performed. The distribution of the wt PDE4B2-GFP (arrowheads) was compared to the distribution in a stable Jurkat cell line transfected with the GFP control plasmid that was detected homogeneously throughout the cell. T cells attached to a poly-l-lysine matrix were viewed with a Zeiss LSM510 confocal microscope (63× objective) capturing both green fluorescent laser filter detection and bright phase. The experiments were repeated three times.
It was important to establish whether the wt PDE4B2-GFP chimera possessed enzymatic activity. This was investigated with PDE assays of cell lysates from noninduced and doxycycline-induced T-cell transfectants (Fig. 3). Induction of PDE4B2-GFP expression with doxycycline correlated with an increase in PDE activity over the basal PDE activity provided by endogenous PDE3B, PDE4A, and PDE7 (16, 42). The inducible PDE activity was inhibited by rolipram (10 μM), a selective PDE4 inhibitor, corroborating that this activity was due to the overexpressed PDE4B2 transgene. In transfected noninduced wt PDE4B2-GFP-expressing cells, endogenous PDE4 activity, as measured by rolipram sensitivity, was approximately 14% (data not shown). In contrast, in doxycycline-induced PDE4B2-GFP-expressing cells, about 70% of the PDE activity was rolipram sensitive, suggesting that 56% of the total PDE4 activity in induced cells can be attributed to recombinant PDE4B2-GFP. Based on this, we concluded that the PDE4B2-GFP fusion protein was functional as a PDE.
FIG. 3.
The wt PDE4B2-GFP fusion protein has rolipram-sensitive PDE activity. The PDE activity of the stable Jurkat T-cell clone transfected with wt PDE4B2-GFP (2 × 105 cells) was determined following 24 h of treatment with different concentrations of doxycycline (0 to 5,000 ng/ml). The increase in PDE activity upon doxycycline induction was inhibited by the addition of 10 μM rolipram, a PDE4-specific inhibitor. The data are representative of those from three different experiments. Error bars indicate standard deviations.
De novo expression of wt PDE4B2-GFP enhances TCR-dependent T-cell activation in the presence of costimulation.
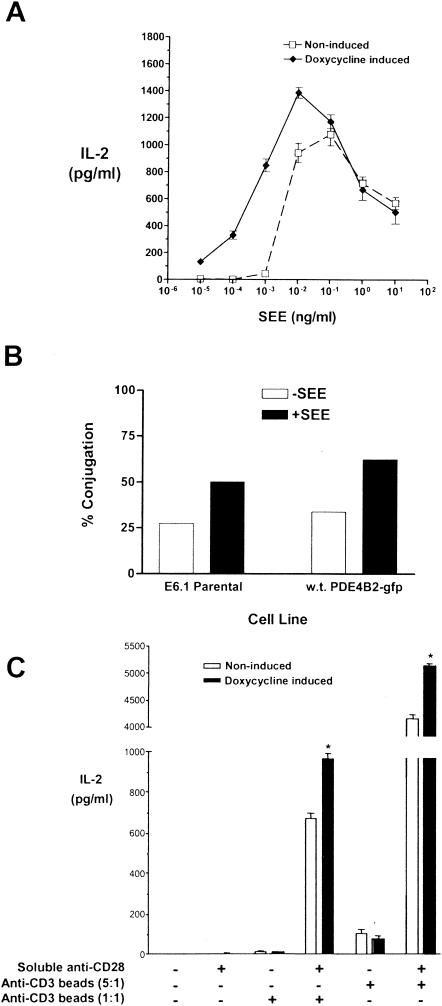
To study the effects of de novo expression of PDE4B2 on T-cell responsiveness, we first looked at the effect of its expression on IL-2 production following T-cell stimulation with SEE and LG2 APCs (Fig. 4A). We observed that expression of PDE4B2 correlated with a decrease in the threshold of T-cell activation, as illustrated by a shift to the left of the IL-2 production curve and by a net increase in IL-2 production. Induction with different concentrations of doxycycline (0.5 to 2 μg/ml) generated the same profile of IL-2 response (data not shown). Addition of doxycycline to nontransfected Jurkat T-cell cultures did not affect their functional responsiveness (data not shown). The enhanced responsiveness of the PDE4B2-GFP-expressing T cells to SEE and APC stimulation was not simply due to an increased level of T-cell-APC conjugation upon PDE4B2-GFP overexpression, as determined by quantitation of the conjugates by two-color flow cytometry (Fig. 4B), since both PDE4B2-expressing and control cell types exhibited similar proportional increases in T-cell-APC conjugates in the presence of SEE.
FIG. 4.
PDE4B2 expression enhances IL-2 production by host T cells in response to specific antigen stimulation. (A) Enhancement of SEE-induced IL-2 production by de novo expression of PDE4B2. T cells stably transfected with wt PDE4B2-GFP and induced with doxycycline (1 μg/ml, 24 h prior assay) or noninduced were stimulated for 14 h with LG2 cells pretreated with SEE for 24 h prior to the assay at a T-cell/APC ratio of 5:1. Doxycycline and SEE levels were maintained throughout the assay where appropriate. After the 14-h stimulation, culture supernatants were collected and IL-2 production was measured by ELISA. (B) The enhanced T-cell response seen in PDE4B2-GFP-expressing T cells is not due to increased conjugate formation. Parallel cultures of wt PDE4B2-GFP-transfected T cells and their parentalJurkat E6.1 counterparts were maintained with doxycycline (1 μg/ml) for 24 h, stained with calcein-acetoxymethyl, and then mixed with hydroethidine-stained APCs at a 1:1 ratio. Cells were coincubated for 30 min at 37°C before paraformaldehyde fixation prior to measurement by two-color flow cytometry. The percentage of conjugated T cells is the number of red-green events divided by the total number of green events times 100. The results are representative of those from three experiments. (C) Enhancement of IL-2 production in response to TCR-CD28 coligation. Jurkat T cells were incubated without or with (to induce wt PDE4B2-GFP expression) doxycycline (1 μg/ml) for 24 h. At that time, noninduced and doxycycline-induced wt PDE4B2-GFP T cells were subjected to stimulation with anti-CD3 antibody-coated beads and soluble anti-CD28 antibody for 14 h, at which time culture supernatants were collected for analysis of IL-2 production. Stimulations involved anti-CD3 antibody-coated beads at a bead/cell ratio of either 5:1 or 1:1 and soluble anti-CD28 antibody at 5 μg/ml as indicated. *, P < 0.005. Doxycycline was maintained within the appropriate cultures during induction (24 h) and during T-cell stimulation (14 h). The data are representative of those from three different experiments. Error bars indicate standard deviations.
