Abstract
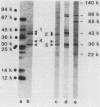
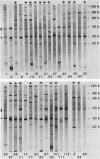
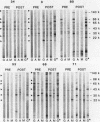
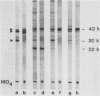
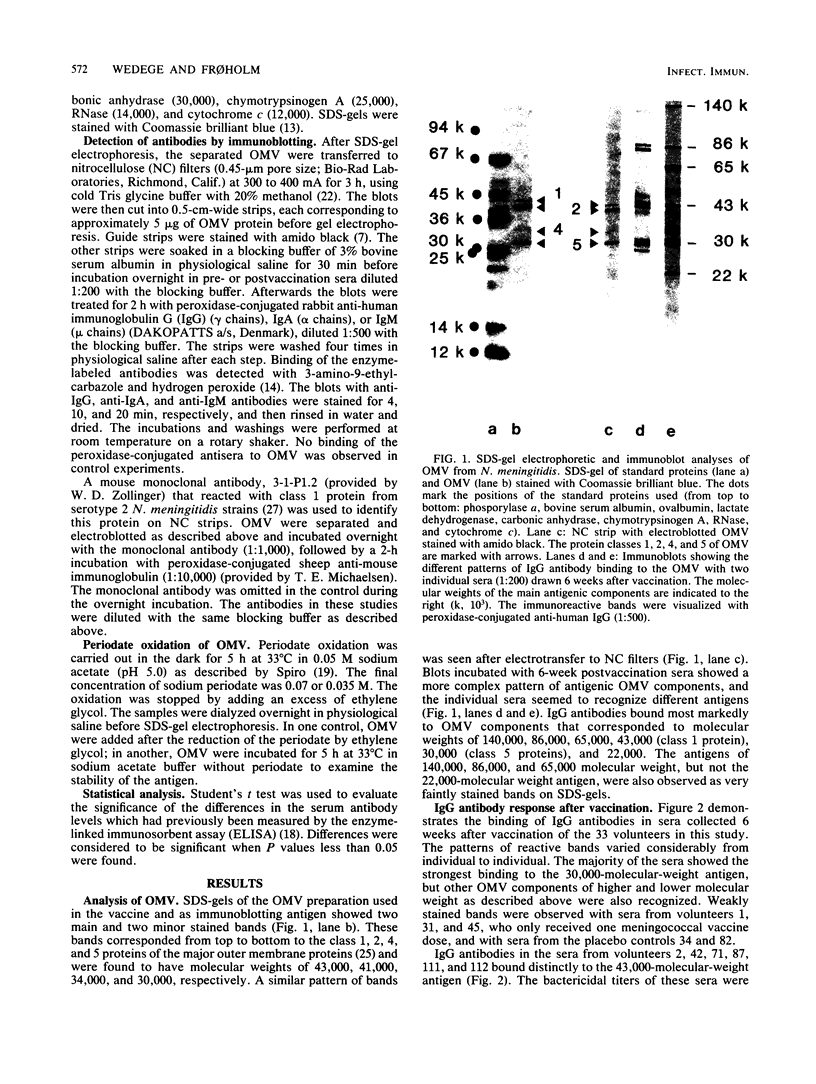
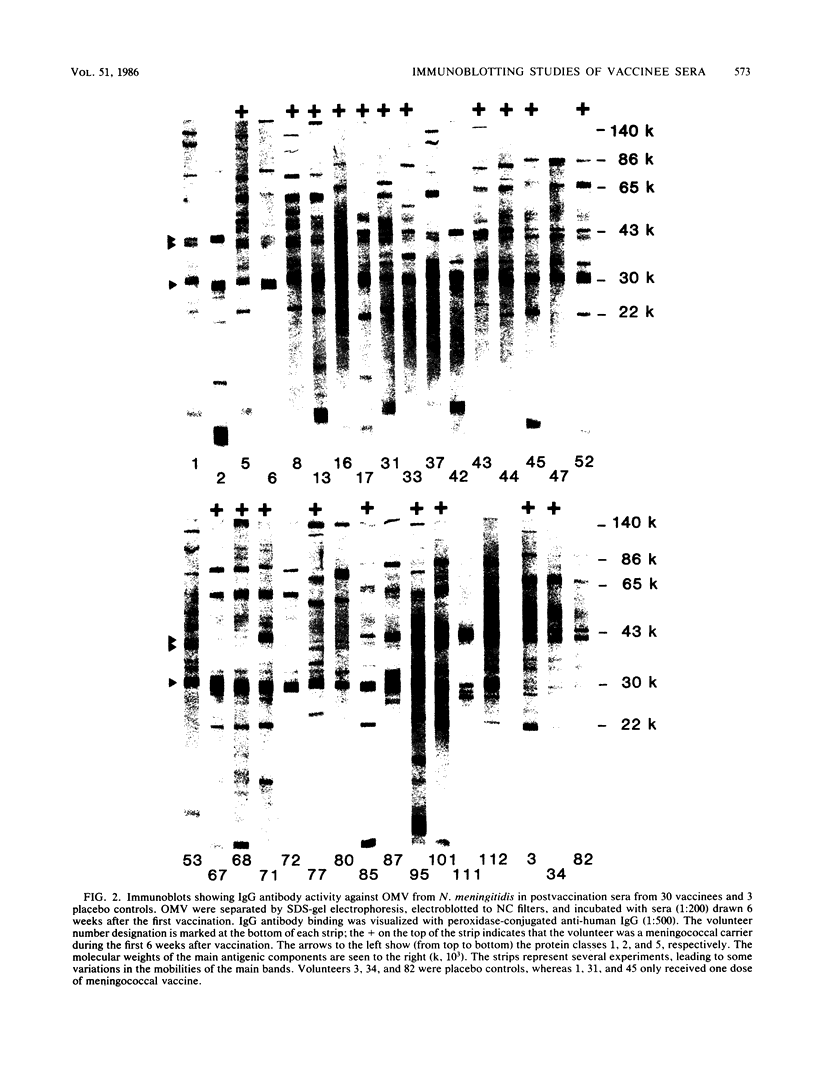
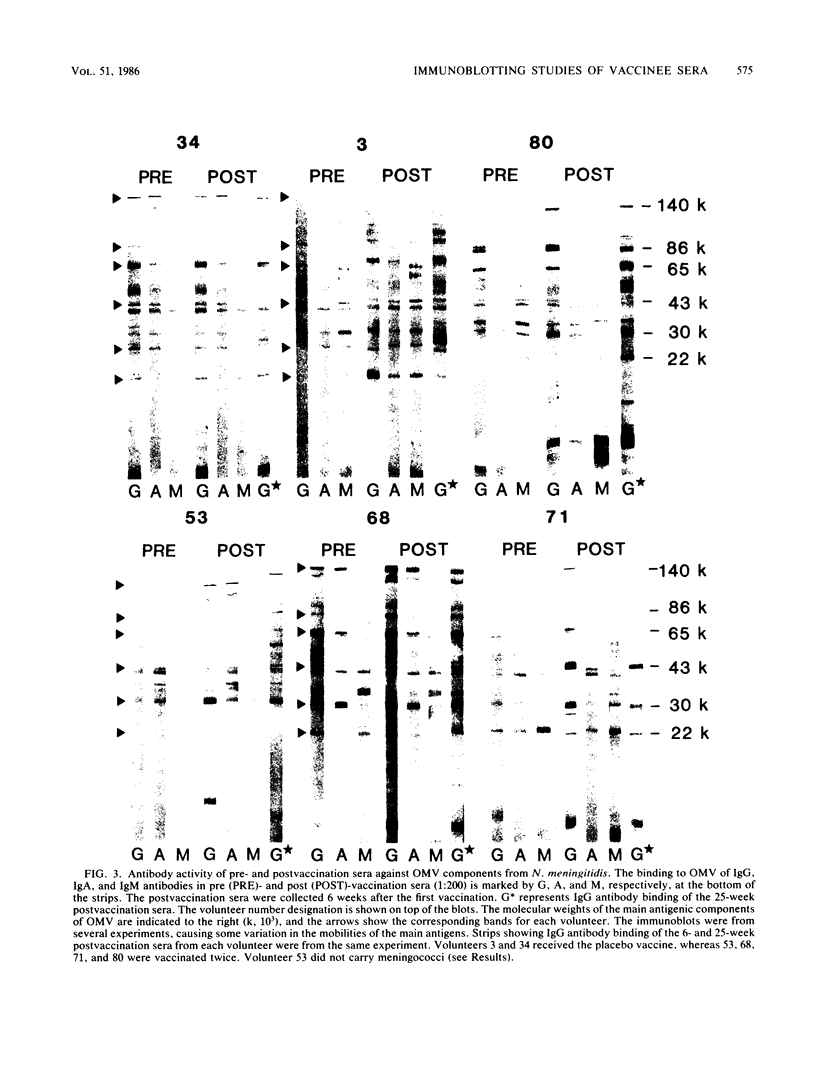
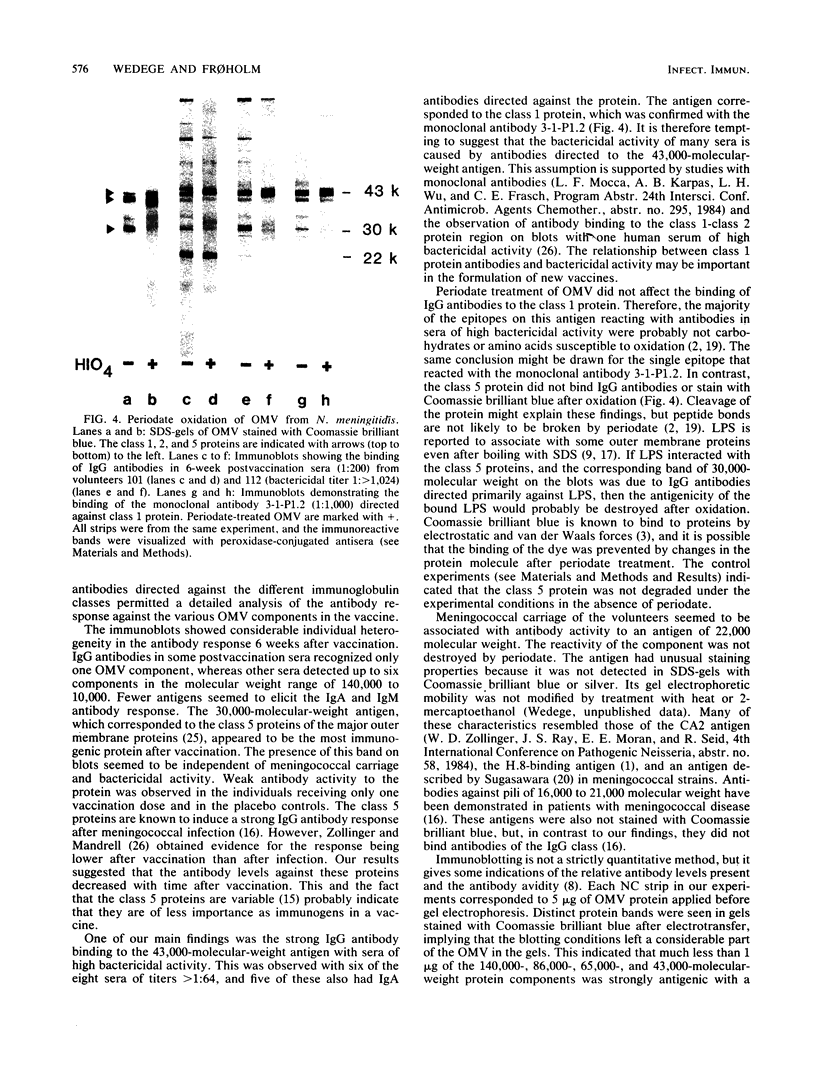
The antibody response of 30 volunteers vaccinated with a complex of group B polysaccharide and outer membrane vesicles (OMV) from serotype 2a Neisseria meningitidis and of 3 individuals who received a placebo vaccine was determined by immunoblotting. OMV were separated by sodium dodecyl sulfate-gel electrophoresis and electrotransferred to nitrocellulose filters. Binding of immunoglobulin G (IgG), IgA, and IgM antibodies in the human sera to OMV components was detected with class-specific peroxidase-conjugated antibodies. The immunoblotting results were also related to the bactericidal activity of the sera and the meningococcal carrier status of the volunteers. Before vaccination weakly reactive bands in the molecular weight range of 140,000 to 10,000 were observed on the blots. Sera from carriers showed more marked bands. Individual patterns of increased reactivity were seen 6 weeks after vaccination. The main immunoreactive components of OMV corresponded to a molecular weight of 43,000 (class 1 protein), 30,000 (class 5 proteins), and 22,000. IgG antibodies in postvaccination sera of high bactericidal titers showed distinct binding to the 43,000-molecular-weight antigen. Meningococcal carriers had antibodies against an antigen of 22,000 molecular