Abstract
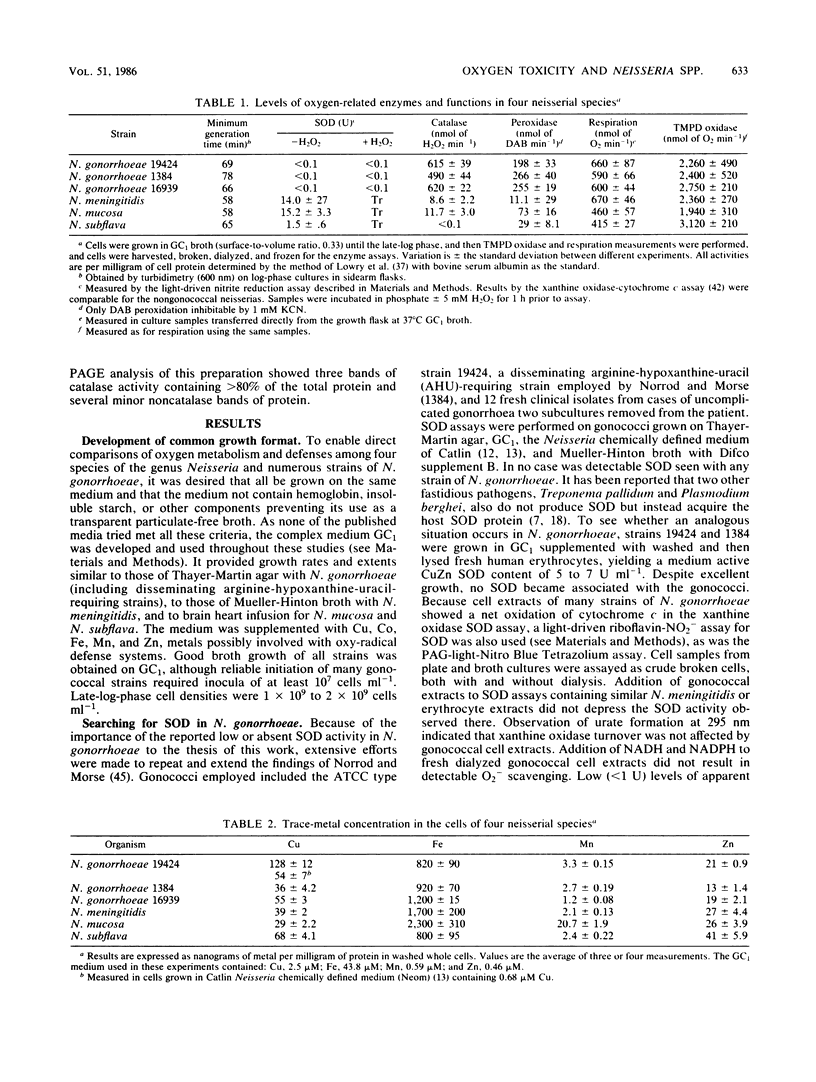
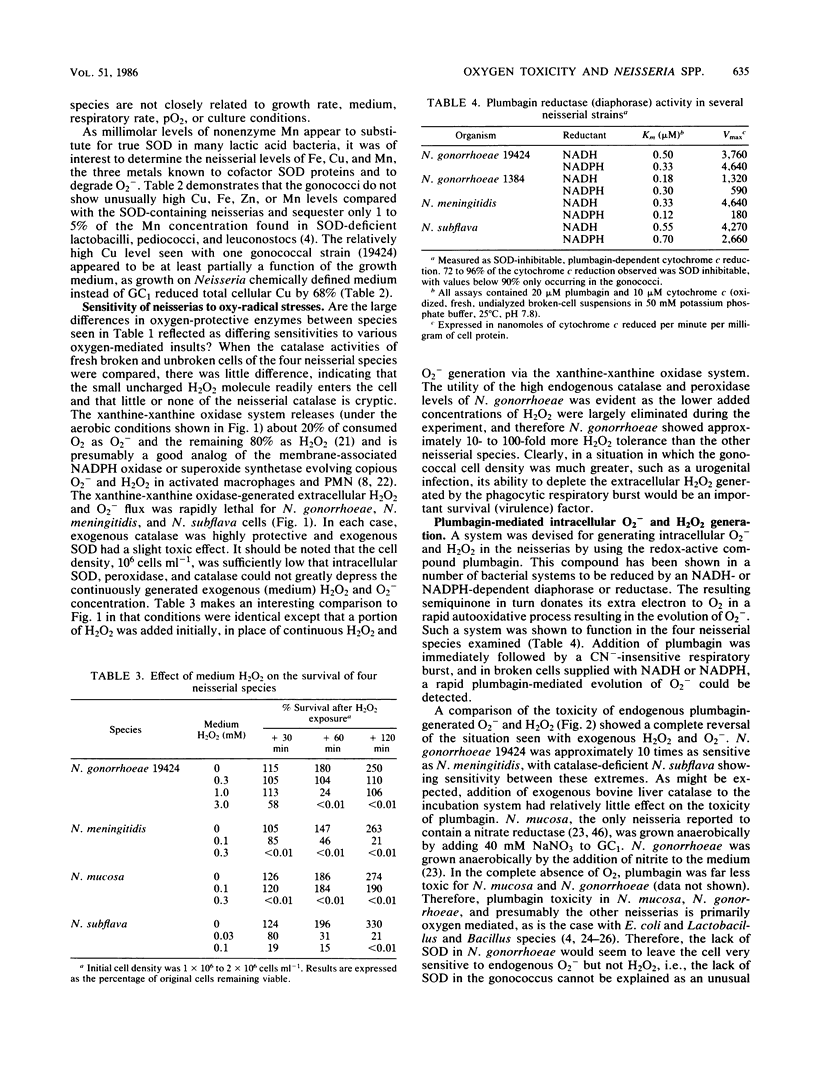
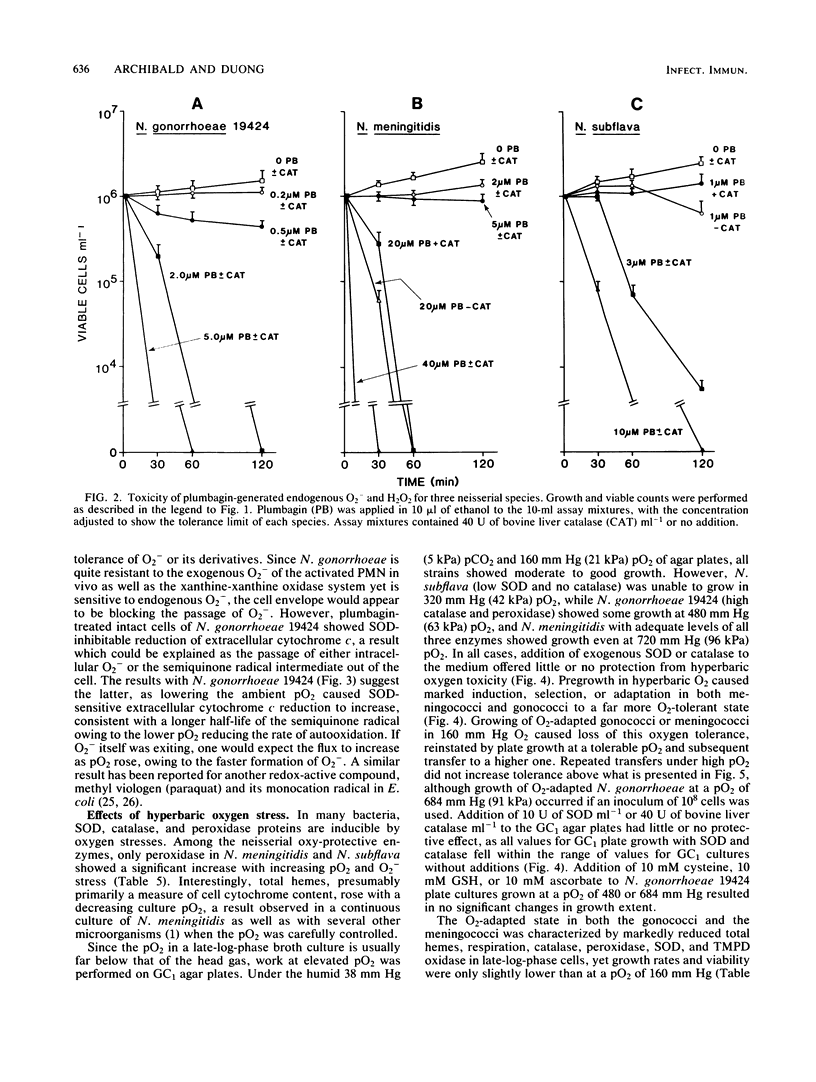
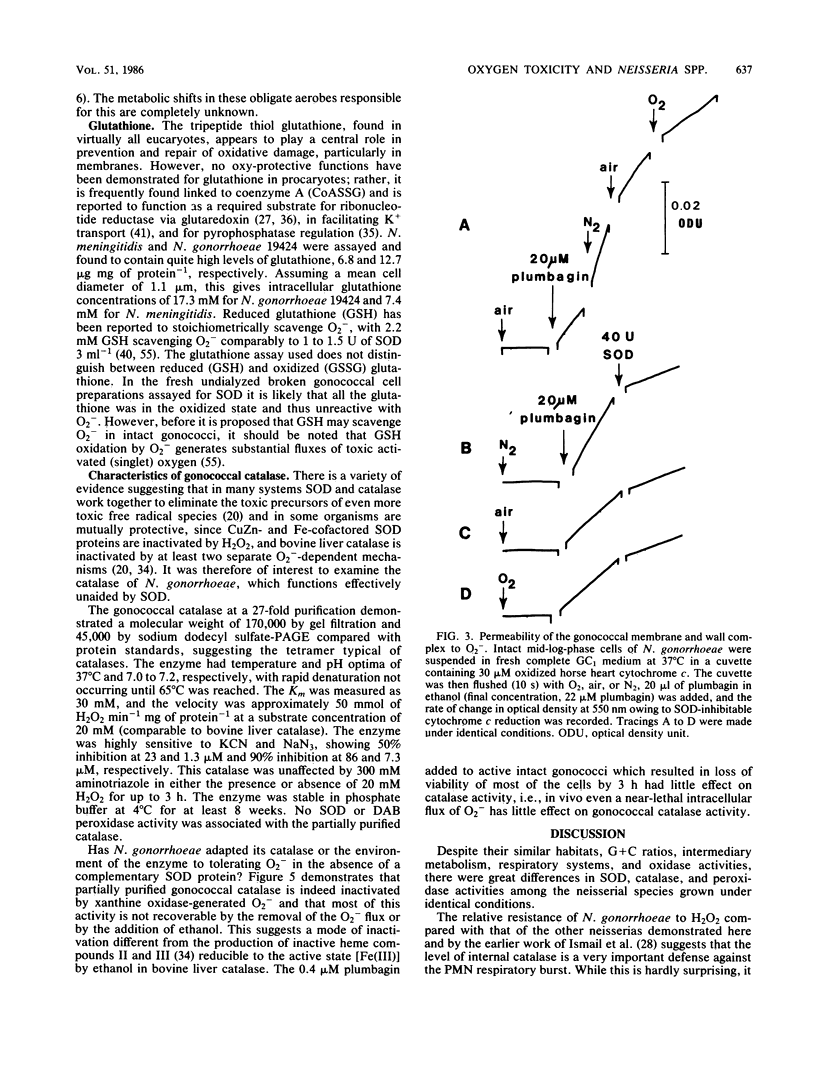
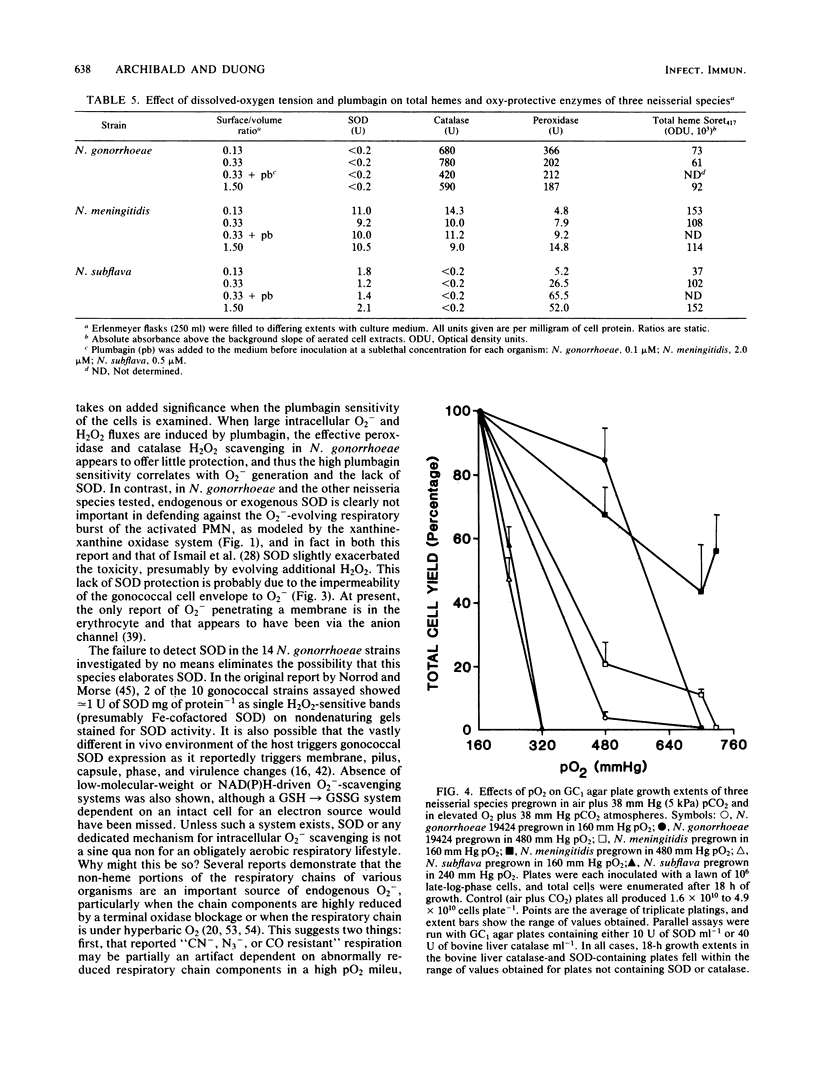
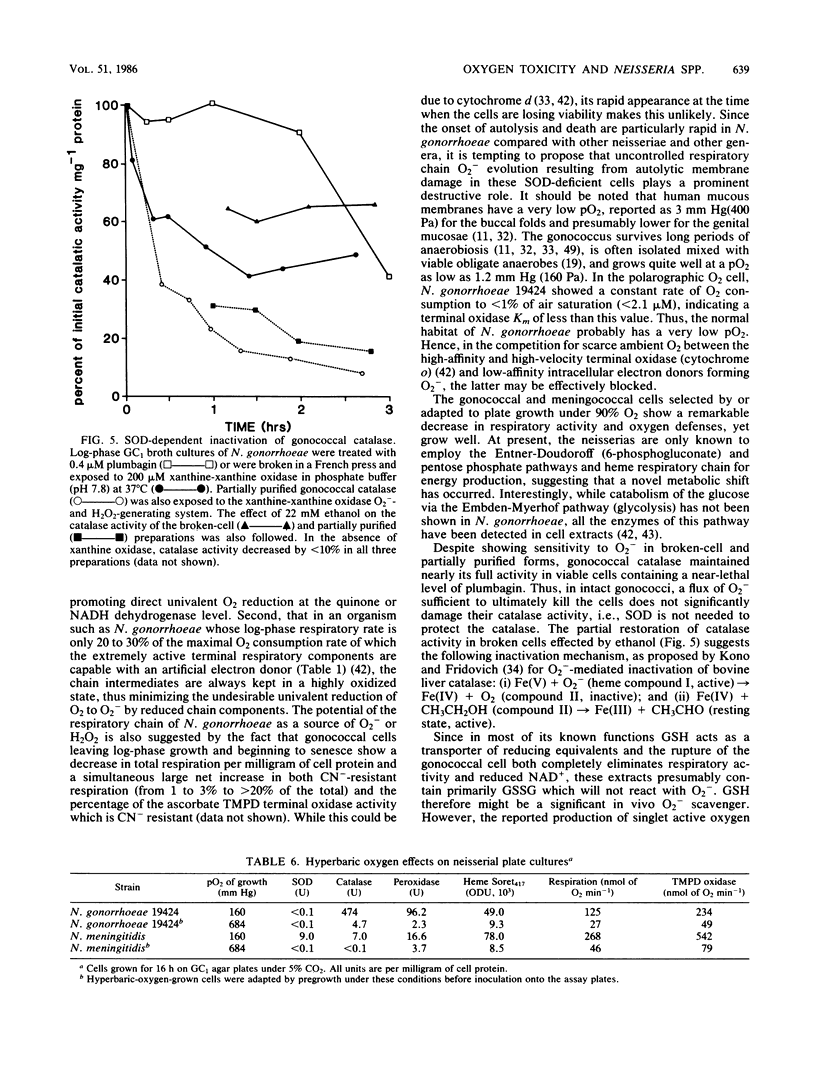
Among aerotolerant cells, Neisseria gonorrhoeae is very unusual because despite its obligately aerobic lifestyle and frequent isolation from purulent exudates containing polymorphonuclear leukocytes vigorously evolving O2- and H2O2, it contains no superoxide dismutase (SOD). Strains (14) of N. gonorrhoeae were compared with each other and with strains of Neisseria meningitidis, Neisseria mucosa, and Neisseria subflava under identical growth conditions for their contents of the oxy-protective enzymes catalase, peroxidase, and SOD, as well as respiratory chain proteins and activity. The absence of SOD from N. gonorrhoeae strains was demonstrated under a variety of oxygen-stress conditions. The neisserial species showed very different SOD, catalase, and peroxidase profiles. These profiles correlated well with the tolerance of the species to various intra- and extracellular oxygen insults. The high tolerance of N. gonorrhoeae for extracellular O2- and H2O2 appeared to be due to very high constitutive levels of peroxidase and catalase activity combined with a cell envelope impervious to O2-. Nevertheless, N. gonorrhoeae 19424 was much more sensitive to an intracellular flux of O2- than were the other (SOD-containing) neisserial species. The responses of N. gonorrhoeae and N. meningitidis respiratory and oxy-protective enzymes to growth under