The enhanced responsiveness to SEE stimulation in PDE4B2-expressing T cells was also seen when these T cells were activated with monoclonal antibodies against CD3 and CD28. Using this stimulation system, we observed that expression of PDE4B2 significantly and consistently enhanced IL-2 production in response to CD3-CD28 coligation (Fig. 4C). Interestingly, expression of PDE4B2 did not induce IL-2 production in response to CD3 ligation alone in the absence of CD28 ligation, suggesting that the effect of de novo PDE4B2 expression cannot substitute for CD28-mediated costimulation but adds to it. Again, there was no enhancing effect of doxycycline on the responsiveness of parental (nontransfected) Jurkat E6.1 T cells under the specific stimulation conditions shown in Fig. 4C (data not shown). The enhanced IL-2 responsiveness observed in doxycycline-induced T cells upon activation with SEE and LG2 APCs was attributable to PDE4B2 expression, since it was eliminated by the addition of rolipram (data not shown).
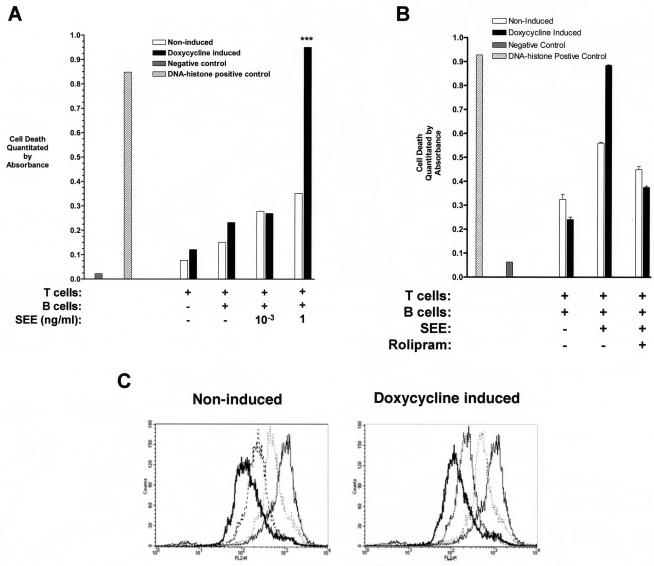
The increase in IL-2 production by PDE4B2-expressing T cells in response to low concentrations of SEE contrasted with a significant decrease in IL-2 production by these cells at higher levels of SEE (Fig. 4A). This suggested that the responding T cells were undergoing activation-induced death. To formally test this possibility, we examined the incidence of apoptosis in these cultures. As demonstrated in Fig. 5A, when the T cells induced to express PDE4B2 were exposed to high concentrations of SEE (1 ng/ml), they exhibited a significantly higher degree of apoptosis than matched, uninduced T cells not expressing PDE4B2. The enhanced apoptosis was not seen in the PDE4B2-expressing T cells when they were stimulated by low concentrations (10−3 ng/ml) of SEE, in the presence of LG2 APCs alone, or in cultures containing only the T cells. The increased activation-induced apoptosis in PDE4B2-expressing T cells stimulated with high concentrations of SEE was inhibited by rolipram (Fig. 5B). To exclude the possibility that overexpression of PDE4B2-GFP was affecting cell cycling, we looked at the kinetics of cell cycling by flow cytometry analysis of CMTMR-labeled T cells upon expression of wt PDE4B2-GFP. Tracking of CMTMR-labeled, PDE4B2-expressing and non-PDE4B2-expressing T cells over 24-, 48-, and 72-h periods by FACS did not reveal any significant difference in the cell division-cycling profiles of the two cell populations (Fig. 5C).
FIG. 5.
Enhanced activation-induced apoptosis in SEE-stimulated T cells expressing PDE4B2. (A) Jurkat T cells were incubated without or with (to induce wt PDE4B2-GFP expression) doxycycline (1 μg/ml) for 24 h. At that time, noninduced and doxycycline-induced wt PDE4B2-GFP T cells were subjected to stimulation with LG2 APCs (T-cell/APC ratio of 5:1) and different SEE concentrations (1 or 10−3 ng/ml) for 16 h. Cell lysates from these cultures were prepared, and cytoplasmic histone-associated DNA fragments indicative of apoptotic death were quantitated by ELISA. ***, P < 0.001. (B) Same experimental design as for panel A except that T cells were stimulated with LG2 APCs and SEE (1 ng/ml) in the absence or presence of 10 μM rolipram for the duration of the stimulation (16 h). (C) Cycling of nonstimulated, stably transfected wt PDE4B2-GFP T cells is not affected by wt PDE4B2-GFP expression. T cells were labeled with the nonspecific intracellular stain CMTMR, and cell cycling was determined by flow cytometry at 0 (grey line), 24 (dotted line), 48 (dashed line), and 72 (thick line) h. These experiments were performed in triplicate.
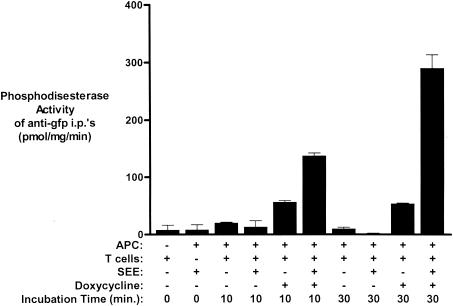
The enhanced IL-2 response seen in T cells expressing PDE4B2 upon activation with SEE and LG2 APCs correlated with an increase in PDE4B2 activity as indicated by the PDE activity detected in GFP immunoprecipitates after SEE stimulation (Fig. 6). We observed that by 10 min following stimulation with SEE and LG2 APCs, PDE4B2-GFP-expressing T cells had significantly higher PDE4B2 activity than was observed in doxycycline-induced, nonstimulated T cells or noninduced, transfected T cells (Fig. 6). The PDE activity further increased at 30 min (Fig. 6) and returned to basal levels by 18 h poststimulation (data not shown). Together, our findings demonstrate that T-cell activation through the TCR induces a specific increase in PDE4B2 activity that correlates with increased IL-2 production and activation-induced apoptosis.
FIG. 6.
Enhanced PDE4B2 activity following SEE stimulation of T cells expressing PDE4B2. PDE activity was quantitated in anti-GFP immunoprecipitates (i.p.'s) (i.e., specific PDE activity of PDE4B2-GFP) from wt PDE4B2-GFP-transfected Jurkat T cells (0.8 × 106) not induced or induced with doxycycline (2 μg/ml for 24 h prior to the assay) following 10 or 30 min of exposure to LG2 APCs (0.4 × 106) preincubated with SEE (10 ng/ml, 24 h prior to the assay). Concomitant assessment of the PDE activity of additional control immunoprecipitates was performed with doxycycline-induced and noninduced T-cell-APC cultures in the absence of SEE and with LG2 APCs and SEE incubated in the absence of T cells. Determination of the PDE4 activity of the various samples was performed in quadruplicate. Error bars indicate standard deviations.
Dynamic distribution of PDE4B2-GFP during TCR-mediated signaling.