weight; in polyacrylamide gels this component did not stain with Coomassie brilliant blue or silver. The marked binding of IgG antibodies to the class 5 proteins decreased considerably between weeks 6 and 25 after vaccination. Periodate oxidation of OMV abolished the binding of IgG antibodies to the class 5 proteins, whereas the antigenicity of the 43,000-molecular-weight (class 1 protein) and 22,000-molecular-weight antigens was unaffected.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLAMP J. R., HOUGH L. THE PERIODATE OXIDATION OF AMINO ACIDS WITH REFERENCE TO STUDIES ON GLYCOPROTEINS. Biochem J. 1965 Jan;94:17–24. doi: 10.1042/bj0940017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G., Black W. J., Nachamkin I., Stewart P. W. Monoclonal antibody that recognizes an outer membrane antigen common to the pathogenic Neisseria species but not to most nonpathogenic Neisseria species. Infect Immun. 1984 Mar;43(3):994–999. doi: 10.1128/iai.43.3.994-999.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Gotschlich E. C. An outer membrane protein of Neisseria meningitidis group B responsible for serotype specificity. J Exp Med. 1974 Jul 1;140(1):87–104. doi: 10.1084/jem.140.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Peppler M. S. Protection against group B Neisseria meningitidis disease: preparation of soluble protein and protein-polysaccharide immunogens. Infect Immun. 