Abstract
Slow nonselective cation conductances play a central role in determining the excitability of many neurons, but heretofore this channel type has not been analyzed at the single-channel level. Neurotensin (NT) excites cultured dopaminergic neurons from the ventral tegmental area primarily by increasing such a cation conductance. Using the outside–out configuration of the patch clamp, we elicited single-channel activity of this NT-induced cation channel. Channel activity was blocked by the nonpeptide NT antagonist SR48692, indicating that the response was mediated by NT receptors. The channel opened in both solitary form and in bursts. The reversal potential was −4.2 ± 1.7 mV, and the elementary conductance was 31 pS at −67 mV with [Na+]o = 140 mM, [Cs+]o = 5 mM, [Na+]i = 88 mM, and [Cs+]i = 74 mM. Thus, the channel was permeable to both Na+ and Cs+. From these characteristics, it is likely that this channel is responsible for the whole-cell current we studied previously. In guanosine 5′-[γ-thio]triphosphate-loaded cells, NT irreversibly activated about half of the channel activity, suggesting that at least part of the response was mediated by a G protein. Similar channel activity could be induced occasionally in the cell-attached configuration by applying NT outside the patch region.
Keywords: cell culture, G protein, ventral tegmental area, outside–out patch, neurotensin antagonist
Neurotensin (NT), a peptide neurotransmitter originally isolated from bovine hypothalamus (1), excites many central nervous system neurons (2–6) including dopaminergic neurons in the ventral tegmental area (VTA) (7–9). The mechanism by which NT excites VTA neurons has been shown to be an overlapping increase of nonselective cation conductance and decrease of inward rectifier K+ conductance (8, 9).
We previously used whole-cell recording to study the nonselective conductance increased by NT in cultured VTA neurons (9). The conductance was equally permeable to Na+, K+, and Cs+, but was impermeable to Cl−, indicating that it was indeed a nonselective cation conductance. Activation of the NT-induced nonselective current did not involve cAMP, cGMP, or internal Ca2+, while the latency of activation was long, indicating the involvement of second messenger(s). Therefore, this NT-induced nonselective cation conductance might be a member of a unique class of slow nonselective cation conductance, different from ligand-gated (10, 11), cyclic nucleotide-gated (12), and calcium-activated nonselective (13) cation conductances.
The purpose of this paper was to characterize the single-channel properties of the nonselective cation conductance induced by NT. We also examined the involvement of G proteins in the NT response.
MATERIALS AND METHODS
VTA neurons were cultured from 2- to 4-day-old postnatal Long-Evans rats (Charles River Breeding Laboratories) as described (14) except that 2% (instead of 5%) rat serum was used, and neurons were dissociated with 12 (instead of 20) units/ml of papain. We recorded from large neurons cultured for ≈2 weeks (soma diameter ≈ 21 μm), ≈75% of which are dopaminergic (9).
Recordings were made using the outside–out or cell-attached configuration of the patch clamp (15). Single-channel currents were recorded on videotape from a List EPC-7 amplifier (List Electronic, Darmstadt, Germany) and analyzed by using pclamp software (Axon Instruments, Foster City, CA) as described (16). NT (10 nM) was applied by pressure ejection (0.5 psi) from glass pipettes with a tip diameter of 3–4 μm, placed ≈45 μm from the patch. Experiments were conduced at ≈23°C. Values are given as mean ± SEM.
The standard pipette solution for whole-cell and outside–out recording contained 144 mM K-d-gluconate, 8.5 mM Na-d-gluconate, 1.5 mM NaCl, 5 mM Hepes·KOH, 0.5 mM EGTA·KOH, 0.25 mM CaCl2, 3 mM MgCl2, 2 mM Na2ATP, and 100 μM Na3GTP (pH 7.2). When guanosine 5′-[γ-thio]triphosphate (GTP[γS]; 100 μM) was used, GTP was omitted. A Cs+-containing internal solution used for determining the reversal potential contained 74 mM CsCl, 80 mM NaCl, 5 mM Hepes·NaOH, 0.5 mM EGTA·NaOH, 0.25 mM CaCl2, 3 mM MgCl2, 2 mM Na2ATP, and 100 μM Na3GTP (pH 7.2).