high and low oxygen tensions were followed, and a novel response, the apparent repression of the respiratory chain intermediates, respiration, and SOD, peroxidase, and catalase activity, was observed. The gonococcal catalase was partially purified and characterized. The results suggest that the very active terminal oxidase, low pO2 natural habitat, O2-stable catalase, and possibly the high glutathione content of the organism explain its aerobic survival in the absence of SOD.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S., DeVoe I. W. Iron in Neisseria meningitidis: minimum requirements, effects of limitation, and characteristics of uptake. J Bacteriol. 1978 Oct;136(1):35–48. doi: 10.1128/jb.136.1.35-48.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Duong M. N. Manganese acquisition by Lactobacillus plantarum. J Bacteriol. 1984 Apr;158(1):1–8. doi: 10.1128/jb.158.1.1-8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981 Jan;145(1):442–451. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J Bacteriol. 1981 Jun;146(3):928–936. doi: 10.1128/jb.146.3.928-936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. The scavenging of superoxide radical by manganous complexes: in vitro. Arch Biochem Biophys. 1982 Apr 1;214(2):452–463. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- Arko R. J., Odugbemi T. Superoxol and amylase inhibition tests for distinguishing gonococcal and nongonococcal cultures growing on selective media. J Clin Microbiol. 1984 Jul;20(1):1–4. doi: 10.1128/jcm.20.1.1-4.