Abstract
Antiapoptotic activity of NF-κB in tumors contributes to acquisition of resistance to chemotherapy. Degradation of IκB is a seminal step in activation of NF-κB. The IκB kinases, IKK1 and IKK2, have been implicated in both IκB degradation and subsequent modifications of NFκB. Using mouse embryo fibroblasts (MEFs) devoid of both IKK1 and IKK2 genes (IKK1/2−/−), we document a novel IκB degradation mechanism. We show that this degradation induced by a chemotherapeutic agent, doxorubicin (DoxR), does not require the classical serine 32 and 36 phosphorylation or the PEST domain of IκBα. Degradation of IκBα is partially blocked by phosphatidylinositol 3-kinase inhibitor LY294002 and is mediated by the proteasome. Free NF-κB generated by DoxR-induced IκB degradation in IKK1/2−/− cells is able to activate chromatin based NF-κB reporter gene and expression of the endogenous target gene, IκBα. These results also imply that modification of NF-κB by IKK1 or IKK2 either prior or subsequent to its release from IκB is not essential for NF-κB-mediated gene expression at least in response to DNA damage. In addition, DoxR-induced cell death in IKK1/2−/− MEFs is enhanced by simultaneous inhibition of NF-κB activation by blocking the proteasome activity. These results reveal an additional pathway of activating NF-κB during the course of anticancer therapy and provide a mechanistic basis for the observation that proteasome inhibitors could be used as adjuvants in chemotherapy.
The transcription factor NF-κB has been documented to play roles in innate and adaptive immune responses (19). Deregulated activity of NF-κB pathway has also been observed and linked to the progression of several human malignancies (27). It is becoming increasingly clear that NF-κB can modulate multiple aspects of cell growth and death (10). Understanding the regulation of NF-κB activity is likely to shed insight into cancer progression and treatment.
NF-κB, a dimer of two similar or heterologous subunits, is predominantly found in the cytoplasm complexed with the IκB family of inhibitory molecules (37). Degradation of IκB proteins in response to stimuli is an obligatory step in NF-κB activation (14). This degradation is mediated by the proteasome and requires that the IκB molecules are phosphorylated at specific serine residues (4). Hence, the role of the IκB kinases (IKKs), IKK1 (also called IKKα) and IKK2 (also called IKKβ), which induce signal-dependent phosphorylation and degradation of IκB molecules, is extensively investigated. The cytoplasmic >700-kDa complex containing IKK1 and IKK2 receives and transmits various stimuli that lead to IκBα degradation and NF-κB DNA binding. Apart from the two IKK enzymes, the IKK complex is believed to consist of other known and unknown proteins which might have regulatory or chaperone-like functions (5, 39). Mice which were homozygous null for both IKK1 and IKK2 die at embryonic day 9.5 (18). Mouse embryo fibroblasts (MEFs) derived from IKK1/2−/− double knockout embryos are severely defective in IκB degradation and NF-κB activation, in response to “classical” stimuli such as cytokines and bacterial products recognized by pattern recognition receptors (19). These results emphasize that IKK1 and IKK2 are absolutely essential for IκB degradation and NF-κB activation (18).
The rate-limiting step in the activation of NF-κB is believed to be its release from the inhibitor IκB molecules, which retain NF-κB in the cytoplasm and additionally inhibit its DNA-binding ability. Defective IκBα activity has been mechanistically linked to the persistent nuclear NF-κB seen in many human tumors (27). However, other genetic modifications of NF-κB subsequent to its release from IκB are now thought to be necessary for generating functional NF-κB activity in the context of transcription of target genes. These changes, which are mainly initiated by selective phosphorylation, increase its interaction with p300/CBP and also the chromatin remodeling factors. Several kinases, including GSK3, NIK, CKII, PKA, PKCζ, TBK/t2k/NAK, and IKK themselves have been thought to be required for modifying NF-κB after its release from IκBα (10). Since the same catalytic activity of IKK is required both for IκBα degradation and NF-κB modification, it has been difficult to dissect the contribution of IKKs in processes subsequent to the release of NF-κB from IκB.
In the present study, using IKK1/2−/− MEFs, we decipher a novel mechanism of IκBα degradation in response to the anticancer agent doxorubicin (DoxR), independent of IKK1 and IKK2. Further, treatment of IKK1/2−/− MEFs with DoxR leads to NF-κB transcriptional activity and, more importantly, this activity correlates with the protection of these MEFs from apoptosis. Our results demonstrate that certain nonclassical stimuli can activate NF-κB upon prolonged treatment and that this “sneak” NF-κB activity might have clinical relevance during the course of long-term chemotherapy.
MATERIALS AND METHODS
Cell culture and reagents.
Spontaneously immortalized MEFs were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (HyClone), 100 μg of penicillin/ml, 100 μg of streptomycin/ml, and 250 ng of amphotericin B (Gibco)/ml. Doxorubicin was purchased from Sigma and dissolved in MilliQ water at a concentration of 10 mg/ml as stock. N-Acetyl-l-alanine (NAA) and N-acetyl-l-cysteine (NAC) were from Sigma and were used at 10 mM after being dissolved in the medium and after the pH was adjusted to 7. Lactacystin and the caspase inhibitor set II kit (catalog no. 218772) were obtained from Calbiochem.
Antibodies.
The antibodies against IKK1, IKK2, p65, IκBα (C-21 and C-15), IκBβ, NEMO, p50, p52, Bax, and p21 were all from Santa Cruz Biotechnology. The p53 protein was detected with an antibody (OPO3) from Calbiochem. Anti-β-actin antibody (Sigma) was used to detect levels of actin which served as loading controls in all of the experiments. Detection of Flag-tagged IKK2 was performed with the Flag-M2 monoclonal antibody from Sigma. Hemagglutinin (HA)-tagged IKK1WT and IKK1KM proteins were detected by using anti-HA antibody (12CA5). All of the phospho-specific antibodies were obtained from Zymed Laboratories.
Western blots and immunoprecipitations.
Typically, cells were cultured in six-well dishes, and confluent cells were treated with DoxR for the indicated times, washed with phosphate-buffered saline (PBS), and harvested in radioimmunoprecipitation assay buffer. Whole-cell extracts were prepared, and lysates were resolved on Bis-Tris sodium dodecyl sulfate-gels (4 to 12%) in morpholinepropanesulfonic acid (MOPS) buffer. After electrophoresis, proteins were transferred on polyvinylidene difluoride membrane (Immobilon P; Millipore), blocked in PBS without Mg2+ and Ca2+ but containing 0.2% Tween 20 and 5% nonfat milk, and probed with the indicated antibodies in PBS without Mg2+ and Ca2+ but containing 0.2% Tween 20 and 1% milk. For detection of phosphorylated IκBα, the membranes were blocked and probed in 3% bovine serum albumin.
For immunoprecipitations, total cell lysates were prepared in a buffer containing 50 mM Tris-HCl (pH 8), 170 mM NaCl, 0.5% Nonidet P-40, and 50 mM NaF supplemented with complete protease inhibitors (Roche) at 4°C. Immunoprecipitations were performed after the lysates were precleared for 1 h by incubation with protein G-Sepharose (Amersham Biosciences). Precleared supernatants were incubated overnight with primary antibodies, as well as protein G-Sepharose beads, at 4°C. Finally, these supernatants were washed four times with a wash buffer containing 10 mM Tris-HCl (pH 8), 250 mM NaCl, 5 mM EDTA, 0.5% Nonidet P-40, and complete protease inhibitors.
Electrophoretic mobility shift assays.