In early experiments, Ca2+ was omitted from the standard external solution because our previous whole-cell data suggested that the NT-induced conductance is blocked by external Ca2+. This nominally Ca2+-free external solution contained 155 mM NaCl, 5 mM KCl, 1.3 mM MgCl2, 5 mM Hepes·NaOH (pH 7.4) and had a Ca2+ concentration of ≈1 μM, measured by Ca2+ electrode. In later experiments, external Ca2+ was buffered to 1 μM with EGTA. This Ca2+-buffered external solution contained 140 mM NaCl, 5 mM KCl, 4.7 mM CaCl2, 5 mM EGTA·NaOH, 1.3 mM MgCl2, and 5 mM Hepes·NaOH (pH 7.4). To determine the reversal potential, we used a Cs+-containing external solution, in which KCl was replaced by CsCl. Tetrodotoxin (0.5 μM) was added for all recordings. The membrane potential was corrected for the liquid junction potential as measured in reference to a saturated KCl microelectrode.
When analyzing burst time, we used a 3-kHz filter (−3 db by 8-pole Bessel filter) with 25-kHz digitization, and the level changes registered if longer than 40 μsec. As will be described, the close time histogram was fit by two exponentials with time constants τg,f (short gaps) and τg,s (long closings), indicating that the channel opened in bursts, with the long openings interrupted by brief closures. After measuring close times, burst times were determining by analyzing the same data file, but this time a level change from the open state was registered only when the transition from the open state lasted longer than a critical time (τc) (17). For the rest of the experiments, the frequency response was 2 kHz with 20-kHz digitization, and level changes registered if longer than 100 μsec.
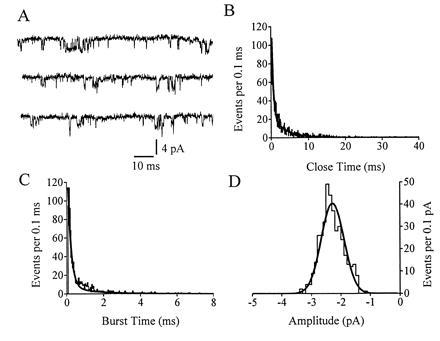
Npo was calculated by (18):
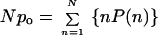 |
in which N is the number of the channels in the patch, po is the open probability of an individual channel, and P(n) is the probability of the record staying at a level at which n channels open simultaneously.
The chemicals used were NT (Peninsula Laboratories) and GTP[γS] (Sigma). The nonpeptide NT antagonist SR48692 was obtained from Sanofi Recherche (Tolouse, France).
RESULTS
NT-Induced Channel Activity.
As previously described (9), NT induced an inward current in whole-cell recording (Fig. 1A). The average whole-cell response induced by 10 nM NT was 240 ± 154 pA (n = 5). NT applied to outside–out membrane patches induced long-lasting channel activity (Fig. 1B1). On an expanded time base, Fig. 1B2 shows that there was no channel activity before NT application, while channel activity occurred after NT was applied (Fig. 1B3). Fig. 1B4 shows the change of Npo induced by NT. Npo reached a peak (≈0.1) 25 sec after NT application, then decreased to zero after ≈60 sec. More than half of the outside–out patches responded to NT (28/52). We also investigated whether the nonselective channel could be induced using the cell-attached configuration, with NT (1 μM) applied outside the pipette. No channel activity was detected in our original experiments (n = 32). However, in later experiments a few cell-attached patches (4/22) responded to NT (Fig. 1C). The rest of the experiments were done in the outside–out configuration.
Figure 1.
NT (10 nM)-induced channel activity. (A) In whole-cell recording, NT elicited an inward current. Holding potential was −79 mV. Depolarizing (20 mV) and hyperpolarizing (−30 mV) command voltages were applied to monitor the conductance. (B1) NT-induced single-channel activity from an outside–out patch held at −80 mV. On an expanded time base, there was no channel activity before NT application (B2), while single-channel openings appeared after NT was applied (B3). (B4) Npo of the NT response in B1 was calculated at 4-sec intervals. The Npo reached a peak (=0.097) then decreased to zero. (C1) Similar channel activity occasionally could be induced in the cell-attached configuration. Potential was held at 30 mV intracellular side more negative than resting potential. NT was applied outside the patch region, and the patch pipette solution contained standard external solution. On an expanded time base, there was no channel activity before NT application (C2), while single-channel openings appeared after NT was applied (C3). The frequency response was 2 kHz (B2, B3, C2, and C3) and 250 Hz (B1 and C1) for display purposes.
