Abstract
Pharmacological studies show that ghrelin stimulates growth hormone release, appetite, and fat deposition, but ghrelin's physiological role in energy homeostasis has not been established. Ghrelin was also proposed to regulate leptin and insulin release and to be important for the normal function of stomach, heart, kidney, lung, testis, and placenta. To help determine a definable physiological role for ghrelin, we generated ghrelin-null mice. In contrast to predictions made from the pharmacology of ghrelin, ghrelin-null mice are not anorexic dwarfs; their size, growth rate, food intake, body composition, reproduction, gross behavior, and tissue pathology are indistinguishable from wild-type littermates. Fasting produces identical decreases in serum leptin and insulin in null and wild-type mice. Ghrelin-null mice display normal responses to starvation and diet-induced obesity. As in wild-type mice, the administration of exogenous ghrelin stimulates appetite in null mice. Our data show that ghrelin is not critically required for viability, fertility, growth, appetite, bone density, and fat deposition and not likely to be a direct regulator of leptin and insulin. Therefore, antagonists of ghrelin are unlikely to have broad utility as antiobesity agents.
Ghrelin is an endogenous ligand for the growth hormone secretagogue receptor (GHS-R). GHS-R was first characterized, cloned, and identified as the receptor for a family of synthetic ligands that restore the age-related decline in pulse amplitude of growth hormone (GH) release by activating hypothalamic neurons (14, 29, 30). To identify an endogenous ligand for the GHS-R, cell lines overexpressing the GHS-R were prepared and used to assay fractionated tissue extracts for activators of the GHS-R. Two natural agonists were identified: ghrelin in stomach extracts and adenosine in hypothalamic extracts (15, 28, 36). Like ghrelin, adenosine shows agonist activity on the GHS-R, stimulates appetite (17), and exhibits cross-desensitization with the synthetic ligands (28, 36). However, in contrast to ghrelin and the synthetic GHS-R agonists, adenosine fails to stimulate GH release from pituitary cells. Hence, ghrelin, as a new hormone and a closer mimetic of the synthetic GHS-R ligands, became the major focus of subsequent research.
Overwhelming attention to ghrelin's role in obesity was initiated by the localization of this peptide in the stomach. Ghrelin levels in plasma are influenced by nutritional status and are believed to regulate GH, appetite, and fat deposition (12, 20, 21, 35, 40). Most intriguing was the observation that low levels of circulating ghrelin correlate with sustained weight loss and reduced appetite in obese humans after gastric bypass surgery (6). However, whether these beneficial changes are a result of reduced ghrelin, rather than alterations in other gut peptides involved in regulation of appetite, is still unclear. The association of ghrelin with obesity was surprising because chronic administration of a synthetic ghrelin agonist, MK-0677, to obese humans produced increases in muscle rather than fat (31).
A reciprocal relationship exists between ghrelin and leptin. Studies in rats show that ghrelin and leptin are secreted episodically during ad libitum feeding and that fasting increases the amplitude of ghrelin secretion but diminishes leptin pulse amplitude (1). In the stomach, ghrelin mRNA increases after the administration of leptin, and in db/db mice lacking the leptin receptor, the level of ghrelin mRNA is low (33). Furthermore, leptin-induced inhibition of food intake is reversed by intracerebroventricular coinjection of ghrelin (26). Electrophysiological studies on arcuate neurons in hypothalamic slices showed that ghrelin increased the electrical activity of neurons that were inhibited by leptin (34), a finding which is consistent with the orexigenic and anorectic effects of ghrelin and leptin. Intriguingly, the hypothalamic action of leptin was reported to reduce bone density in rodents (7); therefore, as a consequence of unopposed leptin, ghrelin-null (ghrelin−/−) mice might exhibit low bone mass.
In rats, ghrelin mRNA levels in the stomach increase after administration of insulin (33). A reciprocal relationship was also identified for ghrelin and insulin in humans (24). Infusion of insulin reduces ghrelin levels in plasma and, after discontinuation of the infusion, the levels of ghrelin return to basal levels. This inverse relationship suggests that insulin is a physiological and dynamic modulator of plasma ghrelin (24). Given the reciprocal relationship between ghrelin and leptin, as well as insulin, it could be predicted that in the absence of ghrelin, leptin, and insulin levels would be insensitive to feeding and fasting.
To help evaluate the potentially broad physiological role of ghrelin, we generated ghrelin−/− mice with a LacZ/Neo cassette replacing the entire coding region of ghrelin. GHS-R is predominantly expressed in the pituitary and brain (9, 11), but ghrelin is expressed in almost all tissues, with the highest expression in the stomach (9, 15). Whether ghrelin is produced in the brain or is transported from peripheral tissues into the brain has been the subject of continued debate. The controversy is based on very weak or almost undetectable ghrelin mRNA signal observed in rat and mouse brains. Clear discrimination between a very weak, and a nonspecific signal could be achieved by the comparison of brains from wild-type and ghrelin−/− mice.
MATERIALS AND METHODS
Animal care.
All animal protocols used were approved by Baylor College of Medicine Animal Care and Use Committee. All mice were housed in a 7-a.m.-to-7-p.m. light cycle B3 barrier facility. All of the experiments were carried out with F3 littermate pairs, and mice were individually caged during the experiments.