Total cellular extracts were prepared in Totex lysis buffer (20 mM HEPES [pH 7.9], 350 mM NaCl, 20% glycerol, 1% Nonidet P-40, 1 mM MgCl2, 0.5 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin/ml). Supernatants from centrifugation at 15,000 × g for 15 min were collected and used for mobility shift assay. The NF-κB and Oct-1 probes were as described previously (36). The end-labeled probes (T4 kinase) were incubated with Totex extracts for 30 min at 37°C. Complexes were separated by electrophoresis on a 5% nondenaturing polyacrylamide gel in 0.5× Tris-borate-EDTA. Dried gels were subjected to phosphorimager analysis.
Cell survival assays.
Cells typically were plated in six well dishes. Confluent cells were treated with DoxR or lactacystin. The percent survival was calculated by trypan blue staining of attached and detached cells.
Metabolic labeling.
Subconfluent 10-cm plates of IKK1/2−/− MEFs were washed twice in warm PBS and starved for 6 h in cysteine- and methionine-free DMEM supplemented with 10% dialyzed fetal bovine serum. Overnight labeling was performed with 0.5 mCi of [35S]methionine (NEN Life Science Products, Inc.) per ml. Cells were then washed with PBS and chased in complete medium with or without DoxR for the given time points in complete medium. After each time point, the cells were washed twice with ice-cold PBS, and cell pellets were frozen on dry ice for later manipulation. Cell pellets were thawed on ice, and whole-cell lysis was performed by adding 500 μl of lysis buffer (20 mM Tris [pH 8.0], 100 mM NaCl, 0.2% sodium deoxycholate, 0.2% Nonidet P-40, 0.2% Triton X-100) containing the protease inhibitors (Roche). Lysates were precleared with protein G-Sepharose for 1 h at 4°C and then incubated with IκBα (C-21) antibody plus protein G-Sepharose overnight at 4°C. The immunoprecipitated pellets were washed thrice and boiled for 10 min in SDS loading buffer, and the eluted proteins were separated on a 4 to 12% Bis-Tris gel in morpholineethanesulfonic acid (MES) buffer (Invitrogen).
RNA extraction and reverse transcription.
After stimulation, cells were harvested and total RNA isolated by using the TriPure reagent (Boehringer Mannheim). RNAs were quantified by analysis at 260 nm. Reverse transcription was performed with RT Superscript II (Invitrogen/Life Technologies) with 4 μg of total RNA and oligo(dT) (Invitrogen/Life Technologies) for 1 h at 42°C, followed by 3 min at 95°C.
Real-time PCR analysis.
The PCR amplification was performed with the following primers: IκBα sense (5′-CGC TTG GTG GAC GAT CG-3′) and antisense (5′-TTG CTC GTA CTC CTC GTC CTT C-3′). The actin gene was amplified as control with the following primers: sense (5′-TTC GTT GCC GGT CCA CA-3′) and antisense (5′-ACC AGC GCA GCG ATA TCG-3′). PCRs were performed by using an ABI Prism 7700 sequence detection system with SYBRGreen PCR master mix (Applied Biosystems, Warrington, United Kingdom).
Retrovirus generation and stable cell selection.
A total of 20 μg of vector plasmid was transfected with 5 μg of an expression vector for vesicular stomatitis virus G protein and 1 μg of a green fluorescent protein (GFP) expression plasmid into a cell line 293 gp/bsr (N. Somia and I. M. Verma, unpublished data). Supernatants containing the viruses were recovered 48 and 72 h later, filtered through a 0.45-μm-pore-size filter, and used to infect MEFs on 3 to 6 successive days.
Adenovirus production and infections.
Recombinant adenovirus (rAD) expression cassettes carrying the cytomegalovirus immediate-early enhancer, chicken β-actin promoter, and rabbit β-globin poly(A) signal were used to express GFP, IKK1WT, IKK1KM, IKK2WT, and IKK2KM proteins. Viruses were generated by homologous recombination in 293 cells (20). rAD vectors do not integrate and replicate in the host cells. Thus, to keep the multiplicity of infection (MOI) constant, only freshly confluent cells were used for infection.
Generation of lentiviral vectors and the IKK1/2−/− reporter cell lines.
The LV-GFP and LV-GFP-p65 constructs were derived from p156RRLsinPPTPGK-eGFP-PRE vector, wherein the transgene is driven by the mouse PGK promoter. The production of lentiviruses was performed according to standard protocols (12). To generate the IKK1/2−/− reporter cell lines, IKK1/2−/− MEFs were infected either with pLV-κBluc or pLV-Mut-κBLuc, lentiviral vectors containing five copies of wild-type (GGGACTTTCC), or mutated (AGAACTTCA) NF-κB binding sites, respectively, and a minimal promoter linked to luciferase reporter gene. Pools of infected cells were used in the assays described.
RESULTS
IκBα degradation in MEFs lacking IKK.
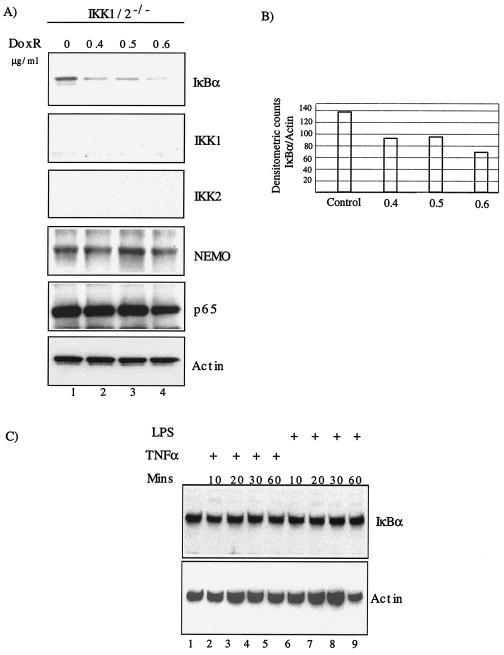
We evaluated IκBα degradation in response to anticancer drug DoxR in IKK1/2−/− MEFs. Upon titration with various doses of DoxR for 24 h, we observed degradation of IκBα (Fig. 1A). Western blot analysis showed no changes in expression of Nemo (IKKγ), p65, or actin. As expected, no IKK1 or IKK2 proteins were detected. The degradation of IκBα was confirmed based on densitometric quantitation of IκBα and actin band intensities (Fig. 1B). Treatment of IKK1/2−/− MEFs with classical short-term inducers of NF-κB activity such as tumor necrosis factor alpha (TNF-α) or lipopolysaccharide (LPS) did not lead to IκBα degradation (Fig. 1C, lanes 1 to 9).
FIG. 1.
DoxR-induced IκBα degradation in IKK1/2−/− MEFs. (A) IKK1/2−/− MEFs were plated in six-well plates and allowed to reach confluence. Cells were treated with indicated doses of DoxR and Western blot was performed 24 h later. (B) Densitometric quantitation of IκBα degradation was carried out by using NIH image software. The ratio of IκBα to actin has been plotted on the y axis. (C) Confluent cells grown in six-well dishes were treated with TNF-α or LPS for the indicated amounts of time. Whole-cell lysates were prepared and subjected to Western blot analysis with the indicated antibodies.