Block by NT Antagonists.
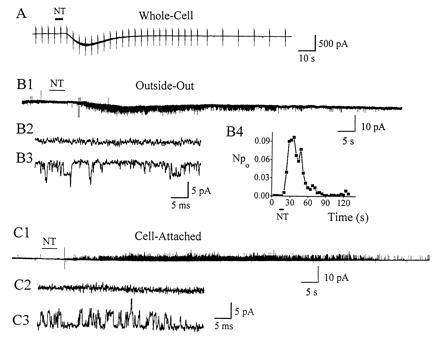
The nonpeptide NT antagonist SR48692 was used to characterize the receptor mediating the NT response. This antagonist competitively inhibits NT binding to the high-affinity binding site present in the rat mesencephalon (19). NT was first applied to the patch to establish a baseline response. Two hundred seconds later, SR48692 (10 nM) was applied for 5 sec, followed in 15–23 sec by a second application of NT. After another 200 sec, NT was applied for a third time (Fig. 2). Using different patches as controls, the solvent alone (0.001% dimethyl sulfoxide) instead of SR48692 was applied before the second NT application. The results were summarized in Fig. 2. For control patches (open bars), the average peak Npo for the first NT response was 0.11 ± 0.03 (n = 5). The second NT response decreased to 0.06 ± 0.02 because of desensitization. The third response decreased further to 0.03 ± 0.01. For the NT antagonist experiment (solid bars), the average peak Npo of the first NT response was 0.14 ± 0.04 (n = 7), fairly close to the response in control patches. After SR48692 was applied, the second NT response was dramatically decreased to 0.007 ± 0.002. Compared with the control, the difference was significant (P = 0.0045). After washout of the NT antagonist, the third response partially recovered, to 0.05 ± 0.02. The single-channel current amplitude was not affected by the NT antagonist (data not shown). These results showed that the channel activity was blocked by the NT antagonist, indicating that NT was acting through NT receptors.
Figure 2.
Effect of NT antagonist. NT (10 nM) was applied three times to elicit first, second, and third responses. Either the NT receptor antagonist SR48692 (10 nM in 0.001% dimethyl sulfoxide) or dimethyl sulfoxide alone (0.001%) as control was applied before the 2nd NT application. In control experiments, NT responses decreased steadily because of desensitization. In contrast, in NT antagonist experiments the second NT response was much decreased by SR48692, and the difference between control and antagonist was very significant (∗, P = 0.0045). After the antagonist was washed away, the third NT response showed partial recovery.
Kinetic Analysis of the Burst Behavior.
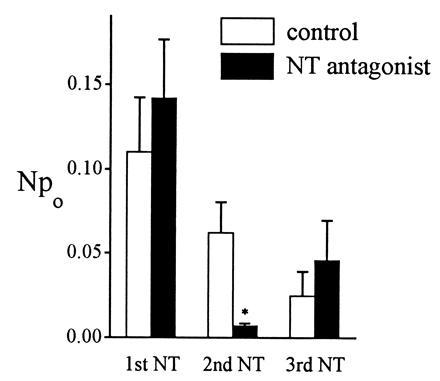
Kinetic properties of the NT-induced channel were studied in standard external and internal solutions (Fig. 3). The NT-induced channel opened in solitary form as well as in bursts, with the long openings interrupted by brief closures (Fig. 3A). The close time histogram is shown in Fig. 3B. This histogram was fit by the sum of two exponentials. The faster component (τg,f) of close time represents the brief closures within a burst. The slower component (τg,s) reflects intervals between individual bursts. The average of τg,f was 0.34 ± 0.02 ms, and the average of τg,s was 8.45 ± 1.30 ms (n = 8). Fig. 3C shows the burst time histogram. Again, the histogram was fit by two exponentials. The faster time constant (τb,f) represents the short openings (short bursts) while the longer time constant (τb,s) represents the long bursts. The average of τb,f was 0.102 ± 0.013 ms with a weight factor (ab,f) of 0.528 ± 0.015, and the average of τb,s was 1.12 ± 0.13 ms. The average burst time (weighted average of fast and slow components) was 0.528 ± 0.046 ms (n = 8).