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin F. E., Barbieri J. T., Corin R. E., Grigas K. E., Cox C. D. Distribution of superoxide dismutase, catalase, and peroxidase activities among Treponema pallidum and other spirochetes. Infect Immun. 1981 Aug;33(2):372–379. doi: 10.1128/iai.33.2.372-379.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Britton L., Malinowski D. P., Fridovich I. Superoxide dismutase and oxygen metabolism in Streptococcus faecalis and comparisons with other organisms. J Bacteriol. 1978 Apr;134(1):229–236. doi: 10.1128/jb.134.1.229-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey S. G., Shafer W. M., Spitznagel J. K. Anaerobiosis increases resistance of Neisseria gonorrhoeae to O2-independent antimicrobial proteins from human polymorphonuclear granulocytes. Infect Immun. 1985 Feb;47(2):401–407. doi: 10.1128/iai.47.2.401-407.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Clare D. A., Duong M. N., Darr D., Archibald F., Fridovich I. Effects of molecular oxygen on detection of superoxide radical with nitroblue tetrazolium and on activity stains for catalase. Anal Biochem. 1984 Aug 1;140(2):532–537. doi: 10.1016/0003-2697(84)90204-5. [DOI] [PubMed] [Google Scholar]
- Devoe I. W., Gilchrist J. E. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973 Nov 1;138(5):1156–1167. doi: 10.1084/jem.138.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Masi A. T. Disseminated gonococcal infection (DGI) and gonococcal arthritis (GCA): I. Bacteriology, epidemiology, host factors, pathogen factors, and pathology. Semin Arthritis Rheum. 1981 Feb;10(3):155–172. doi: 10.1016/s0049-0172(81)80001-7. [DOI] [PubMed] [Google Scholar]