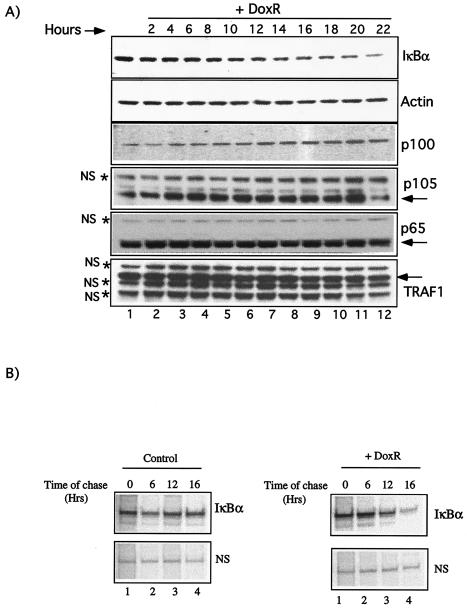
To further characterize the DoxR-induced IκBα degradation in IKK1/2−/− MEFs, we performed a time course. Unlike classical IκBα degradation induced by stimuli such as TNF-α and LPS, the degradation induced by DoxR occurred over several hours (Fig. 2A). Western blot analysis with p65, p100, p105, and TRAF1 antibodies further confirmed the specificity of IκBα degradation observed in our assays. In addition to the levels of five nonspecific proteins indicated by asterisks, levels of other nonrelated proteins, including p300 and tubulin (data not shown), did not change upon DoxR treatment. Densitometric quantitation of IκBα degradation over time was performed by using NIH Image 1.6 software, and the ratios of IκBα/actin and IκBα/p65 were found to be similar (data not shown). To better judge the effect of DoxR on IκBα degradation, we also performed a pulse-chase experiment with or without DoxR (Fig. 2B). Treatment with DoxR clearly reduced the levels of IκBα within 16 h (Fig. 2B, lanes 1 to 4). Real-time PCR analysis (see Fig. 7E) suggested that the reduction in levels of IκBα protein was due to its degradation and not due to a decrease in the transcription of its mRNA or nonspecific protein turnover. We conclude that long-term treatment with topoisomerase poisons such as DoxR induces IκBα protein degradation that is independent of IκB kinase activity.
FIG. 2.
Time course of DoxR-induced IκBα degradation in IKK1/2−/− MEFs. (A) Confluent IKK1/2−/− MEFs were treated with 0.7 μg of DoxR/ml for the indicated time in hours, and a Western blot was performed with the indicated antibodies. The levels of nonspecific proteins (NS) are indicated by asterisks. (B) Pulse-chase analysis of IκBα levels in the absence or presence of DoxR. IKK1/2−/− MEFs were starved and subsequently labeled with 35S protein labeling mix (NEN). Chase with or without 0.7 μg of DoxR/ml was performed for the indicated durations. The levels of a nonspecific (NS) protein are shown as a loading control.
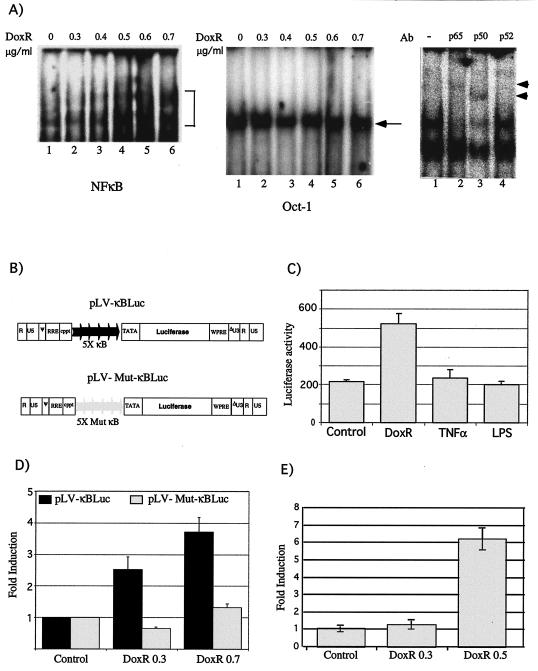
FIG. 7.
NF-κB activity in response to DoxR. (A) IKK1/2−/− MEFs were treated with indicated doses of DoxR for 12 h and analyzed for gel shift activity by using a NF-κB consensus oligonucleotide (left panel) or Oct-1 oligonucleotide (middle panel). Supershift analysis with antibodies to p50, p65, and p52 was performed on extracts derived from cells treated with 0.7 μg of DoxR/ml (right panel). (B) Construction of LV-κBluc and LV-Mut-κBluc vectors. The positions of the κB binding sites, along with the TATA box, have been indicated. (C) IKK1/2−/− MEFs were transduced with LV-κBLuc, and pools of these cells were treated for 18 h with DoxR (0.3 μg/ml), TNF-α (10 ng/ml), and LPS (30 ng/ml). Luciferase activity was evaluated in a 96-well plate reader. (D) IKK1/2−/− MEFs were either transduced with LV-κBLuc or LV-Mut-κBluc vectors, and pools of these cells were treated for 18 h with DoxR before luciferase activity was measured. (E) Real-time PCR analysis for IκBα. IKK1/2−/− MEFs were treated with the indicated doses of DoxR, and the levels of IκBα and actin messages were determined by real-time PCR analysis. The ratios of IκBα levels to actin levels were quantitated and are indicated as a fold change on the y axis.
DoxR-induced IκBα degradation does not require serine 32/36 phosphorylation or the PEST domain.
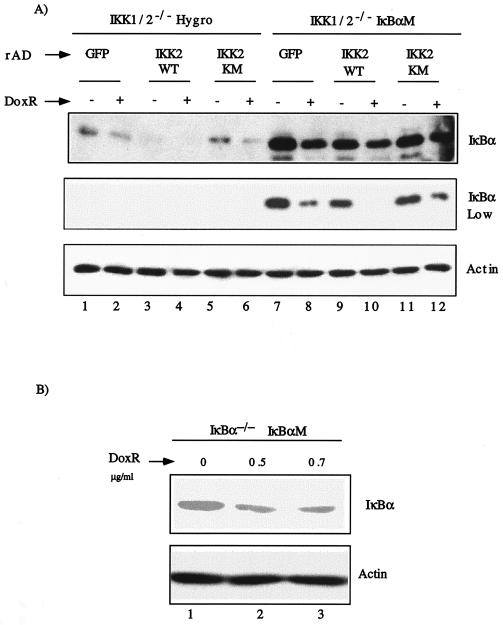
To further characterize DoxR induced IκBα degradation in IKK1/2−/− MEFs, we evaluated the role of inducible and constitutive phosphorylations of IκBα at positions 32/36/283/288/291/293/296, which play a role in its degradation (25). We tested whether IκBαM (36), an IκBα molecule wherein all of the serine and threonine residues at the above-mentioned positions are converted to alanines, is degraded by DoxR. IKK1/2−/− MEFs were transduced with retroviral constructs pLXSH or pLXSH-IκBαM to generate stable cell lines (IKK1/2−/− Hygro and IKK1/2−/− IκBαM, respectively). Since the endogenous IκBα and the mutant IκBαΜ do not differ in size, we induced the degradation of endogenous IκBα by overexpression of IKK2WT with rAD-IKKWT. Mere transduction with rAD-IKK2WT led to degradation of endogenous IκBα (Fig. 3A, lane 3) but not of IκBαM (Fig. 3A, lane 9). As a control, cells were infected with rAD-IKK2KM (kinase inactive IKK2) or rAD-GFP (a virus expressing GFP). Treatment with DoxR induced endogenous IκBα degradation (compare lanes 1 and 2 or lanes 5 and 6; top IκBα panel). Since all IκBα in lanes 9 and 10 is IκBαM, it is evident that DoxR also induced degradation of IκBαM (compare lanes 9 and 10; see the low exposure panel). These results suggest that phosphorylation of serine 32 and 36 is not essential for DoxR-induced IκBα degradation. In addition, phosphorylations of the residues in the C-terminal PEST domain of IκBα are also not required for its degradation induced by DoxR. The levels of Flag-tagged IKK2WT expressed in IKK1/2−/− Hygro and IKK1/2−/− IκBαM cells were comparable (data not shown).
FIG. 3.