Figure 3.
Kinetic analysis with standard external and internal solutions. (A) The NT (10 nM)-induced channel opened in solitary form as well as in bursts. (B–D) Analysis of the data shown in record A. (B) Close time histogram. (C) Burst time histogram. (D) Histogram of burst amplitudes; only openings lasting longer than one-half of the average burst time were included (following the procedure of ref. 20). The frequency response was 3 kHz (−3 db). The potential was held at −80 mV.
Fig. 3D shows a histogram of burst amplitude that was fit by a single Gaussian distribution. The average single-channel current at a holding potential of −80 mV was −2.85 ± 0.38 pA (n = 8). Based on this value and the average peak inward current (≈2500 pA) induced by NT in whole-cell recordings (9), we have estimated that 1 μM NT opened ≈900 channels per neuron during the peak response (or ≈0.6 channels/μm2, assuming the neuron is sphere with an average diameter of 21 μm). This channel density is on the same order as that of an inward rectifier K channel (1.3 channels/μm2) in frog skeletal muscle (21).
Current–Voltage Relation of the Channel.
The current–voltage relation of the NT-induced channel activity was first examined in standard internal and external solutions. However, channel activity of unknown origin occurred at depolarized potentials, obscuring the NT response. This compelled us to determine the reversal potential of the NT-induced channels using Cs+ in place of K+ to suppress K+ conductance, and increasing internal Na+ to shift the reversal potential toward negative potential. An example of single-channel records from a patch held at various membrane potentials is shown in Fig. 4A. Before NT application (Fig. 4A1) there were no channel openings, while the NT-induced channel activity is shown in Fig. 4A2, as well as in Fig. 4A3 on an expanded time base. The relation between amplitude and voltage was plotted in Fig. 4B. On average, the reversal potential was −4.2 ± 1.7 mV (n = 6), which was between ECs (−90 mV) and ENa (+13 mV). The unitary chord conductance (γ) was 31 pS at −67 mV using the Cs+-containing solutions.
Figure 4.

Current–voltage relation. Single-channel records from a patch held at various membrane potentials are shown (A1–A3). (A1) No channel opened before NT application. (A2) Channel activity was induced by NT (10 nM). (A3) Segments of A2 on an expanded time base. The current through the NT-induced channels reversed from inward at −67 mV to outward at 33 mV. The current-voltage relation for the patch in A was plotted in B. Cs+-containing external and internal solutions were used.
We also estimated what the elementary conductance of the nonselective channel would be in the standard external and internal solutions, containing normal K+ and Na+ concentrations. From the single-channel amplitude (−2.85 pA) and the permeability ratio data from whole-cell experiments (9), the elementary chord conductance at −80 mV would be 36 pS, not much different from the value measured in the Cs+-containing solutions. This value is close to the conductance of cyclic nucleotide-gated channels (20–25 pS in photoreceptor, see ref. 12; 40 pS in olfactory epithelium, see ref. 22) and calcium-activated nonselective channels (20–30 pS, see ref. 13).
NT-Induced Irreversible Activation in GTP[γS].
The signal transduction mechanism of the NT-induced nonselective cation channel was characterized by using GTP[γS], a nonhydrolyzable GTP analogue. The pipette solution contained either GTP[γS] (100 μM), or, for control, GTP (100 μM), and the time course of Npo after NT application was calculated (Fig. 5). In control, the Npo reached maximum and decreased to almost 0% at 80 sec. In contrast, the Npo of the patch with GTP[γS] reached the peak and then decreased to about 40% of the peak, where it remained steady. This irreversible component of channel activity indicated that at least some of the NT-induced nonselective cation channels were linked to a signal transduction pathway involving G proteins.
Figure 5.
