Abstract
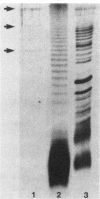
Lipopolysaccharide (LPS) from Haemophilus pleuropneumoniae 1536, serotype 2, was isolated and purified by a procedure designed to be equally satisfactory for both smooth- and rough-type LPS. The LPS yield was 53%. Analysis of the preparations revealed that protein, nucleic acid, and cellular phospholipid contamination was negligible (less than 0.1%). Analysis of the sugar content of the LPS by gas-liquid chromatography and colorimetric analysis revealed the presence of rhamnose, mannose, galactose, glucose, heptose, glucosamine, galactosamine, and 3-deoxy-D-manno-2-octulosonic acid. The heptose and glucose contents appeared to be unusually high. The fatty acids of the LPS consisted of a mixture of C14:0 and C16:0 in a ratio of about 4.5:1 (50% of the total) and 3-hydroxy C14:0. When used as a preparatory dose for the dermal Shwartzman reaction, as little as 10 micrograms of the LPS injected intradermally in rabbits produced reddening and swelling. After intravenous injection of a 100-micrograms LPS provoking dose, necrosis was observed at all intradermal injection sites. Limulus amebocyte lysate gelation was observed with an LPS concentration as low as 0.5 ng/ml. A typical biphasic fever response was noted in rabbits injected with as little as 0.25 ng of LPS per kg of body weight.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afzal M., Tengerdy R. P., Squire P. G., Ellis R. P. Characterization of Brucella ovis lipopolysaccharide and its use for diagnosis of ram epididymitis by enzyme-linked immunosorbent assay. J Clin Microbiol. 1984 Dec;20(6):1159–1164. doi: 10.1128/jcm.20.6.1159-1164.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade H., Galanos C., Lüderitz O. Differential determination of the 3-Deoxy-D-mannooctulosonic acid residues in lipopolysaccharides of Salmonella minnesota rough mutants. Eur J Biochem. 1983 Mar 1;131(1):195–200. doi: 10.1111/j.1432-1033.1983.tb07249.x. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichberg J., Mokrasch L. C. Interference by oxidized lipids in the determination of protein by the Lowry procedure. Anal Biochem. 1969 Sep;30(3):386–390. doi: 10.1016/0003-2697(69)90131-6. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Flesher A. R., Insel R. A. Characterization of lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1978 Dec;138(6):719–730. doi: 10.1093/infdis/138.6.719. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Herd J. K. Interference of hexosamines in the Lowry reaction. Anal Biochem. 1971 Dec;44(2):404–410. doi: 10.1016/0003-2697(71)90227-2. [DOI] [PubMed] [Google Scholar]
- Inzana T. J. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983 Sep;148(3):492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- Inzana T. J., Seifert W. E., Jr, Williams R. P. Composition and antigenic activity of the oligosaccharide moiety of Haemophilus influenzae type b lipooligosaccharide. Infect Immun. 1985 May;48(2):324–330. doi: 10.1128/iai.48.2.324-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Dur A., Chaby R., Szabó L. Isolation of two protein-free and chemically different lipopolysaccharides from Bordetella pertussis phenol-extracted endotoxin. J Bacteriol. 1980 Jul;143(1):78–88. doi: 10.1128/jb.143.1.78-88.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Parr T. R., Jr, Bryan L. E. Lipopolysaccharide composition of three strains of Haemophilus influenzae. Can J Microbiol. 1984 Sep;30(9):1184–1187. doi: 10.1139/m84-185. [DOI] [PubMed] [Google Scholar]
- Raichvarg D., Brossard C., Agneray J. Chemical composition and biological activities of a phenol-water extract from Haemophilus influenzae type a. Infect Immun. 1979 Nov;26(2):415–421. doi: 10.1128/iai.26.2.415-421.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp V. J., Ross R. F., Erickson B. Z. Serotyping of Haemophilus pleuropneumoniae by rapid slide agglutination and indirect fluorescent antibody tests in swine. Am J Vet Res. 1985 Jan;46(1):185–192. [PubMed] [Google Scholar]
- SHOPE R. E. PORCINE CONTAGIOUS PLEUROPNEUMONIA. I. EXPERIMENTAL TRANSMISSION, ETIOLOGY, AND PATHOLOGY. J Exp Med. 1964 Mar 1;119:357–368. doi: 10.1084/jem.119.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Wilkinson S. G., Galbrath L. Studies of lipopolysaccharides from Pseudomonas aeruginosa. Eur J Biochem. 1975 Mar 17;52(2):331–343. doi: 10.1111/j.1432-1033.1975.tb04001.x. [DOI] [PubMed] [Google Scholar]
- Wright B. G., Rebers P. A. Procedure for determining heptose and hexose in lipopolysaccharides. Modification of the cysteine-sulfuric acid method. Anal Biochem. 1972 Oct;49(2):307–319. doi: 10.1016/0003-2697(72)90433-2. [DOI] [PubMed] [Google Scholar]