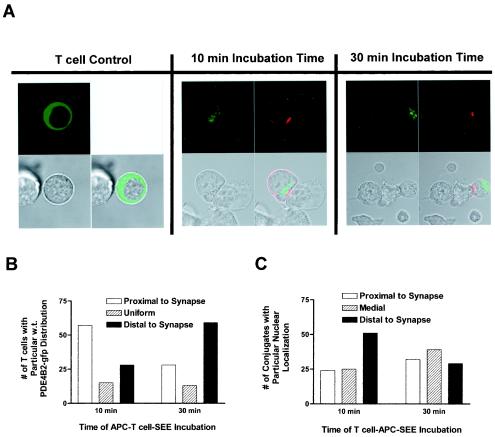
The increase in PDE4B2 activity in response to TCR-dependent stimulation was compatible with the claim that this enzyme compartmentalized to the vicinity of engaged TCRs, at the interface between the T cell and the APC, known as the immunological synapse. Therefore, we examined the distribution of PDE4B2-GFP in relation to the immunological synapse during SEE-induced T-cell activation. Under resting conditions, PDE4B2-GFP was distributed in the periphery of the cytoplasm (Fig. 2B and 7A). However, as TCR signaling progressed, we observed that PDE4B2-GFP redistributed in the T cell in relation to the immunological synapse (Fig. 7A). After 10 min of TCR-dependent stimulation, a time in which TCR redistribution to the T-cell-APC interface was not complete, the majority of PDE4B2-GFP localized in an area underneath the immature synapse in most of the T cells interacting with APC. In contrast, after 30 min of TCR-dependent stimulation, a time at which TCR clustering at the immunological synapse was apparent, we observed that PDE4B2-GFP was distributed predominantly in the antipodal pole, distal to the maturing synapse, in most T cells. A quantitative assessment of this distribution is shown in Fig. 7B. Proximal distribution was defined as when more than 50% of the GFP signal was located on the side of the synapse, distal distribution was when more than 50% of the GFP signal was distributed in the pole of the T cell distal to the synapse, and uniform distribution was the state in which the GFP signal was equally distributed in the synapse pole and the antipodal pole. The apparent change in the distribution of PDE4B2-GFP during the TCR-dependent stimulation with SEE and APCs was not due to nuclear movement, since tracking of the stained nucleus under the same SEE-APC stimulation conditions did not reveal any specific pattern of directionality (Fig. 7C). Furthermore, the pattern of PDE4B2-GFP distribution in T cells was not due to GFP itself, since this molecule distributed homogeneously in the T cell (Fig. 2A) and stimulation had no effect on this distribution (data not shown).
FIG. 7.
Kinetics of wt PDE4B2-GFP redistribution during early TCR signaling and immunological synapse formation. (A) Confocal analysis of PDE4B2-GFP distribution during synapse formation. Doxycycline-induced T cells stably transfected with wt PDE4B2-GFP (1 μg of doxycycline per ml for 24 h) were incubated with SEE-treated LG2 APCs (100 ng of SEE per ml for 24 h) for 10 or 30 min (in the continuing presence of doxycycline). The T-cell control was induced, nonstimulated T cells. Subsequently, the T-cell-APC conjugates attached to the polylysine-coated confocal dishes were fixed and stained with PE-conjugated anti-CD3. CD3 location and GFP signal were assessed with a Zeiss LSM510 confocal microscope (63× objective). Upper left panels, green fluorescence; upper right panels, red fluorescence; lower left panels, bright phase; lower right panels, overlays of all three pictures. (B) Quantitation of wt PDE4B2-GFP distribution during synapse formation. One hundred immunological synapses were analyzed, and the distribution of wt PDE4B2-GFP was noted. Proximal distribution was defined as 50% or greater GFP signal in the half of the T cell close to the T-cell-APC interface. Distal distribution was defined as 50% or greater GFP signal in the half of the T cell antipolar to the synapse. Uniform distribution was defined as GFP signal homogeneously distributed throughout the T cell. (C) Fluorescence microscope analysis of nuclear distribution during synapse formation. T cells and LG2 APCs were prepared and coincubated as described for panel A with the exception that before the T-cell-APC coincubation for either 10 or 30 min, the doxycycline-induced T cells stably transfected with wt PDE4B2-GFP were stained with Hoechst 33342 nuclear stain. The nuclear distribution and PE-labeled TCR (CD3) of 100 T-cell-APC conjugates were visualized with a Zeiss FSET01 fluorescence microscope (63× objective), and the distribution of stained nucleus with relation to the PE-labeled TCR was noted. Proximal distribution is defined as when the nucleus is found in the half of the T cell that juxtaposes the synapse, while distal distribution is defined as when the nucleus is found in the half of the T cell that is polar to the synapse. Medial location was defined as central location of the nucleus. All confocal microscopy studies for this figure were performed in triplicate, and the photographs are representative of all three viewings.
The UCR2 domain of PDE4B2 is required for enhancement of T-cell activation.
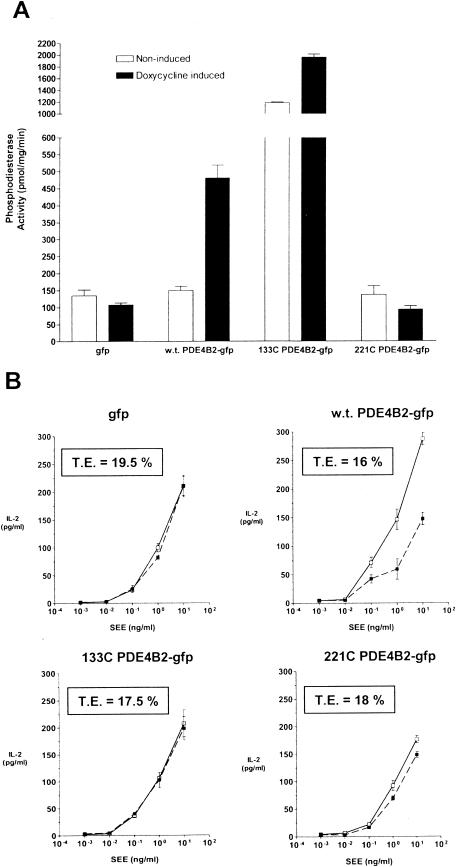
The observation of dynamic redistribution of PDE4B2 during TCR-dependent stimulation was consistent with specific intracellular targeting of this enzyme. To corroborate the importance of such compartmentalization, we deleted the N terminus of PDE4B2, since this region regulates the molecular interactions and intracellular targeting of PDE enzymes (4, 11, 17, 21, 24). Two mutant forms of PDE4B2 were studied (Fig. 1A). One is a mutant that lacks the first 133 amino acids of the UCR2 domain of PDE4B2 but still possesses a fully functional PDE catalytic domain that has been crystallized (133C PDE4B2-GFP) (56). The other is a mutant that lacks the entire UCR2 and linking region (221C PDE4B2-GFP). When Jurkat T cells transiently transfected with 133C PDE4B2-GFP were tested for PDE activity, we observed a high level of PDE activity (Fig. 8A). Such an increase in PDE activity following deletion and/or mutation of the N terminus has been previously reported for PDE3 and PDE4 (24, 25, 43). Despite the dramatic increase in PDE activity, this mutant failed to enhance the production of IL-2 in response to SEE and APCs (Fig. 8B). The possibility that the high PDE activity in this mutant causes desensitization of the T cell is unlikely, since the IL-2 responsiveness to SEE for this mutant was not different from that seen in cells transiently transfected with wt PDE4B2 under basal conditions, despite an additional increase in PDE activity following doxycycline induction. The 221C PDE4B2-GFP did not exhibit significant PDE activity and had no effect on T-cell activation (Fig. 8). Transfection efficiencies, as determined by fluorescence microscopy, were comparable among the different groups. Together, these findings demonstrate that the enhancing effect of PDE4B2 on T-cell activation is not the result of a nonspecific increase of PDE activity but requires the UCR2 of PDE4B2.