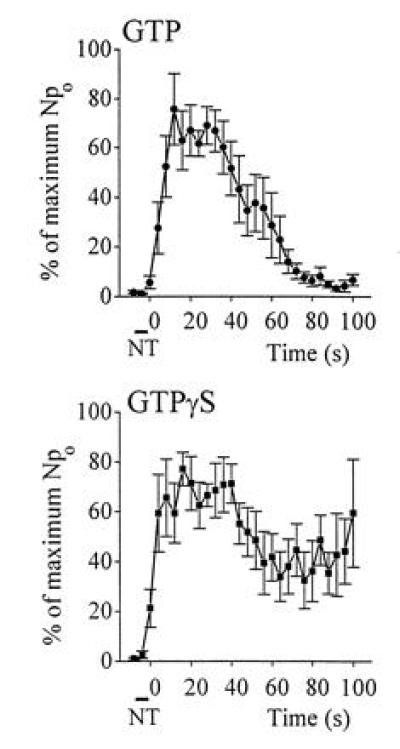
NT-induced irreversible channel activity in GTP[γS]. Standard external solution was used. Internal solutions contained either GTP (100 μM) for control, or GTP[γS] (100 μM) for experiments. For each individual patch, after NT was applied, the time course of Npo was calculated at 4-sec interval. Npo was then normalized referring to the peak as 100%. In control (GTP-loaded patches), the average Npo reached a maximum and decreased to almost 0% at ≈80 sec (n = 6). In contrast, the average Npo of the patch with GTP[γS] reached a peak, then decreased to about 40% of peak and remained steady (n = 6). NT was applied during the time indicated.
DISCUSSION
Comparison of Single-Channel with Whole-Cell Current.
The characteristics of the channel induced by NT were very similar to those of the whole-cell current previously examined. Both are mediated by NT receptors, as indicated by inhibition by the NT antagonist SR48692. The reversal potential for the single-channel current (−4.2 ± 1.7 mV) was close to the reversal potential in the whole cell (−7 ± 2 mV) using about the same internal and external solutions. Furthermore, the single-channel activity and the whole-cell current had a very similar time course (Fig. 1). These findings suggest that we recorded the same channels that produced the NT-induced whole-cell current.
Signal Transduction.
About half of the channel activity induced by NT was irreversibly activated by GTP[γS], suggesting that at least part of the signal transduction mechanism involves G proteins. This agrees with previous work showing that the cloned NT receptor belongs to the family of G protein-coupled receptors (23), and that NT excites VTA neurons by activating a pertussis toxin-insensitive G protein (8), probably Gαq/11 (24). The portion of the NT-induced channel activity that was not irreversibly activated by GTP[γS] might have been inactivated by an unknown mechanism, or possibly this activity might not be G protein-related.
The NT-induced channel activity was recorded in more than half (28/52) of the outside–out patches. When outside–out patches are formed, soluble second messengers, such as Ca2+, cAMP, and cGMP are believed to be dialyzed out of the patch. Thus, the outside–out results suggest that NT induced the channels locally. NT receptors and the nonselective cation channels we studied might be coupled by a G protein acting through a membrane-delimited pathway, similar to muscarinic K+ channels (25–27). Alternatively, other unknown messengers might be involved, but any such messengers are not dialyzed out by the pipette solution.
We occasionally (4/54) could elicit the nonselective channel in the cell-attached configuration when NT was applied outside the patch region. This might indicated that a “diffusible” messenger activated these channels. This does not necessarily contradict the apparently local effect of NT in outside–out recordings; the same pathway activated in outside–out patches might have the mobility to cross the barrier of the recording electrode. On the other hand, the cell attached activity might represent a distinct, diffusible pathway, independent from the activity observed in outside–out patches.
Burst Analysis.
The NT-induced channel opened in bursts, as do ligand-gated (17, 28) and cyclic nucleotide-gated nonselective cation channels (29) (Bursts also occur in inward rectifier K+ channels; see refs. 16 and 30). The mechanism of bursts for the nicotinic acetylcholine receptor channel was proposed by Colquhoun and Sakmann (17) to be a long opening interrupted by gaps from the channel briefly closing several times during a single acetylcholine receptor occupancy. The bursts we observed could represent the nonselective cation channel opening and closing several times during a single messenger binding. On the other hand, bursts can also result from divalent ion block as in the case of the N-methyl-d-aspartate receptor (31) and cyclic nucleotide-gated nonselective channels (32).