- Elstner E. F., Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem. 1976 Feb;70(2):616–620. doi: 10.1016/0003-2697(76)90488-7. [DOI] [PubMed] [Google Scholar]
- Fairfield A. S., Meshnick S. R., Eaton J. W. Malaria parasites adopt host cell superoxide dismutase. Science. 1983 Aug 19;221(4612):764–766. doi: 10.1126/science.6348944. [DOI] [PubMed] [Google Scholar]
- Fontaine E. A., Taylor-Robinson D., Hanna N. F., Coufalik E. D. Anaerobes in men with urethritis. Br J Vener Dis. 1982 Oct;58(5):321–326. doi: 10.1136/sti.58.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Gabig T. G., Babior B. M. The O2(-) -forming oxidase responsible for the respiratory burst in human neutrophils. Properties of the solubilized enzyme. J Biol Chem. 1979 Sep 25;254(18):9070–9074. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979 Sep;196(2):385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Paraquat and Escherichia coli. Mechanism of production of extracellular superoxide radical. J Biol Chem. 1979 Nov 10;254(21):10846–10852. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of superoxide dismutase in Escherichia coli. Induction by methyl viologen. J Biol Chem. 1977 Nov 10;252(21):7667–7672. [PubMed] [Google Scholar]
- Hög J. O., Holmgren A., D'Silva C., Douglas K. T., Seddon A. P. Glutathione derivatives as inhibitors of glutaredoxin and ribonucleotide reductase from Escherichia coli. FEBS Lett. 1982 Feb 8;138(1):59–61. doi: 10.1016/0014-5793(82)80394-3. [DOI] [PubMed] [Google Scholar]