FIG.8.
UCR2 of PDE4B2 is required for the enhancement of T-cell activation. (A) PDE activity of T cells transiently transfected with wt PDE4B2-GFP, 133C PDE4B2-GFP, or 221C PDE4B2-GFP. PDE activity in cell lysates obtained from these Jurkat T cells cultured in the absence or presence of doxycycline was quantitated and normalized as described in Materials and Methods. As a control, cells transiently transfected with GFP alone were used. The transiently transfected T-cell lines were induced appropriately at 0.75 μg of doxycycline per ml for 24 h prior to PDE activity quantitation of the cell lysates. The data are representative of those from three separate transient transfections. (B) The enhancing effect of PDE4B2 on IL-2 production requires an intact UCR2. IL-2 production by these transient transfectants in response to SEE and LG2 APCs, both under basal conditions and following doxycycline induction, was determined by ELISA. The transiently transfected T-cell lines were induced as appropriate at 0.75 μg of doxycycline per ml for 24 h prior to exposure to SEE-pretreated (24 h prior assay) LG2 cells at a T-cell/LG2 APC ratio of 5:1. With doxycycline levels being maintained (0.75 μg/ml) throughout the assay where appropriate, culture supernatants were collected after 14 h and quantitated for IL-2 production. Individual transfection efficiencies (T.E.) were determined by fluorescence microscopy and counting of 10 representative fields of 100 cells each. The data are representative of those from three separate transient transfections. Error bars indicate standard deviations.
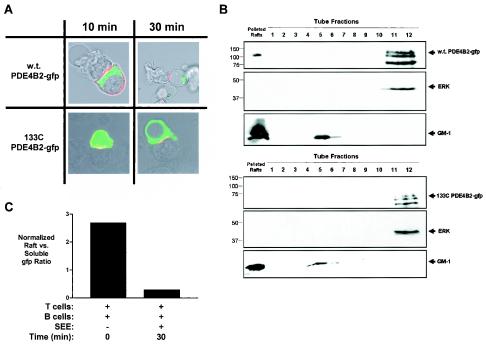
To document the intracellular targeting of wt PDE4B2-GFP and its loss in the 133C PDE4B2-GFP mutant, we examined the distribution of these PDE4B2 forms during T-cell activation by confocal microscopy. In contrast to the wt PDE4B2-GFP redistribution, the 133C mutant did not change location during SEE stimulation (Fig. 9A). In addition, and since we failed to see an association between the TCR and PDE4B2-GFP by conventional coprecipitation studies, we decided to establish the subcellular compartmentalization of PDE4B2 in relation to lipid rafts. These membrane microdomains have been linked to signaling from surface receptors, including the TCR, and are enriched in molecules involved in signaling from these receptors (10). We observed that wt PDE4B2-GFP was detectable both in the soluble fraction and in the lipid raft fraction (Fig. 9B). In contrast, the 133C mutant was observed only in the soluble fraction. Together, the results from confocal microscopy and subcellular fractionation demonstrate the differential intracellular targeting and subcellular compartmentalization of the wt PDE4B2 and the 133C mutant PDE4B2 chimeric molecules. In addition, we examined the changes in the distribution of PDE4B2 during T-cell activation. We found that there was a decrease in the presence of PDE4B2 in lipid rafts but not in the soluble fraction with time (Fig. 9C). The basis for such a decrease is presently under investigation and may be due to targeting to the cytoskeletal fraction.
FIG. 9.
wt PDE4B2-GFP is found within the immunological synapse and lipid rafts, while the N-terminal deletion form of PDE4B2 (133C) is not. (A) Localization of wt PDE4B2-GFP or 133C PDE4B2-GFP in relation to the T-cell-APC interface was determined by confocal microscopy. Doxycycline-induced (1 μg of doxycycline per ml for 24 h) T cells stably transfected with wt PDE4B2-GFP or 133C PDE4B2-GFP were exposed for either 10 or 30 min to LG2 APCs preincubated with 100 ng of SEE per ml (24 h prior to the assay). Subsequently, the T-cell-APC conjugates attached to the polylysine-coated confocal dishes were fixed and stained with PE-conjugated anti-CD3. CD3 location (red fluorescence) and GFP signal (green fluorescence) were assessed with a Zeiss LSM510 confocal microscope (63× objective). (B) Differential targeting of wt PDE4B2-GFP and 133C PDE4B2-GFP was determined for resting T cells by performing a lipid raft fractionation. T cells (100 × 106) stably transfected with wt PDE4B2-GFP or 133C PDE4B2-GFP were induced with 2 μg of doxycycline per ml for 48 h. Lysates were fractionated by sucrose gradient centrifugation to isolate the lipid raft compartments. The pelleted rafts and 20-μl aliquots of the 1-ml fractions were immunoblotted for GFP (upper panels), total ERK (middle panels), and GM-1 (lower panels). (C) The amount of PDE4B2-GFP in lipid rafts decreases upon stimulation. PDE4B2-GFP-transfected T cells were stimulated with APCs and SEE (100 ng/ml). Lipid raft and soluble fractions were isolated as described for panel B and sequentially immunoblotted for GFP, ERK-1 and -2, and GM-1. Signals were quantitated by densitometry, and the GFP signal was normalized for each fraction by using the GM-1 and total ERK signals as controls for rafts and soluble fractions, respectively.
Endogenous PDE4B2 in primary human T cells localizes in lipid raft microdomains on the cell membrane.
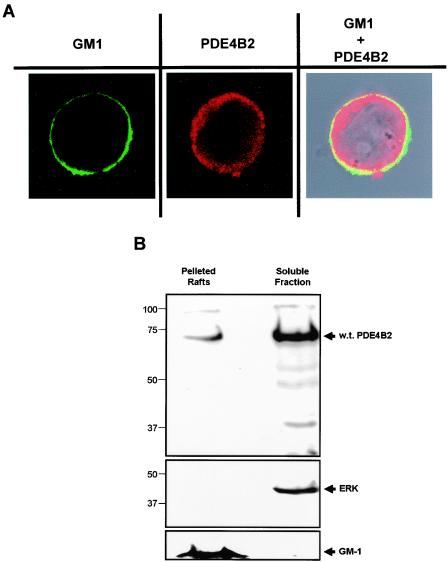
To confirm that the previous results were applicable to normal human T cells, we assessed the distribution of endogenous PDE4B2 in primary T cells by confocal microscopy and subcellular fractionation. We observed that endogenous PDE4B2 localized in the periphery of T cells and colocalized with GM-1, a marker of lipid rafts (Fig. 10A). Although most of the endogenous PDE4B2 was localized within the soluble fraction, we consistently detected a pool within the lipid rafts of human T cells (Fig. 10B). Together, the results from confocal microscopy and subcellular fractionation validate the intracellular targeting and subcellular compartmentalization reported here for the recombinant PDE4B2-GFP in Jurkat T cells.