Significance of the Slow Nonselective Cation Channel.
Brain neurons constantly receive slow excitatory and slow inhibitory signals from neighboring neurons, and the interplay of these signals sets neuronal excitability. Many transmitters, including muscarine, substance P, luteinizing hormone-releasing hormone, and NT, induce slow excitation in various neurons by dual ionic mechanisms: inhibition of a resting K+ conductance and activation of a slow nonselective cation conductance (6, 8, 9, 33–36). The transmitter-modulated K+ channels (inward rectifier K+ channels) have been studied at the single-channel level (16, 30, 37), and several have recently been cloned (38–40). In contrast, the nonselective cation channels activated by slow transmitters have not, until now, been examined at the single-channel level, and the first member of this family remains to be cloned. We previously showed that these cation channels are different from ligand-gated, cyclic nucleotide-gated, and calcium-activated nonselective cation conductances (9). It is likely that the nonselective cation channels activated by other transmitters are in the same family as those activated by NT. Thus, to our knowledge, we have for the first time analyzed the single-channel properties of a slow nonselective cation channel that plays a central role in determining neuronal excitability.
Acknowledgments
We thank Ms. Linda Johnston for technical help. This work was supported by National Science Foundation Grant IBN 9319456 and National Institutes of Health Grant F30MH10167.
Footnotes
Abbreviations: NT, neurotensin; VTA, ventral tegmental area; GTP[γS], guanosine 5′-[γ -thio]triphosphate.
References
- 1.Carraway R, Leeman S E. J Biol Chem. 1973;248:6854–6861. [PubMed] [Google Scholar]
- 2.Miletić V, Randić M. Brain Res. 1979;169:600–604. doi: 10.1016/0006-8993(79)90412-8. [DOI] [PubMed] [Google Scholar]
- 3.Henry J L. Ann NY Acad Sci. 1982;400:216–226. doi: 10.1111/j.1749-6632.1982.tb31571.x. [DOI] [PubMed] [Google Scholar]
- 4.Baldino F, Jr, Wolfson B. Brain Res. 1985;325:161–170. doi: 10.1016/0006-8993(85)90312-9. [DOI] [PubMed] [Google Scholar]
- 5.Audinat E, Hermel J M, Crépel F. Exp Brain Res. 1989;78:358–368. doi: 10.1007/BF00228907. [DOI] [PubMed] [Google Scholar]
- 6.Farkas R H, Nakajima S, Nakajima Y. Proc Natl Acad Sci USA. 1994;91:2853–2857. doi: 10.1073/pnas.91.7.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercuri N B, Stratta F, Calabresi P, Bernardi G. Neurosci Lett. 1993;153:192–196. doi: 10.1016/0304-3940(93)90320-k. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Z-G, Pessia M, North R A. J Physiol (London) 1994;471:119–129. doi: 10.1113/jphysiol.1994.sp020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farkas R H, Chien P-Y, Nakajima S, Nakajima Y. J Neurophysiol. 1996;76:1968–1981. doi: 10.1152/jn.1996.76.3.1968. [DOI] [PubMed] [Google Scholar]
- 10.Jonas P. In: Nonselective Cation Channels: Pharmacology, Physiology, and Biophysics. Siemen D, Hescheler J, editors. Basel: Birkhauser; 1993. pp. 61–76. [Google Scholar]
- 11.Sargent P B. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- 12.Yau K-W, Baylor D A. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- 13.Partridge L D, Swandulla D. Trends Neurosci. 1988;11:69–72. doi: 10.1016/0166-2236(88)90167-1. [DOI] [PubMed] [Google Scholar]
- 14.Masuko S, Nakajima S, Nakajima Y. Neuroscience. 1992;49:347–364. doi: 10.1016/0306-4522(92)90101-7. [DOI] [PubMed] [Google Scholar]
- 15.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 16.Grigg J J, Kozasa T, Nakajima Y, Nakajima S. J Neurophysiol. 1996;75:318–328. doi: 10.1152/jn.1996.75.1.318. [DOI] [PubMed] [Google Scholar]