IκBα degradation in response to DoxR does not require phosphorylations on serine 32 and 36 and in the PEST domain. (A) IKK1/2−/− MEFs were infected with either pLXSH or pLXSH-IκBαM retroviruses and stably selected using hygromycin. Mixed populations of IKK1/2−/− Hygro (lanes 1 to 6) and IKK1/2−/− IκBαM cells (lanes 7 to 12) were infected with rAD, expressing GFP (rAD-GFP), IKK2WT (rAD-IKK2WT), or IKK2KM (rAD-IKK2KM). At 48 h postinfection cells were either left untreated or treated with 0.4 μg of DoxR/ml. Expression of IKK2 and IκBα proteins was measured by using the Flag M2 and IκBα (C21) antibodies, respectively. Actin levels were used to normalize for protein loading. (B) IκBα−/− MEFs were infected with retroviruses expressing the dominant-negative form of IκBα, IκBαM. Stable pools of cells were generated as described previously and plated in six-well dishes. Confluent cells were treated with 0.5 and 0.7 μg of DoxR/ml for 24 h. Lysates were probed with C21 antibody to detect IκBαM.
We also reconstituted the IκBα−/− MEFs with IκBαM using retroviruses. Upon treatment with DoxR, IκBαM was degraded (Fig. 3B, lanes 2 and 3). These results further support the notion that DoxR-induced IκBα degradation does not require either the inducible phosphorylation of serines 32 and 36 or the C terminus phosphorylation sites of IκBα.
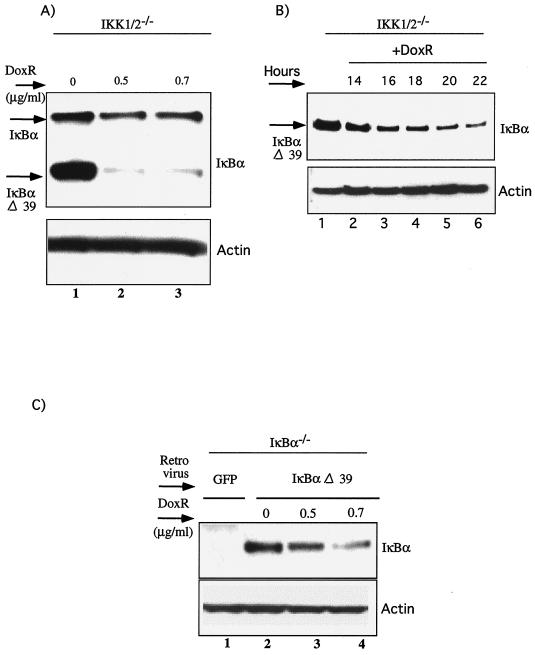
To further evaluate the role of the C-terminal PEST domain of IκBα, we transduced IKK1/2−/− MEFs with a retrovirus expressing a C-terminal truncation mutant of IκBα, IκBαΔ39, which removes the PEST domain. Surprisingly, removal of the PEST domain enhanced IκBα degradation (Fig. 4A, lanes 1 to 3). However, the kinetics of degradation of IκBαΔ39 (Fig. 4B, lanes 1 to 6) and wild-type IκBα (Fig. 2A, lanes 1 to 12) were similar. The ability of IκBαΔ39 mutant to be degraded efficiently was confirmed by its reconstitution in IκBα−/− MEFs (Fig. 4C; lanes 2 to 4).
FIG. 4.
IκBα degradation in response to DoxR does not require the PEST domain. (A) IKK1/2−/− MEFs were infected with retroviruses expressing IκBαΔ39, which is lacking the last 39 amino acids of IκBα. Cells were selected on hygromycin, mixed pools of cells were treated with the indicated doses of DoxR for 24 h, and IκBα degradation was evaluated by probing with IκBα C15 antibody. (B) Time course of IκBαΔ39 degradation in IKK1/2−/− MEFs. Confluent cells were treated with 0.5 μg of DoxR/ml for the indicated durations in hours and Western blot analysis for IκBαΔ39 was performed with IκBα C15 antibody. (C) IκBα−/− MEFs were infected with retroviruses expressing either GFP (as a control) or a virus expressing the IκBαΔ39. Stable pools of cells were generated as described previously and plated in six-well dishes. Confluent cells were treated with 0.5 and 0.7 μg of DoxR/ml for 24 h. Lysates were probed with C15 antibody to detect IκBαΔ39.
IκBα degradation by DoxR is insensitive to reactive oxygen species (ROS) and is not mediated by p53.
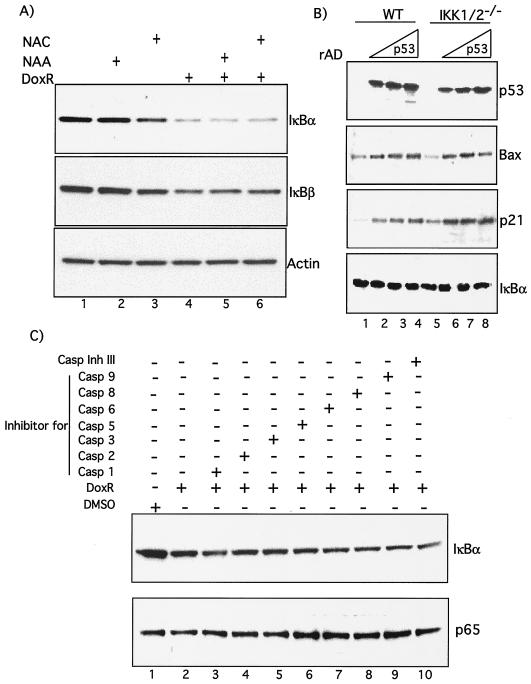
To better understand the molecular mechanism of IKK independent IκBα degradation, we evaluated the role of ROS that are activated by DNA damage and have been implicated in modulation of the NF-κB pathway (9). Western blot analysis demonstrated that IκBα degradation by DoxR was not inhibited by the ROS scavenger NAC (Fig. 5A, compare lanes 4 and 6) or its nonthiol counterpart, NAA (Fig. 5A, compare lanes 4 and 5). Like IκBα, degradation of IκBβ by DoxR was also not blocked by treatment with NAC (Fig. 5A).
FIG. 5.
IκBα degradation by DoxR is not mediated by ROS, p53, or caspases. (A) Confluent IKK1/2−/− MEFs were treated with 0.7 μg of DoxR/ml and 10 mM NAA or NAC as indicated. After 24 h the cells were lysed, and Western blots were performed. (B) IKK1/2−/− MEFs were cultured in six-well dishes and infected with increasing MOIs of rAD-p53 or rAD-GFP in 300 μl of DMEM with 5% fetal calf serum and antibiotics. The plates were rocked every 10 min, and the infection was allowed to proceed for 2 h, after which the cells were replenished with medium without removing the virus. This medium was changed after overnight incubation at 37°C with fresh medium. Cells were lysed and analyzed by Western blot analysis between 48 and 60 h postinfection. (C) IKK1/2−/− MEFs were treated either with DoxR alone or in combination with various caspase inhibitors, as indicated, for 24 h and then analyzed by Western blotting. Rows: Casp 1, caspase-1 inhibitor VI, Z-YVAD-FMK; Casp 2, caspase-2 inhibitor I, Z-VDVAD-FMK; Casp 3, caspase-3 inhibitor II, Z-DEVD-FMK; Casp 5, caspase-5 inhibitor I, Z-WEHD-FMK; Casp 6, caspase-6 inhibitor I, Z-VEID-FMK; Casp 8, caspase-8 inhibitor II, Z-IETD-FMK; Casp 9, caspase-9 inhibitor I, Z-LEHD-FMK. All are cell-permeable, irreversible, caspase inhibitors of various caspases as indicated by their number. Caspase inhibitor III (Casp Ihn III), Boc-d-FMK, is a broad-spectrum caspase inhibitor. More information regarding these inhibitors can be obtained on the Calbiochem information sheet.