FIG. 10.
Endogenous PDE4B2 is found within lipid rafts of human peripheral blood T-cell blasts. (A) Localization of endogenous PDE4B2 in resting T-cell blasts was assessed by confocal microscopy. Lipid rafts were identified by using fluorescein isothiocyanate-labeled cholera toxin, which binds to GM-1 (left panel). The PDE4B2 distribution (center panel) was determined by using a polyclonal anti-PDE4B2 rabbit serum and an Alexa Fluor 633-labeled secondary antibody. The GM-1-PDE4B2 overlay is shown in the right panel. T cells (106) attached to a poly-l-lysine matrix were examined with a Zeiss LSM510 confocal microscope (63× objective). (B) Endogenous PDE4B2 is detected in the lipid raft compartment of T-cell blasts. Lysates from 250 × 106 T cells were fractionated by sucrose gradient centrifugation to isolate the lipid raft fraction. The pelleted rafts and the soluble fraction were immunoblotted for PDE4B2 (upper panel), for total ERK (middle panel), or for GM-1 (lower panel).
DISCUSSION
Very little is known about the molecular mechanisms involved in the tight regulation of cAMP levels during T-cell activation through the TCR. Abundant evidence implicates a biochemical pathway that coordinates the activation of PDEs of the PDE3, PDE4, and PDE7 families to limit the magnitude and the duration of the increase in cAMP levels resulting from TCR signaling (6, 18, 39, 49). However, direct proof for the existence of such a pathway is still missing, and the particular PDEs that are involved remain uncharacterized. Here, we describe an inducible experimental system to study the biological effects of de novo expression of a PDE in T cells and correlate these effects with the amount of enzyme being expressed and its spatiotemporal distribution under conditions of T-cell activation. Using this system, we show that de novo expression of PDE4B2, a PDE previously reported to be associated with the TCR complex (3), enhances T-cell activation and increases the production of IL-2 and the subsequent activation-induced apoptosis in response to stimulation with SEE and APCs. Such an enhanced response correlates with an increase in PDE4B2 activity triggered by TCR signaling. We also demonstrate that PDE4B2 partitions in the soluble fraction and in lipid rafts and redistributes upon T-cell stimulation first to the vicinity of the immunological synapse and subsequently to the antipodal pole of the synapse, implying sequential changes in the location of PDE-dependent signaling events from the immunological synapse to other parts of the cell.
The enhancing effect of PDE4B2 on T-cell activation was apparent under conditions in which CD3 and CD28 signaling was not limiting. However, de novo expression of PDE4B2 was not sufficient to obviate the need for CD28 costimulation, because it did not have any effect when only CD3 was ligated. These findings extend the previous observation that PDE7 and PDE8, other PDEs expressed in T lymphocytes, participate in CD3- and CD28-induced proliferation (19, 28). It is of interest that PDE4B2 had an effect on T-cell activation above and beyond that contributed by endogenous PDEs, including PDE7 and PDE8. However this effect is not just the result of providing more PDE activity to the T cell, since no enhancement of IL-2 production was seen in the presence of untargeted PDE activity due to the lack of the specific UCR2 of PDE4B2. Based on our evidence linking PDE4B2 with TCR signaling (3) and the increase in the activity of the PDE4B2-GFP construct beyond that of the endogenous PDEs, we propose that upon TCR engagement, the enhancing effect of de novo expression of PDE4B2 on SEE-induced T-cell activation results from an increased availability of appropriately targeted PDE activity. This claim is consistent with the lack of such an enhancing effect in the absence of the UCR2 region of PDE4B2 (a region of the molecule involved in the regulation of PDE4 localization and interactions [4, 21]) despite the high catalytic activity of this mutant.
The molecular basis of the PDE4B2 effect on TCR-dependent T-cell activation is not known. Although we have reported phosphorylation of PDE4B2 upon TCR ligation in primary cells (3), we failed to see it consistently in the chimeric PDE4B2 form (data not shown), which may be due to the GFP fusion close to the tyrosine phosphorylation site on the C terminus of PDE4B2. However, previous studies have shown that in T cells, cAMP-elevating agents, such as phorbol 12-myristate 13-acetate and forskolin, suppresses tyrosine phosphorylation and activation of phospholipase C γ-1 (PLCγ-1) in response to TCR signaling (37). This mechanism may involve the activation of Rap-1 through protein kinase A (PKA) activation of Src kinases (41). Given the critical role of PLCγ1 in T-cell proliferation and differentiation, a lack of PLCγ-1 activity would lead to a decreased amount of IL-2 being produced. Therefore, one would expect that an increase in PDE4B2 activity leads to a concomitant decrease in cAMP levels within T cells and thus increases PLCγ1 activity. An additional mechanism that may explain the increased IL-2 production upon stimulation of the PDE4B2-expressing T cells may be related to the inhibitory Src kinase Csk. It has been shown that high levels of cAMP in T cells increase PKA-dependent serine phosphorylation and activation of Csk (54). The increased Csk activity results in tyrosine phosphorylation of residue Y505 of Lck and subsequent inhibition of Lck- and TCR-mediated signaling (54). Thus, attenuation of cAMP levels in the T cell by PDE4B2 could lead to reduced activity of Csk and consequently to an increase in Lck activity that would enable effective TCR-mediated signaling and production of IL-2.
In this paper, we describe the distribution of PDE4B2 during T-cell activation. In resting T cells, we found that PDE4B2 was distributed preferentially in peripheral areas of the cytosol as determined by confocal microscopy. The predominant cytoplasmic distribution of PDE4B2-GFP correlated biochemically with the partitioning of PDE4B2-GFP to the soluble subcellular fraction (31, 44). However, a consistent pool of wt PDE4B2 was found within membrane lipid raft microdomains. The physiological validity of such a distribution was confirmed in primary human T cells by confocal microscopy and by subcellular fractionation. The small pool of wt PDE4B2 in lipid rafts in resting cells may be determined by the potential myristoylation sites present in the N terminus of PDE4B2 (3), and this would explain why lipid raft association is lost in the 133C mutant form of PDE4B2. Our combined data also suggest that lipid raft association of PDE4B2 may be required for initial modulation of the T-cell response following specific stimulation by SEE and APCs. The requirement for appropriate compartmentalization of PDE4B2 in T-cell regulation and signaling was suggested by modulation of IL-2 expression in the presence of wt PDE4B2-GFP following specific T-cell stimulation but lack of IL-2 modulation in the presence of the 133C mutant, despite its significantly high levels of catalytic PDE activity.