- Ismail G., Sawyer W. D., Wegener W. S. Effect of hydrogen peroxidase and superoxide radical on viability of Neisseria gonorrhoeae and related bacteria. Proc Soc Exp Biol Med. 1977 Jun;155(2):264–269. doi: 10.3181/00379727-155-39786. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Aston P. R., Old L. Enzymatic oxidation of tetramethyl-p-phenylenediamine and p-phenylenediamine by the electron transport particulate fraction of Azotobacter vinelandii. J Bacteriol. 1967 Mar;93(3):1069–1078. doi: 10.1128/jb.93.3.1069-1078.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtshuk P., Milligan T. W. Preliminary characterization studies on the Neisseria catarrhalis respiratory electron transport chain. J Bacteriol. 1974 Oct;120(1):552–555. doi: 10.1128/jb.120.1.552-555.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtshuk P., Milligan T. W. Quantitation of the tetramethyl-p-phenylenediamine oxidase reaction in Neisseria species. Appl Microbiol. 1974 Dec;28(6):1079–1081. doi: 10.1128/am.28.6.1079-1081.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Crawford J. A., Callaway C. S. Cultivation of Neisseria gonorrhoeae under low-oxygen conditions. J Clin Microbiol. 1983 Jul;18(1):178–184. doi: 10.1128/jcm.18.1.178-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenimer E. A., Lapp D. F. Effects of selected inhibitors on electron transport in Neisseria gonorrhoeae. J Bacteriol. 1978 May;134(2):537–545. doi: 10.1128/jb.134.2.537-545.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982 May 25;257(10):5751–5754. [PubMed] [Google Scholar]
- Lahti R., Suonpä M. Role of glutathione in the regulation of inorganic pyrophosphatase activity in Streptococcus faecalis. J Gen Microbiol. 1982 May;128(5):1023–1026. doi: 10.1099/00221287-128-5-1023. [DOI] [PubMed] [Google Scholar]