To exclude the possibility that delayed degradation of IκBα may be a result of DoxR-induced apoptosis we evaluated the role of p53 and caspases in this degradation. NF-κB and p53 are simultaneously turned on in response to anticancer agents such as DoxR, and it has been reported that p53 induces NF-κB activation in certain cell types (28). To determine whether increased levels of p53 contribute to IκBα degradation, we overexpressed p53 by using an adenovirus (rAD-p53). Expression of p53 led to the concomitant upregulation of its target genes p21, Bax and mdm2 in both WT and IKK1/2−/− MEFs. However, in our assays, even such overexpression of p53 did not lead to IκBα degradation (Fig. 5B, lanes 1 to 8) or NF-κB DNA binding (data not shown) in either wild-type or IKK1/2−/− MEFs. Similarly, a range of caspase inhibitors were unable to prevent IκBα degradation in response to DoxR (Fig. 5C, compare lanes 1 to 10). Levels of p65 are shown as loading control. Taken together, these results indicate that increased apoptosis might not be responsible for the IκBα degradation induced in IKK1/2−/− MEFs by DoxR.
DoxR-mediated IκBα degradation is partially blocked by LY294002.
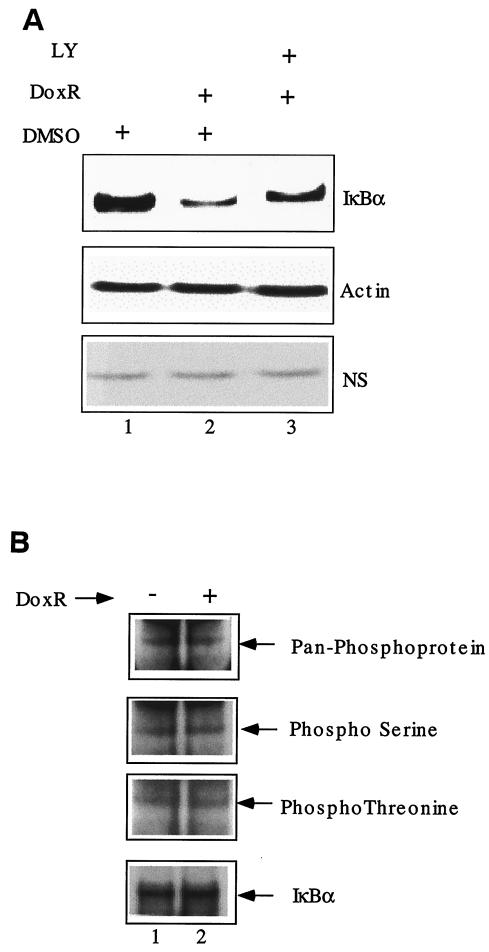
To better understand the molecular mechanism of IKK-independent IκBα degradation, we evaluated the role of phosphatidylinositol 3-kinases (PI3-kinases) in this process. Treatment with LY294002, a known inhibitor of PI3-kinase activity, partially blocked the IκBα degradation in response to DoxR (Fig. 6A, compare lanes 2 and 3). We suggest that PI3-kinases whose activity is LY294002 sensitive may be involved the IKK-independent IκBα degradation in a direct or indirect manner.
FIG. 6.
Role of other kinases in DoxR-mediated IκBα degradation. (A) IKK1/2−/− MEFs were treated with 0.7 μg of DoxR/ml, alone or in combination with LY294002 (80 μM). Western blot analysis was performed 24 h later. The levels of actin and a nonspecific (NS) protein were used as loading controls. (B) IKK1/2−/− MEFs were treated with 0.7 μg of DoxR/ml, along with the proteasome inhibitor lactacystin (20 μM), for 10 h. Treated and untreated cells were lysed, and IκBα was immunoprecipitated. The immunoprecipitated material was analyzed by Western blot with the indicated phospho-specific antibodies. The levels of total IκBα were analyzed by using the C21 antibody.
Apart from IKK1 and IKK2, other kinases have been documented to phosphorylate IκBα in vitro. To test whether IKK-independent phosphorylation of IκBα precedes its degradation, we evaluated the phosphorylation of IκBα in response to DoxR. IKK1/2−/− MEFs were treated with DoxR and lactacystin (see Fig. 8), a proteasome inhibitor, for 10 h to accumulate any modified IκBα species before degradation. Treated and untreated cells were lysed and IκBα was immunoprecipitated. The immunoprecipitated material was analyzed in a Western blot analysis with various phospho-specific antibodies (Fig. 6B, lanes 1 and 2). We did not find any detectable changes in phosphorylation status of IκBα by this method. More sensitive tools are required to detect modifications of IκBα that mark it for degradation in an IKK-independent manner.
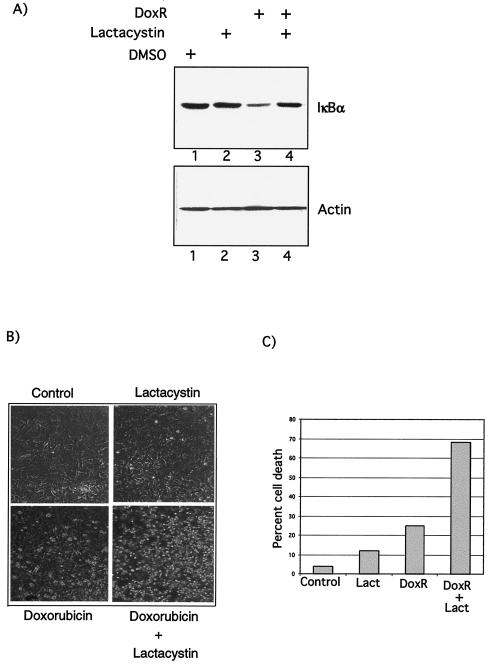
FIG. 8.
Functional relevance of NF-κB activity induced by DoxR. (A) DoxR-mediated IκBα degradation requires the proteasome. IKK1/2−/− MEFs were treated either with dimethyl sulfoxide, lactacystin (20 μM), or 0.7 μg of DoxR/ml alone or in combination as indicated. Lysates were prepared 24 h after treatment, and Western blot analysis was performed as described previously. (B) IKK1/2−/− MEFs were treated either with DoxR (0.7 μg/ml), lactacystin (20 μM), or a combination of the two drugs. Cells were visualized 24 h later. Detached and apoptotic cells are more refractile than the attached cells. (C) Quantitation of cell death induced by DoxR and lactacystin. Cell survival of attached and detached cells was measured after 24 to 30 h of treatment by using a trypan blue viability assay. This figure is a representative of three independent assays.
DoxR-induced IκBα degradation leads to NF-κB-dependent reporter gene expression.
We next investigated whether degradation of IκBα in response to DoxR could also lead to activation of NF-κB. Gel shift with consensus NF-κB oligonucleotide probe shows that treatment of IKK1/2−/− MEFs with DoxR led to increased NF-κB DNA-binding activity (Fig. 7A, left panel, compare lanes 1 to 6). Binding to the Oct-1 probe (middle panel) is used as a loading control. Further analysis of the DNA-binding complexes induced in response to DoxR by supershift assay indicated that they were composed of p65 and p50 subunits (Fig. 7A, right panel). To investigate whether DoxR induced IκBα degradation led to functional NF-κB transcriptional activity, we constructed lentiviruses carrying the luciferase reporter gene under the control of either five consensus or mutated NF-κB binding sites (Fig. 7B). IKK1/2−/− cells were transduced with LV-κBLuc or LV-Mut-κBLuc to obtain integrated copies of luciferase reporter gene. We consistently found that treatment of LV-κBLuc-transduced IKK1/2−/− cells with DoxR did lead to an increase in NF-κB-dependent reporter gene activity (Fig. 7C). In contrast, treatment of these cells with TNF-α and LPS treatment did not show detectable NF-κB reporter gene activity (Fig. 7C). Further, treatment of LV-Mut-κBLuc-transduced IKK1/2−/− MEFs with DoxR did not lead to increase in luciferase activity (Fig. 7D). Real-time PCR analysis revealed that treatment of DoxR does indeed induce the expression of IκBα, a known NF-κB target gene (Fig. 7E). We thus conclude that treatment of IKK1/2−/− MEFs with DoxR over a long time period does generate IKK-independent NF-κB transcriptional activity.