1982 Jul;37(1):271–280. doi: 10.1128/iai.37.1.271-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøholm L. O., Berdal B. P., Bøvre K., Gaustad P., Harboe A., Holten E., Høiby E. A., Lystad A., Omland T., Frasch C. E. Meningococcal group B vaccine trial in Norway 1981--1982. Preliminary report of results available November 1982. NIPH Ann. 1983 Dec;6(2):133–138. [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to a positively charged membrane filter. Anal Biochem. 1982 Aug;124(2):396–405. doi: 10.1016/0003-2697(82)90056-2. [DOI] [PubMed] [Google Scholar]
- Griffiths E., Stevenson P., Thorpe R., Chart H. Naturally occurring antibodies in human sera that react with the iron-regulated outer membrane proteins of Escherichia coli. Infect Immun. 1985 Mar;47(3):808–813. doi: 10.1128/iai.47.3.808-813.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J. Analyses of gonococcal lipopolysaccharide in whole-cell lysates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis: stable association of lipopolysaccharide with the major outer membrane protein (protein I) of Neisseria gonorrhoeae. Infect Immun. 1984 Oct;46(1):202–212. doi: 10.1128/iai.46.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Use of a zwitterionic detergent for the restoration of the antibody-binding capacity of electroblotted meningococcal outer membrane proteins. J Immunol Methods. 1984 Feb 24;67(1):1–11. doi: 10.1016/0022-1759(84)90080-2. [DOI] [PubMed] [Google Scholar]
- Orstavik K. H. Alloantibodies to factor IX in Haemophilia B characterized by crossed immunoelectrophoresis and enzyme-conjugated antisera to human immunoglobulins. Br J Haematol. 1981 May;48(1):15–23. [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Immunogenicity of meningococcal antigens as detected in patient sera. Infect Immun. 