- Loewen P. C. Identification of a coenzyme A--glutathione disulfide (DSI), a modified coenzyme A disulfide (DSII), and a NADPH-dependent coenzyme A--glutathione disulfide reductase in E. coli. Can J Biochem. 1977 Oct;55(10):1019–1027. doi: 10.1139/o77-152. [DOI] [PubMed] [Google Scholar]
- Lynch R. E., Cole B. C. Mycoplasma pneumoniae: a prokaryote which consumes oxygen and generates superoxide but which lacks superoxide dismutase. Biochem Biophys Res Commun. 1980 Sep 16;96(1):98–105. doi: 10.1016/0006-291x(80)91186-9. [DOI] [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978 Jul 10;253(13):4697–4699. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Meury J., Kepes A. Glutathione and the gated potassium channels of Escherichia coli. EMBO J. 1982;1(3):339–343. doi: 10.1002/j.1460-2075.1982.tb01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Cacciapuoti A. F., Lysko P. G. Physiology of Neisseria gonorrhoeae. Adv Microb Physiol. 1979;20:251–320. doi: 10.1016/s0065-2911(08)60209-x. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrod P., Morse S. A. Absence of superoxide dismutase in some strains of Neisseria gonorrhoeae. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1287–1294. doi: 10.1016/0006-291x(79)91176-8. [DOI] [PubMed] [Google Scholar]
- Saginur R., Clecner B., Portnoy J., Mendelson J. Superoxol(catalase)test for identification of Neisseria gonorrhoeae. J Clin Microbiol. 