DoxR-mediated IκBα degradation requires the proteasome.
To decipher the mechanism of DoxR-induced IκBα degradation, we investigated the role of the proteasome, which is known to be essential for IκBα degradation in response to the canonical stimuli. DoxR-mediated IκBα degradation in IKK1/2−/− cells was significantly reduced upon incubation of cells with lactacystin, a natural, irreversible and highly specific nonpeptide proteasome inhibitor (Fig. 8A, compare lanes 3 and 4). This increase in IκBα stability was not due to an increase in steady-state levels of IκBα since the treatment of cells with lactacystin alone did not cause a detectable increase in the levels of IκBα in IKK1/2−/− MEFs (Fig. 8A, compare lanes 1 and 2).
We next tested the functional relevance of NF-κB activity induced by DoxR in IKK1/2−/− MEFs. Blocking of NF-κB activation by treatment of lactacystin dramatically increased DoxR-induced cell death in IKK1/2−/− MEFs (Fig. 8B). Quantitation of cell death is shown in Fig. 8C. We conclude that the IKK1- and IKK2-independent NF-κB activity in IKK1/2−/− cells could potentially contribute to prevent or decrease apoptosis in response to DoxR.
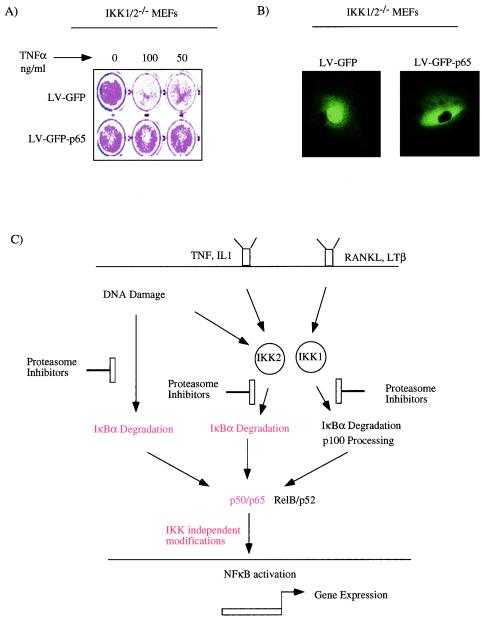
Free p65 can protect IKK1/2−/− MEFs against TNF-α-induced cell death.
To conclusively show that IKK1 and IKK2 mediated modification is not obligatory for p65 function, we tested whether p65 expression in IKK1/2−/− MEFs would protect them against TNF-α-induced apoptosis. IKK1/2−/− MEFs were transduced either with LV-GFP or LV-GFP-p65 at an MOI of 50, and pools of cells were treated with various doses of TNF-α. Unlike the control IKK1/2−/− MEFs, cells expressing the GFP-p65 fusion protein were significantly protected against TNF-induced cell death (Fig. 9A). To rule out gross overexpression of p65 that leads to constitutive nuclear activity of p65, we analyzed the localization of ectopic GFP-p65. Deconvolution microscopy revealed that the ectopically expressed p65 is primarily cytoplasmic (Fig. 9B). We propose that modification of p65 by IKK1 or IKK2 is not obligatory for its function, as judged by its ability to activate antiapoptotic genes and protect against TNF-α-induced apoptosis.
FIG. 9.
(A) Free p65 can protect against TNF-α-induced apoptosis in the absence of IKK activity. IKK1/2−/− MEFs transduced with LV-GFP or LV-GFP-p65 were treated with TNF-α, 48 h after transduction. Cell survival was estimated by staining the cells with crystal violet 48 h after TNF-α treatment. (B) Subcellular localization of GFP-p65. Live IKK1/2−/− cells transduced with LV-GFP or LV-GFP-p65 were imaged at ×60 magnification by using a deconvolution microscope. (C) Model based on our studies. Cytokines such as TNF-α and interleukin-1 primarily employ IKK2, and lymphotoxin β (LTβ) and RANK ligand (RANKL) use IKK1 to activate NF-κB. Unlike these stimuli, DoxR can activate NF-κB in an IKK1- and IKK2-independent manner. Despite their different origins, all of the pathways of NF-κB activation are blocked by proteasome inhibitors. Based on data presented in this report, functional activation by p65/p50 complexes (shown in red) generated after TNF-α signaling (A) or DoxR induction (Fig. 7C and E) do not require further modification by IKK1 or IKK2.
DISCUSSION
Apart from the p53 status, additional mutations and genetic variability, such as amplification of Mdm2 (24) and HER-2/neu (43), and mutations/deletions in INK4a/ARF loci (30), are crucial modifiers of a tumor's response to chemotherapy. Deregulated activity of the transcription factor NF-κB has frequently been observed in several cancers (22, 33). The NF-κB pathway impinges on multiple aspects of cell growth and apoptosis and has been shown to protect against various apoptotic stimuli (1), including chemotherapeutic agents (7, 8). Hence, understanding the regulatory mechanisms that control NF-κB in tumors is of paramount interest.
Multiple modes of NF-κB activation.
A plethora of external signals employ NF-κB to carry out their instructive functions in vertebrate and nonvertebrate cells (31). Hence, only stimuli that activate NF-κB in a rapid manner are considered to be physiological. Most rapid-acting stimuli, such as cytokines, activate NF-κB mainly through IKK2. Recent evidence has suggested that exclusively IKK1-dependent, IKK2-independent pathways of NF-κB activation also exist (26). These IKK1-dependent NF-κB activation pathways are crucial for lymphoid development and organogenesis (26). As expected, the MEFs derived from IKK1/2−/− mice are refractory to most of the canonical NF-κB inducers (18). It is, however, possible that there are stimuli that activate NF-κB independently of both IKK1 and IKK2 and that this NF-κB activity generated upon prolonged treatment does have physiological relevance.
In the present study, we report that prolonged treatment of IKK1/2−/− MEFs with DoxR led to degradation of IκBα (Fig. 1A) and IκBβ (Fig. 5A). Surprisingly, IκBα degradation by DoxR did not require serine 32 and 36 phosphorylation (Fig. 3) or the PEST domain of IκBα (Fig. 4). The degradation observed in our assays was not mediated by ROS (Fig. 5A), p53 (Fig. 5B), or caspases (Fig. 5C). We further show that the IκB degradation in the absence of IKK1 or IKK2 activity is mediated by the proteasome (Fig. 8A). IKK-independent IκBα degradation pathways have previously been described (2, 17). Although these degradation pathways (2, 17) did not require the serine 32 and 36 phosphorylation, the kinetics of degradation described are different than those observed in our assays. Unlike IKK-independent IκBα degradation, the properties and putative roles of free NF-κB in the complete absence of IKK1 or IKK2 activity have not been documented before. We document that the free NF-κB generated by DoxR-mediated IκB degradation is able to activate NF-κB-dependent reporter gene integrated in the chromosomes (Fig. 7C) and also induce the expression of at least one physiological target gene, IκBα (Fig. 7E). Furthermore, our preliminary results suggest that PI3-kinases might be involved in IκBα degradation in the absence of IKK (Fig. 6A). Based on the established role of ATM and ATR kinases in NF-κB activation (16), we believe that PI3-kinases might act as upstream sensors of DoxR-mediated DNA damage and might not be direct IκBα kinases in our assays.
IKK not needed for p65 function.