Following T-cell stimulation through the TCR, PDE4B2 undergoes intracellular redistribution. TCR signaling induces significant cytoskeletal rearrangement and polarization of the T cell that culminates in the formation of a mature immunological synapse between the T cell and the APC within 30 min of TCR ligation (15, 20, 33, 53). One could argue that the changes in the distribution of PDE4B2-GFP reported here reflect just its passive movement forced by intracellular reorganization. However, this is unlikely, since this pattern of distribution was seen at low levels of PDE4B2-GFP expression, which would not affect the intracellular space for organelle movement. In addition, the changes in PDE4B2-GFP localization at early and late time points of T-cell activation were complete in most T cells and did not correlate with modifications in the location of the nucleus, which in Jurkat T cells occupies the majority of the intracellular space. Thus, we propose that the changes in the distribution of PDE4B2-GFP in relation to the immunological synapse reflect active compartmentalization of PDE4B2 to sites where cAMP is generated following TCR engagement.
The proposed compartmentalization of PDE4B2-GFP close to the immunological synapse and to sites of maximal cAMP generation at early time points of T-cell activation is particularly attractive. Multiple studies have shown that the peak production of cAMP is reached after 5 min of TCR ligation (3, 8, 26). This time frame is compatible with our finding of PDE4B2-GFP aggregation in the periphery of the synapse after the onset of T-cell stimulation. It is also consistent with the finding of PDE4B2 within lipid rafts, since these microdomains cluster at the immunological synapse upon T-cell stimulation (55) and subsequently decrease. A similar compartmentalization phenomenon has been suggested from the interaction between β-arrestin and PDE4D, which determines recruitment to engaged receptors and sites of localized PKA activity and subsequent cAMP degradation (38). Our proposal is also compatible with data suggesting the formation of compartmentalized cAMP microdomains in a gradient-like fashion (57). These cAMP “pockets” may trigger the redistribution of PDE4B2 to the area where cAMP is generated, as cAMP-dependent activation of PKA has been implicated in clustering of CD3, CD4, and CD8 at the cell surface following TCR ligation (23).
A surprising finding of our studies is the accumulation of PDE4B2-GFP in the antipodal pole of the T cell at a time in which lipid raft association is also decreased (Fig. 9C). This antipodal localization has been reported for some surface receptors, and, more importantly, for some second messengers, as activation proceeds following T-cell stimulation. For example, it has been recently reported that following TCR signaling, some cell surface receptors such as CD43, CD44, and CD50, and specific pools of ezrin/radixin/moesin proteins that anchor these receptors to the cytoskeleton, redistribute to the antipodal pole of the T cell (2, 14, 40, 45, 51). Also, following TCR ligation, the second messenger PIP3 accumulates not only in the T-cell-APC interface but also in the antipodal pole of the T cell (12). Since the times at which these changes were observed are within the required TCR signaling window for T-cell commitment to proliferation and differentiation (up to 30 min), we conclude that the antipodal pole of the T cell may be an important signaling site during T-cell activation and that the signaling events at that location may include the generation of cAMP and accumulation of active PDE4B2 (as suggested by the sustained increased in PDE4B2 activity at a time point of such redistribution [Fig. 6]).
In summary, we have shown that de novo expression of PDE4B2 in T cells is associated with enhanced responsiveness to TCR stimulation. Such an effect is not due to changes in the cycling ability of the T cell or to a nonspecific increase of PDE activity but correlates with TCR-induced activation of PDE4B2 and its compartmentalization within lipid rafts and dynamic targeting within the T cell during T-cell activation. Our data provide, for the first time, direct evidence that the specific involvement of PDE4B2 in TCR signaling and T-cell activation requires selective intracellular targeting of this enzyme in a dynamic fashion.
Acknowledgments
We thank Gregory J. Kato and Donald H. Maurice for helpful comments on the manuscript. We also thank the members of the Madrenas laboratory (in particular Kristi A. McConnell, Peter J. Darlington, and John Johnson) for fruitful discussions on PDEs.
This work was supported by grants from the Canadian Institutes of Health Research (CIHR), the Kidney Foundation of Canada, and the Multi-Organ Transplant Program of the London Health Sciences Centre. MG.K. is the recipient of a CIHR M.D./Ph.D. studentship, and J.M. holds a Canada Research Chair in Transplantation and Immunobiology.
REFERENCES
- 1.Aandahl, E. M., W. J. Moretto, P. A. Haslett, T. Vang, T. Bryn, K. Tasken, and D. F. Nixon. 2002. Inhibition of antigen-specific T cell proliferation and cytokine production by protein kinase A type I. J. Immunol. 169:802-808. [DOI] [PubMed] [Google Scholar]
- 2.Allenspach, E. J., P. Cullinan, J. Tong, Q. Tang, A. G. Tesciuba, J. L. Cannon, S. M. Takahashi, R. Morgan, J. K. Burkhardt, and A. I. Sperling. 2001. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity 15:739-750. [DOI] [PubMed] [Google Scholar]
- 3.Baroja, M. L., L. B. Cieslinski, T. J. Torphy, R. L. Wange, and J. Madrenas. 1999. Specific CD3 epsilon association of a phosphodiesterase 4B isoform determines its selective tyrosine phosphorylation after CD3 ligation. J. Immunol. 162:2016-2023. [PubMed] [Google Scholar]
- 4.Beard, M. B., A. E. Olsen, R. E. Jones, S. Erdogan, M. D. Houslay, and G. B. Bolger. 2000. UCR1 and UCR2 domains unique to the cAMP-specific phosphodiesterase family form a discrete module via electrostatic interactions. J. Biol. Chem. 275:10349-10358. [DOI] [PubMed] [Google Scholar]
- 5.Bielekova, B., A. Lincoln, H. McFarland, and R. Martin. 2000. Therapeutic potential of phosphodiesterase-4 and -3 inhibitors in Th1-mediated autoimmune diseases. J. Immunol. 164:1117-1124. [DOI] [PubMed] [Google Scholar]