1982 Mar;15(3):475–477. doi: 10.1128/jcm.15.3.475-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short H. B., Clark V. L., Kellogg D. S., Jr, Young F. E. Anaerobic survival of clinical isolates and laboratory strains of Neisseria gonorrhoea: use in transfer and storage. J Clin Microbiol. 1982 May;15(5):915–919. doi: 10.1128/jcm.15.5.915-919.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. C., Silver L. E., Clark V. L., Young F. E. Construction and characterization of a new shuttle vector, pLES2, capable of functioning in Escherichia coli and Neisseria gonorrhoeae. Gene. 1983 Nov;25(2-3):241–247. doi: 10.1016/0378-1119(83)90228-7. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Touati D. Cloning and mapping of the manganese superoxide dismutase gene (sodA) of Escherichia coli K-12. J Bacteriol. 1983 Sep;155(3):1078–1087. doi: 10.1128/jb.155.3.1078-1087.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens J. F., Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980 Nov 1;191(2):421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefers H., Sies H. Oxidation of glutathione by the superoxide radical to the disulfide and the sulfonate yielding singlet oxygen. Eur J Biochem. 1983 Dec 1;137(1-2):29–36. doi: 10.1111/j.1432-1033.1983.tb07791.x. [DOI] [PubMed] [Google Scholar]
- Whitelam G. C., Codd G. A. A rapid whole-cell assay for superoxide dismutase. Anal Biochem. 1982 Mar 15;121(1):207–212. doi: 10.1016/0003-2697(82)90577-2. [DOI] [PubMed] [Google Scholar]
- Young H., Harris A. B., Tapsall J. W. Differentiation of gonococcal and non-gonococcal neisseriae by the superoxol test. Br J Vener Dis. 1984 Apr;60(2):87–89. doi: 10.1136/sti.60.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]