Cytoplasmic retention of NF-κB by IκB is the major mechanism that controls its activity (37). The role of IκBα in controlling NF-κB activity was underscored by the observation that nondegradable versions of this molecule (dominant-negative IκBα mutants) block NF-κB activation (36). Although it is widely held that the mere degradation of IκB and the subsequent release of NF-κB is sufficient to activate this important signaling cascade, several lines of evidence point to the fact that additional regulatory mechanisms are involved. First, it has been documented that phosphorylation of p65, an NF-κB subunit, increases its DNA binding in vitro (21, 23). Second, p300/CBP and p/CAF histone acetyltransferases preferentially associate with phosphorylated p65 (42). Third, the phenotypic similarity and/or an apparent defect in NF-κB activation in mice that are null for kinases such as GSK3β (11), Tbk/t2k/NAK (3, 35), and PKCζ (15) make it attractive to include them in the pathway. In addition, the PKA catalytic subunit (41) and CKII have been shown to directly associate with the NF-κB:IκB complex (38). Finally, the demonstration that NF-κB is acetylated and that acetylation and deacetylation control its subcellular distribution brings into play additional factors that might regulate this pathway (6).
Intriguingly, the IKKs have been assigned a role in modifying p65/RelA subsequent to its release from IκB (13, 29, 32). Our observations that free p65 can activate NF-κB-dependent reporter gene (Fig. 7C), activate a physiological target gene such as IκBα (Fig. 7E), and also protect against TNF-α-induced apoptosis (Fig. 9A) in IKK1/2−/− MEFs calls into question the role of IKKs in modifying p65. It is possible that, unlike their role in IκB degradation by canonical stimuli, there is redundancy in the activity of these kinases with regard to NF-κB modification. It is not clear whether all kinases implicated in NF-κB phosphorylation act sequentially or modify independent sites simultaneously. At least PKCζ appears to regulate NF-κB phosphorylation both directly and through regulation of IKKs (15). The role of these additional mechanisms might simply be to fine-tune the response to a certain stimuli. This case is best exemplified by the Nik−/− mice; these mice are viable, and cells derived from them activate NF-κB in response to all of the stimuli tested except lymphotoxin-β (40). Alternatively, these mechanisms might be required to control the strength or the quality of NF-κB response in select cell types. Understanding the chronology of events that lead to the generation of active NF-κB molecules after IκB degradation will provide more targets for therapeutic intervention.
Implication for chemotherapy.
We have earlier demonstrated that, compared to wild-type MEFs, IKK1/2−/− MEFs are more sensitive to cell death and p53 induction in response to DoxR (34). Reconstitution of IKK2 but not IKK1 can block this sensitivity to p53 stabilization and repress DoxR-induced cell death measured between 60 and 72 h. We proposed that the IKK1/2−/− MEFs fail to activate IKK2-dependent antiapoptotic genes which might be required for protection against cell death. The findings presented in this paper suggest that even in the absence of IKK1 and IKK2 activity, a small fraction of NF-κB is activated. Further, this activation generates functional transcriptional activity (Fig. 7C and E) as measured within 24 h of treatment. Since proteasome inhibition sensitized IKK1/2−/− MEFs for cell death within 24 h (Fig. 8B and C), we believe that the DoxR-induced NF-κB activity observed during the same time scale in IKK1/2−/− cells could potentially protect against DoxR-induced cell death measured between 24 and 30 h (Fig. 8B and C). In tumors with additional mutations, a delay in apoptosis by this residual NF-κB activity could permit other genetic modifications that might ultimately make the tumor more resistant to chemotherapy. Since the level of protection required upon prolonged treatment with DoxR might require additional genes, which are regulated by IKK2, IKK1/2−/− MEFs are more sensitive (compared to wild-type MEFs) to DoxR-induced cell death measured at 72 h (34). The exact mechanism of IKK2-dependent compared to IKK2-independent protection against DoxR-induced cell death would need the identification NF-κB target genes turned on in the presence or absence of IKK2. As shown by the distinct phenotypes of mice lacking distinct NF-κB subunits, the activation of different NF-κB dimers in presence or absence of IKK2 could dictate the type of genes that are activated. Clearly, deciphering the temporal regulation of the number and the amount of target genes expressed would help understanding the combination of genes which decide the outcome of a cells response to chemotherapy.
Based on the crucial role of IKK2 in regulating NF-κB activity in response to anticancer drugs (34), designing IKK2-specific inhibitors could be instrumental in selectively blocking NF-κB. However, IKK2 inhibitors would not block IKK-independent NF-κB activity described in the present study. As in the case of NF-κB activation by classical stimuli (14), the IKK-independent NF-κB activation is also blocked by proteasome inhibitors (Fig. 8A). We propose that the efficient augmentation of chemotherapy induced cell death by proteasome inhibitors (7) is reflective of their ability to block NF-κB activity generated by the IKK-dependent and -independent pathways. Thus, our results suggest that proteasome inhibition rather than IKK inhibition is likely to be a better means to adjuvant chemotherapy. A model based on our studies is presented in Fig. 9C. In summary, our results provide evidence for generation of IKK-independent NF-κB activity in tumors during long-term chemotherapy. Further evaluation of more physiological stimuli that could generate similar activity might also give insights regarding the persistent NF-κB activity observed in human malignancies.
Acknowledgments
We thank Bert Vogelstein and Wafik El-Deiry for the rAD-p53 virus and Matt Weitzman for helpful discussions.
V.T. is supported by a carrier development fellowship from the Leukemia and Lymphoma Society. V.B. is supported by a fellowship from La Ligue Nationale contre le Cancer. Q.L. is a special fellow of the Leukemia and Lymphoma Society. I.M.V. is an American Cancer Society Professor of Molecular Biology and is supported by grants from the NIH, the Larry L. Hillblom Foundation, Inc., the Lebensfeld Foundation, the Wayne and Gladys Valley Foundation, and the H. N. and Frances C. Berger Foundation.