- 6.Bloom, T. J., and J. A. Beavo. 1996. Identification and tissue-specific expression of PDE7 phosphodiesterase splice variants. Proc. Natl. Acad. Sci. USA 93:14188-14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns, F., A. Z. Zhao, and J. A. Beavo. 1996. Cyclic nucleotide phosphodiesterases: gene complexity, regulation by phosphorylation, and physiological implications. Adv. Pharmacol. 36:29-48. [DOI] [PubMed] [Google Scholar]
- 8.Butman, B. T., T. Jacobsen, O. G. Cabatu, and L. Y. Bourguignon. 1981. The involvement of cAMP in lymphocyte capping. Cell. Immunol. 61:397-403. [DOI] [PubMed] [Google Scholar]
- 9.Chau, L. A., J. A. Bluestone, and J. Madrenas. 1998. Dissociation of intracellular signaling pathways in response to partial agonist ligands of the T cell receptor. J. Exp. Med. 187:1699-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherukuri, A., M. Dykstra, and S. K. Pierce. 2001. Floating the raft hypothesis: lipid rafts play a role in immune cell activation. Immunity 14:657-660. [DOI] [PubMed] [Google Scholar]
- 11.Conti, M., and S. L. Jin. 1999. The molecular biology of cyclic nucleotide phosphodiesterases. Prog. Nucleic Acid Res. Mol. Biol. 63:1-38. [DOI] [PubMed] [Google Scholar]
- 12.Costello, P. S., M. Gallagher, and D. A. Cantrell. 2002. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat. Immunol. 3:1082-1089. [DOI] [PubMed] [Google Scholar]
- 13.Darlington, P. J., M. L. Baroja, T. A. Chau, E. Siu, V. Ling, B. M. Carreno, and J. Madrenas. 2002. Surface cytotoxic T lymphocyte-associated antigen 4 partitions within lipid rafts and relocates to the immunological synapse under conditions of inhibition of T cell activation. J. Exp. Med. 195:1337-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delon, J., K. Kaibuchi, and R. N. Germain. 2001. Exclusion of CD43 from the immunological synapse is mediated by phosphorylation-regulated relocation of the cytoskeletal adaptor moesin. Immunity 15:691-701. [DOI] [PubMed] [Google Scholar]
- 15.Delon, J., S. Stoll, and R. N. Germain. 2002. Imaging of T-cell interactions with antigen presenting cells in culture and in intact lymphoid tissue. Immunol. Rev. 189:51-63. [DOI] [PubMed] [Google Scholar]
- 16.Erdogan, S., and M. D. Houslay. 1997. Challenge of human Jurkat T-cells with the adenylate cyclase activator forskolin elicits major changes in cAMP phosphodiesterase (PDE) expression by up-regulating PDE3 and inducing PDE4D1 and PDE4D2 splice variants as well as down-regulating a novel PDE4A splice variant. Biochem. J. 321:165-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis, S. H., I. V. Turko, and J. D. Corbin. 2001. Cyclic nucleotide phosphodiesterases: relating structure and function. Prog. Nucleic Acid Res. Mol. Biol. 65:1-52. [DOI] [PubMed] [Google Scholar]
- 18.Giembycz, M. A., C. J. Corrigan, J. Seybold, R. Newton, and P. J. Barnes. 1996. Identification of cyclic AMP phosphodiesterases 3, 4 and 7 in human CD4+ and CD8+ T-lymphocytes: role in regulating proliferation and the biosynthesis of interleukin-2. Br. J. Pharmacol. 118:1945-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glavas, N. A., C. Ostenson, J. B. Schaefer, V. Vasta, and J. A. Beavo. 2001. T cell activation up-regulates cyclic nucleotide phosphodiesterases 8A1 and 7A3. Proc. Natl. Acad. Sci. USA 98:6319-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grakoui, A., S. K. Bromley, C. Sumen, M. M. Davis, A. S. Shaw, P. M. Allen, and M. L. Dustin. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science 285:221-227. [DOI] [PubMed] [Google Scholar]
- 21.Houslay, M. D., and D. R. Adams. 2003. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem. J. 370:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houslay, M. D., M. Sullivan, and G. B. Bolger. 1998. The multienzyme PDE4 cyclic adenosine monophosphate-specific phosphodiesterase family: intracellular targeting, regulation, and selective inhibition by compounds exerting anti-inflammatory and antidepressant actions. Adv. Pharmacol. 44:225-342. [DOI] [PubMed] [Google Scholar]
- 23.Kammer, G. M., C. A. Boehm, S. A. Rudolph, and L. A. Schultz. 1988. Mobility of the human T lymphocyte surface molecules CD3, CD4, and CD8: regulation by a cAMP-dependent pathway. Proc. Natl. Acad. Sci. USA 85:792-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenan, Y., T. Murata, Y. Shakur, E. Degerman, and V. C. Manganiello. 2000. Functions of the N-terminal region of cyclic nucleotide phosphodiesterase 3 (PDE 3) isoforms. J. Biol. Chem. 275:12331-12338. [DOI] [PubMed] [Google Scholar]
- 25.Kovala, T., B. D. Sanwal, and E. H. Ball. 1997. Recombinant expression of a type IV, cAMP-specific phosphodiesterase: characterization and structure-function studies of deletion mutants. Biochemistry 36:2968-2976. [DOI] [PubMed] [Google Scholar]
- 26.Ledbetter, J. A., M. Parsons, P. J. Martin, J. A. Hansen, P. S. Rabinovitch, and C. H. June. 1986. Antibody binding to CD5 (Tp67) and Tp44 T cell surface molecules: effects on cyclic nucleotides, cytoplasmic free calcium, and cAMP-mediated suppression. J. Immunol. 137:3299-3305. [PubMed] [Google Scholar]
- 27.Lerner, A., D. H. Kim, and R. Lee. 2000. The cAMP signaling pathway as a therapeutic target in lymphoid malignancies. Leuk. Lymphoma 37:39-51. [DOI] [PubMed] [Google Scholar]
- 28.Li, L., C. Yee, and J. A. Beavo. 1999. CD3- and CD28-dependent induction of PDE7 required for T cell activation. Science 283:848-851. [DOI] [PubMed] [Google Scholar]
- 29.Manganiello, V. C., and E. Degerman. 1999. Cyclic nucleotide phosphodiesterases (PDEs): diverse regulators of cyclic nucleotide signals and inviting molecular targets for novel therapeutic agents. Thromb. Haemost. 82:407-411. [PubMed] [Google Scholar]
- 30.McLaughlin, M. M., L. B. Cieslinski, M. Burman, T. J. Torphy, and G. P. Livi. 1993. A low-Km, rolipram-sensitive, cAMP-specific phosphodiesterase from human brain. Cloning and expression of cDNA, biochemical characterization of recombinant protein, and tissue distribution of mRNA. J. Biol. Chem. 268:6470-6476. [PubMed] [Google Scholar]
- 31.McPhee, I., L. Pooley, M. Lobban, G. Bolger, and M. D. Houslay. 1995. Identification, characterization and regional distribution in brain of RPDE-6 (RNPDE4A5), a novel splice variant of the PDE4A cyclic AMP phosphodiesterase family. Biochem. J. 310:965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michie, A. M., G. Rena, M. M. Harnett, and M. D. Houslay. 1998. Upregulation of cAMP-specific PDE-4 activity following ligation of the TCR complex on thymocytes is blocked by selective inhibitors of protein kinase C and tyrosyl kinases. Cell Biochem. Biophys. 28:161-185. [DOI] [PubMed] [Google Scholar]