- 17.Colquhoun D, Sakmann B. J Physiol (London) 1985;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedrich F, Paulmichl M, Kolb H A, Lang F. J Membr Biol. 1988;106:149–155. doi: 10.1007/BF01871397. [DOI] [PubMed] [Google Scholar]
- 19.Gully D, Canton M, Boigegrain R, Jeanjean F, Molimard J-C, Poncelet M, Gueudet C, Heaulme M, Leyris R, Brouard A, Pelaprat D, Labbé- Jullié C, Mazella J, Soubrié P, Maffrand J-P, Rostene W, Kitabgi P, Le Fur G. Proc Natl Acad Sci USA. 1993;90:65–69. doi: 10.1073/pnas.90.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colquhoun D, Sigworth F J. In: Single-Channel Recording. Sakmann B, Neher E, editors. New York: Plenum; 1995. pp. 483–587. [Google Scholar]
- 21.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer; 1992. pp. 315–336. [Google Scholar]
- 22.Firestein S, Zufall F, Shepherd G M. J Neurosci. 1991;11:3565–3572. doi: 10.1523/JNEUROSCI.11-11-03565.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka K, Masu M, Nakanishi S. Neuron. 1990;4:847–854. doi: 10.1016/0896-6273(90)90137-5. [DOI] [PubMed] [Google Scholar]
- 24.Wang H L, Wu T. Mol Brain Res. 1996;36:29–36. doi: 10.1016/0169-328x(95)00235-k. [DOI] [PubMed] [Google Scholar]
- 25.Soejima M, Noma A. Pflügers Arch. 1984;400:424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- 26.Reuveny E, Slesinger P A, Inglese J, Morales J M, Iñiguez-Lluhi J A, Lefkowitz R J, Bourne H R, Jan Y N, Jan L Y. Nature (London) 1994;370:143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- 27.Wickman K D, Iñiguez-Lluhi J A, Davenport P A, Taussig R, Krapivinsky G B, Linder M E, Gilman A G, Clapham D E. Nature (London) 1994;368:255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- 28.Cull-Candy S G, Parker I. Nature (London) 1982;295:410–412. doi: 10.1038/295410a0. [DOI] [PubMed] [Google Scholar]
- 29.Hanke W, Cook N L, Kaupp U B. Proc Natl Acad Sci USA. 1988;85:94–98. doi: 10.1073/pnas.85.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakmann B, Noma A, Trautwein W. Nature (London) 1983;303:250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- 31.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Nature (London) 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 32.Haynes L W, Kay A R, Yau K-W. Nature (London) 1986;321:66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- 33.Jan L Y, Jan Y N. J Physiol (London) 1982;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murase K, Ryu P D, Randić M. J Neurophysiol. 1989;61:854–865. doi: 10.1152/jn.1989.61.4.854. [DOI] [PubMed] [Google Scholar]
- 35.Shen K-Z, North R A. Neuroscience. 1992;50:345–353. doi: 10.1016/0306-4522(92)90428-5. [DOI] [PubMed] [Google Scholar]
- 36.Koyano K, Velimirović B M, Grigg J J, Nakajima S, Nakajima Y. Eur J Neurosci. 1993;5:1189–1197. doi: 10.1111/j.1460-9568.1993.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 37.Miyake M, Christie M J, North R A. Proc Natl Acad Sci USA. 1989;86:3419–3422. doi: 10.1073/pnas.86.9.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dascal N, Schreibmayer W, Lim N F, Wang W, Chavkin C, DiMagno L, Labarca C, Kieffer B L, Gaveriaux-Ruff C, Trollinger D, Lester H A, Davidson N. Proc Natl Acad Sci USA. 1993;90:10235–10239. doi: 10.1073/pnas.90.21.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubo Y, Baldwin T J, Jan Y N, Jan L Y. Nature (London) 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- 40.Krapivinsky G, Gordon E A, Wickman K, Velimirović B, Krapivinsky L, Clapham D E. Nature (London) 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]