1983 Apr;40(1):398–406. doi: 10.1128/iai.40.1.398-406.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman J. T., de Marie S., Zanen H. C. Variability of low-molecular-weight, heat-modifiable outer membrane proteins of Neisseria meningitidis. Infect Immun. 1980 Dec;30(3):642–648. doi: 10.1128/iai.30.3.642-648.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenqvist E., Tjade T., Frøholm L. O., Frasch C. E. An ELISA study of the antibody response after vaccination with a combined meningococcal group B polysaccharide and serotype 2 outer membrane protein vaccine. NIPH Ann. 1983 Dec;6(2):139–149. [PubMed] [Google Scholar]
- Sugasawara R. J. Recognition of serogroup A Neisseria meningitidis serotype antigens by human antisera. Infect Immun. 1985 Apr;48(1):23–28. doi: 10.1128/iai.48.1.23-28.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Boykins R., Frasch C. E. Heterogeneity and variation among Neisseria meningitidis lipopolysaccharides. J Bacteriol. 1983 Aug;155(2):498–504. doi: 10.1128/jb.155.2.498-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E., Mocca L. F. Five structural classes of major outer membrane proteins in Neisseria meningitidis. J Bacteriol. 1981 Apr;146(1):69–78. doi: 10.1128/jb.146.1.69-78.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Moran E. E., Connelly H., Mandrell R. E., Brandt B. Monoclonal antibodies to serotype 2 and serotype 15 outer membrane proteins of Neisseria meningitidis and their use in serotyping. Infect Immun. 1984 Oct;46(1):260–266. doi: 10.1128/iai.46.1.260-266.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]