- 33.Monks, C. R., B. A. Freiberg, H. Kupfer, N. Sciaky, and A. Kupfer. 1998. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395:82-86. [DOI] [PubMed] [Google Scholar]
- 34.Moon, E. Y., and A. Lerner. 2003. PDE4 inhibitors activate a mitochondrial apoptotic pathway in chronic lymphocytic leukemia cells that is regulated by protein phosphatase 2A. Blood 101:4122-4130. [DOI] [PubMed] [Google Scholar]
- 35.Morgan, M. M., C. M. Labno, G. A. Van Seventer, M. F. Denny, D. B. Straus, and J. K. Burkhardt. 2001. Superantigen-induced T cell:B cell conjugation is mediated by LFA-1 and requires signaling through Lck, but not ZAP-70. J. Immunol. 167:5708-5718. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa, R., M. B. Streiff, A. Bugayenko, and G. J. Kato. 2002. Inhibition of PDE4 phosphodiesterase activity induces growth suppression, apoptosis, glucocorticoid sensitivity, p53, and p21(WAF1/CIP1) proteins in human acute lymphoblastic leukemia cells. Blood 99:3390-3397. [DOI] [PubMed] [Google Scholar]
- 37.Park, D. J., H. K. Min, and S. G. Rhee. 1992. Inhibition of CD3-linked phospholipase C by phorbol ester and by cAMP is associated with decreased phosphotyrosine and increased phosphoserine contents of PLC-gamma 1. J. Biol. Chem. 267:1496-1501. [PubMed] [Google Scholar]
- 38.Perry, S. J., G. S. Baillie, T. A. Kohout, I. McPhee, M. M. Magiera, K. L. Ang, W. E. Miller, A. J. McLean, M. Conti, M. D. Houslay, and R. J. Lefkowitz. 2002. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science 298:834-836. [DOI] [PubMed] [Google Scholar]
- 39.Robicsek, S. A., D. K. Blanchard, J. Y. Djeu, J. J. Krzanowski, A. Szentivanyi, and J. B. Polson. 1991. Multiple high-affinity cAMP-phosphodiesterases in human T-lymphocytes. Biochem. Pharmacol. 42:869-877. [DOI] [PubMed] [Google Scholar]
- 40.Roumier, A., J. C. Olivo-Marin, M. Arpin, F. Michel, M. Martin, P. Mangeat, O. Acuto, A. Dautry-Varsat, and A. Alcover. 2001. The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity 15:715-728. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt, J. M., and P. J. Stork. 2002. PKA phosphorylation of Src mediates cAMP's inhibition of cell growth via Rap1. Mol. Cell 9:85-94. [DOI] [PubMed] [Google Scholar]
- 42.Seybold, J., R. Newton, L. Wright, P. A. Finney, N. Suttorp, P. J. Barnes, I. M. Adcock, and M. A. Giembycz. 1998. Induction of phosphodiesterases 3B, 4A4, 4D1, 4D2, and 4D3 in Jurkat T-cells and in human peripheral blood T-lymphocytes by 8-bromo-cAMP and Gs-coupled receptor agonists. Potential role in beta2-adrenoreceptor desensitization. J. Biol. Chem. 273:20575-20588. [DOI] [PubMed] [Google Scholar]
- 43.Shakur, Y., K. Takeda, Y. Kenan, Z. X. Yu, G. Rena, D. Brandt, M. D. Houslay, E. Degerman, V. J. Ferrans, and V. C. Manganiello. 2000. Membrane localization of cyclic nucleotide phosphodiesterase 3 (PDE3). Two N-terminal domains are required for the efficient targeting to, and association of, PDE3 with endoplasmic reticulum. J. Biol. Chem. 275:38749-38761. [DOI] [PubMed] [Google Scholar]
- 44.Shakur, Y., M. Wilson, L. Pooley, M. Lobban, S. L. Griffiths, A. M. Campbell, J. Beattie, C. Daly, and M. D. Houslay. 1995. Identification and characterization of the type-IVA cyclic AMP-specific phosphodiesterase RD1 as a membrane-bound protein expressed in cerebellum. Biochem. J. 306:801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw, A. S. 2001. FERMing up the synapse. Immunity 15:683-686. [DOI] [PubMed] [Google Scholar]
- 46.Skalhegg, B. S., and K. Tasken. 2000. Specificity in the cAMP/PKA signaling pathway. Differential expression, regulation, and subcellular localization of subunits of PKA. Front. Biosci. 5:D678-D693. [DOI] [PubMed] [Google Scholar]
- 47.Strathdee, C. A., M. R. McLeod, and J. R. Hall. 1999. Efficient control of tetracycline-responsive gene expression from an autoregulated bi-directional expression vector. Gene 229:21-29. [DOI] [PubMed] [Google Scholar]
- 48.Sun, Y., L. Li, F. Lau, J. A. Beavo, and E. A. Clark. 2000. Infection of CD4+ memory T cells by HIV-1 requires expression of phosphodiesterase 4. J. Immunol. 165:1755-1761. [DOI] [PubMed] [Google Scholar]
- 49.Tenor, H., L. Staniciu, C. Schudt, A. Hatzelmann, A. Wendel, R. Djukanovic, M. K. Church, and J. K. Shute. 1995. Cyclic nucleotide phosphodiesterases from purified human CD4+ and CD8+ T lymphocytes. Clin. Exp. Allergy 25:616-624. [DOI] [PubMed] [Google Scholar]
- 50.Thompson, W. J., C. P. Ross, W. J. Pledger, S. J. Strada, R. L. Banner, and E. M. Hersh. 1976. Cyclic adenosine 3′:5′-monophosphate phosphodiesterase. Distinct forms in human lymphocytes and monocytes. J. Biol. Chem. 251:4922-4929. [PubMed] [Google Scholar]
- 51.Tomas, E. M., T. A. Chau, and J. Madrenas. 2002. Clustering of a lipid-raft associated pool of ERM proteins at the immunological synapse upon T cell receptor or CD28 ligation. Immunol. Lett. 83:143-147. [DOI] [PubMed] [Google Scholar]
- 52.Torphy, T. J., and C. Page. 2000. Phosphodiesterases: the journey towards therapeutics. Trends Pharmacol. Sci. 21:157-159. [DOI] [PubMed] [Google Scholar]
- 53.van der Merwe, P. A., S. J. Davis, A. S. Shaw, and M. L. Dustin. 2000. Cytoskeletal polarization and redistribution of cell-surface molecules during T cell antigen recognition. Semin. Immunol. 12:5-21. [DOI] [PubMed] [Google Scholar]
- 54.Vang, T., K. M. Torgersen, V. Sundvold, M. Saxena, F. O. Levy, B. S. Skalhegg, V. Hansson, T. Mustelin, and K. Tasken. 2001. Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T cell receptor. J. Exp. Med. 193:497-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viola, A., S. Schroeder, Y. Sakakibara, and A. Lanzavecchia. 1999. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science 283:680-682. [DOI] [PubMed] [Google Scholar]
- 56.Xu, R. X., A. M. Hassell, D. Vanderwall, M. H. Lambert, W. D. Holmes, M. A. Luther, W. J. Rocque, M. V. Milburn, Y. Zhao, H. Ke, and R. T. Nolte. 2000. Atomic structure of PDE4: insights into phosphodiesterase mechanism and specificity. Science 288:1822-1825. [DOI] [PubMed] [Google Scholar]
- 57.Zaccolo, M., and T. Pozzan. 2002. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295:1711-1715. [DOI] [PubMed] [Google Scholar]