REFERENCES
- 1.Baichwal, V. R., and P. A. Baeuerle. 1997. Activate NF-κB or die? Curr. Biol. 7:R94-R96. [DOI] [PubMed] [Google Scholar]
- 2.Bender, K., M. Gottlicher, S. Whiteside, H. J. Rahmsdorf, and P. Herrlich. 1998. Sequential DNA damage-independent and -dependent activation of NF-κB by UV. EMBO J. 17:5170-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnard, M., C. Mirtsos, S. Suzuki, K. Graham, J. Huang, M. Ng, A. Itie, A. Wakeham, A. Shahinian, W. J. Henzel, A. J. Elia, W. Shillinglaw, T. W. Mak, Z. Cao, and W. C. Yeh. 2000. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-κB-dependent gene transcription. EMBO J. 19:4976-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, K., S. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of IκB-alpha proteolysis by site-specific, signal-induced phosphorylation. Science 267:1485-1488. [DOI] [PubMed] [Google Scholar]
- 5.Chen, G., P. Cao, and D. V. Goeddel. 2002. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol. Cell 9:401-410. [DOI] [PubMed] [Google Scholar]
- 6.Chen, L., W. Fischle, E. Verdin, and W. C. Greene. 2001. Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293:1653-1657. [DOI] [PubMed] [Google Scholar]
- 7.Cusack, J. C., Jr., R. Liu, M. Houston, K. Abendroth, P. J. Elliott, J. Adams, and A. S. Baldwin, Jr. 2001. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-κB inhibition. Cancer Res. 61:3535-3540. [PubMed] [Google Scholar]
- 8.Cusack, J. C., R. Liu, and A. S. Baldwin. 1999. NF-κB and chemoresistance: potentiation of cancer drugs via inhibition of NF-κB. Drug Resist. Update 2:271-273. [DOI] [PubMed] [Google Scholar]
- 9.Garg, A. K., and B. B. Aggarwal. 2002. Reactive oxygen intermediates in TNF signaling. Mol. Immunol. 39:509-517. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-κB puzzle. Cell 109(Suppl.):S81-S96. [DOI] [PubMed] [Google Scholar]
- 11.Hoeflich, K. P., J. Luo, E. A. Rubie, M. S. Tsao, O. Jin, and J. R. Woodgett. 2000. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature 406:86-90. [DOI] [PubMed] [Google Scholar]
- 12.Ikawa, M., V. Tergaonkar, A. Ogura, N. Ogonuki, K. Inoue, and I. M. Verma. 2002. Restoration of spermatogenesis by lentiviral gene transfer: offspring from infertile mice. Proc. Natl. Acad. Sci. USA 99:7524-7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang, X., N. Takahashi, N. Matsui, T. Tetsuka, and T. Okamoto. 2003. The NF-κB activation in lymphotoxin beta receptor signaling depends on the phosphorylation of p65 at serine 536. J. Biol. Chem. 278:919-926. [DOI] [PubMed] [Google Scholar]
- 14.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 15.Leitges, M., L. Sanz, P. Martin, A. Duran, U. Braun, J. F. Garcia, F. Camacho, M. T. Diaz-Meco, P. D. Rennert, and J. Moscat. 2001. Targeted disruption of the ζPKC gene results in the impairment of the NF-κB pathway. Mol. Cell 8:771-780. [DOI] [PubMed] [Google Scholar]
- 16.Li, N., S. Banin, H. Ouyang, G. C. Li, G. Courtois, Y. Shiloh, M. Karin, and G. Rotman. 2001. ATM is required for IκB kinase (IKKκ) activation in response to DNA double strand breaks. J. Biol. Chem. 276:8898-8903. [DOI] [PubMed] [Google Scholar]
- 17.Li, N., and M. Karin. 1998. Ionizing radiation and short wavelength UV activate NF-κB through two distinct mechanisms. Proc. Natl. Acad. Sci. USA 95:13012-13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, Q., G. Estepa, S. Memet, A. Israel, and I. M. Verma. 2000. Complete lack of NF-κB activity in IKK1 and IKK2 double-deficient mice: additional defect in neurulation. Genes Dev. 14:1729-1733. [PMC free article] [PubMed] [Google Scholar]
- 19.Li, Q., and I. M. Verma. 2002. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2:725-734. [DOI] [PubMed] [Google Scholar]
- 20.Miyake, S., M. Makimura, Y. Kanegae, S. Harada, Y. Sato, K. Takamori, C. Tokuda, and I. Saito. 1996. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc. Natl. Acad. Sci. USA 93:1320-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosialos, G., and T. D. Gilmore. 1993. v-Rel and c-Rel are differentially affected by mutations at a consensus protein kinase recognition sequence. Oncogene 8:721-730. [PubMed] [Google Scholar]
- 22.Nakshatri, H., P. Bhat-Nakshatri, D. A. Martin, R. J. Goulet, and G. W. Sledge. 1997. Constitutive activation of NF-κB during progression of breast cancer to hormone-independent growth. Mol. Cell. Biol. 17:3629-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neumann, M., T. Grieshammer, S. Chuvpilo, B. Kneitz, M. Lohoff, A. Schimpl, B. R. Franza, Jr., and E. Serfling. 1995. RelA/p65 is a molecular target for the immunosuppressive action of protein kinase A. EMBO J. 14:1991-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliner, J. D., K. W. Kinzler, P. S. Meltzer, D. L. George, and B. Vogelstein. 1992. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature 358:80-83. [DOI] [PubMed] [Google Scholar]
- 25.Pando, M. P., and I. M. Verma. 2000. Signal-dependent and -independent degradation of free and NF-κB-bound IκBα. J. Biol. Chem. 275:21278-21286. [DOI] [PubMed] [Google Scholar]
- 26.Pomerantz, J. L., and D. Baltimore. 2002. Two pathways to NF-κB. Mol. Cell 10:693-695. [DOI] [PubMed] [Google Scholar]
- 27.Rayet, B., and C. Gelinas. 1999. Aberrant rel/nfkb genes and activity in human cancer. Oncogene 18:6938-6947. [DOI] [PubMed] [Google Scholar]
- 28.Ryan, K. M., M. K. Ernst, N. R. Rice, and K. H. Vousden. 2000. Role of NF-κB in p53-mediated programmed cell death. Nature 404:892-897. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai, H., H. Chiba, H. Miyoshi, T. Sugita, and W. Toriumi. 1999. IκB kinases phosphorylate NF-κB p65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 274:30353-30356. [DOI] [PubMed] [Google Scholar]
- 30.Sherr, C. J. 2001. The INK4a/ARF network in tumour suppression. Nat. Rev. Mol. Cell. Biol. 2:731-737. [DOI] [PubMed] [Google Scholar]
- 31.Silverman, N., and T. Maniatis. 2001. NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dev. 15:2321-2342. [DOI] [PubMed] [Google Scholar]
- 32.Sizemore, N., N. Lerner, N. Dombrowski, H. Sakurai, and G. R. Stark. 2002. Distinct roles of the IκB kinase alpha and beta subunits in liberating nuclear factor κB (NF-κB) from IκB and in phosphorylating the p65 subunit of NF-κB. J. Biol. Chem. 277:3863-3869. [DOI] [PubMed] [Google Scholar]
- 33.Sovak, M. A., R. E. Bellas, D. W. Kim, G. J. Zanieski, A. E. Rogers, A. M. Traish, and G. E. Sonenshein. 1997. Aberrant nuclear factor-κB/Rel expression and the pathogenesis of breast cancer. J. Clin. Investig. 100:2952-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tergaonkar, V., M. Pando, O. Vafa, G. Wahl, and I. Verma. 2002. p53 stabilization is decreased upon NF-κB activation: a role for NF-κB in acquisition of resistance to chemotherapy. Cancer Cell 1:493-503. [DOI] [PubMed] [Google Scholar]
- 35.Tojima, Y., A. Fujimoto, M. Delhase, Y. Chen, S. Hatakeyama, K. Nakayama, Y. Kaneko, Y. Nimura, N. Motoyama, K. Ikeda, M. Karin, and M. Nakanishi. 2000. NAK is an IκB kinase-activating kinase. Nature 404:778-782. [DOI] [PubMed] [Google Scholar]
- 36.Van Antwerp, D. J., S. J. Martin, T. Kafri, D. R. Green, and I. M. Verma. 1996. Suppression of TNF-α-induced apoptosis by NF-κB. Science 274:787-789. [DOI] [PubMed] [Google Scholar]
- 37.Verma, I. M., J. K. Stevenson, E. M. Schwarz, D. Van Antwerp, and S. Miyamoto. 1995. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 9:2723-2735. [DOI] [PubMed] [Google Scholar]
- 38.Wang, D., S. D. Westerheide, J. L. Hanson, and A. S. Baldwin, Jr. 2000. Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J. Biol. Chem. 275:32592-32597. [DOI] [PubMed] [Google Scholar]
- 39.Yamaoka, S., G. Courtois, C. Bessia, S. T. Whiteside, R. Weil, F. Agou, H. E. Kirk, R. J. Kay, and A. Israel. 1998. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell 93:1231-1240. [DOI] [PubMed] [Google Scholar]
- 40.Yin, L., L. Wu, H. Wesche, C. D. Arthur, J. M. White, D. V. Goeddel, and R. D. Schreiber. 2001. Defective lymphotoxin-beta receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science 291:2162-2165. [DOI] [PubMed] [Google Scholar]
- 41.Zhong, H., H. SuYang, H. Erdjument-Bromage, P. Tempst, and S. Ghosh. 1997. The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89:413-424. [DOI] [PubMed] [Google Scholar]
- 42.Zhong, H., R. E. Voll, and S. Ghosh. 1998. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1:661-671. [DOI] [PubMed] [Google Scholar]
- 43.Zhou, B. P., Y. Liao, W. Xia, Y. Zou, B. Spohn, and M. C. Hung. 2001. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat. Cell Biol. 3:973-982. [DOI] [PubMed] [Google Scholar]