Generation of ghrelin−/− mice.
Two overlapping genomic DNA clones were isolated from Lambda KOS mouse genomic library from 129/SvEv substrain (39) by using exon 4- and 5-specific primers (5′-GTGGTTACTTGTCAGCTGGC and 5′-GCTCCCTTCGATGTTGGCATC). A 5.2-kb LacZ/Neo selection cassette was inserted as an SfiI fragment to replace a 315-bp ghrelin genomic fragment that includes exons 2 and 3 after yeast-mediated recombination (39). The targeting construct consisted of 3.0- and 4.5-kb homologous regions of genomic DNA at 5′ and 3′ ends of the LacZ/Neo cassette, respectively (Fig. 1A). The positive embryonic stem (ES) cell clones (129/SvEv) were selected by Southern blot analysis. The targeting efficiency was very high (60%). One of the identified positive ES cell clones was injected into blastocysts to generate chimeras. The chimeric mice were bred with C57BL/6 to generate F1 progeny.
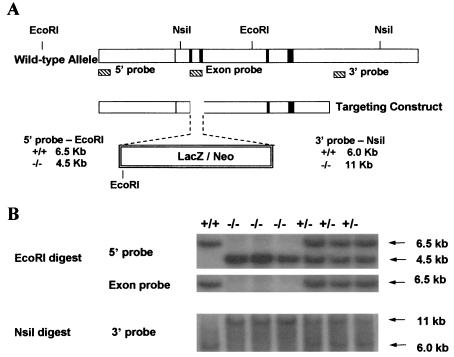
FIG. 1.
(A) Schematic diagram of the derivation of ghrelin−/− mice by homologous recombination. The wild-type allele is shown at the top, and five exons are indicated by a vertical line or rectangular boxes. The targeting construct is shown below. (B) Southern blot analysis of DNA from mice derived from mating heterozygotes.
PCR amplification and Southern blot analysis.
Southern blot analysis was performed for ES cell selection and mouse genotyping with a 5′ internal probe and a 3′ external probe (25). The 5′ internal probe (362 bp) was amplified by PCR with 5′-GCCACACCTTCTGATAGCAG and 5′-GAGAGAGATTACATGGCTCTAG; the 3′ external probe (317 bp) was amplified by PCR with 5′-GGATCCTGTTCAAACAACATAG and 5′-ATACTGCTCACTGGCTGGCTTC. The exon probe (275 bp) within the deleted coding region was amplified by 5′-GCTCTGGATGGACATGGCC in exon 2 and 5′-TGATCTCCAGCTCCTCCTC in exon 3 (Fig. 1A). With the 5′ internal probe, EcoRI-digested genomic DNA showed a 6.5-kb fragment for the wild-type allele and 4.5-kb fragment for the mutant allele; with the 3′ external probe, NsiI-digested genomic DNA showed a 6.0-kb fragment for the wild-type allele and a 11-kb fragment for the mutant allele; and with the exon probe, the 6.5-kb wild-type fragment was absent in −/− mice (Fig. 1B). The symbols used here are defined as foloows: +/+, wild-type; −/−, homozygote; and +/−, heterozygote.
RT-PCR analysis.
Total RNA from stomach and brain was isolated from individual mice. A total of 20 ng of total RNA was used in semiquantitative reverse transcription-PCR (RT-PCR). A 348-bp fragment for ghrelin was amplified by 5′-TTGAGCCCAGAGCACCAGAAA in exon 2 and 5′-AGTTGCCGAGGAGGCTGAG in exon 5. The PCR primers for LacZ were 5′-GAGGCTGAAGTTCAGATGTGCGGCG and 5′-CCTCGAATCAGCAACGGCTTGCCG which specifically amplifies a 338-bp bacterial LacZ fragment.
Immunofluorescence analysis.
Eight-week-old mice were perfused with 4% paraformadehyde; stomachs were additionally fixed in paraformadehyde overnight at 4°C and then embedded in paraffin. Then, 5-μm sections were analyzed. The sections were incubated for 1 h at room temperature with 10% goat serum to block nonspecific binding and then further incubated with affinity-purified rabbit polyclonal ghrelin antibody (diluted 1:500) overnight at 4°C. After a wash in phosphate-buffered saline, the sections were incubated for 1 h at room temperature in the dark in goat anti-rabbit fluorescent secondary antibody Alexa Fluor 594 (diluted 1:500; Molecular Probes). β-Galactosidase staining was carried out on nearby tissue sections from the same mouse according to manufacturer's instructions (Specialty Media).
Bone density and body composition.
Bone density and body composition were analyzed by PIXImus densitometer (Lunar Corp.). Mice (8 weeks old) were anesthetized during the procedure.
Body weight and food intake analysis.
All of the experimental mice were provided with ad libitum access to water and regular chow (PicoLab 5053; 20.0% protein, 5.4% fat) or special diet. Body weight and food intake were measured every other week.
Evaluation of appetite during fasting and refeeding.
For measurement of food intake after fasting, the animals (20 weeks old) were weighed, and the chow was removed. After 24 h, the animals were weighed and then given a weighed amount of chow, and the food intake was measured at 2, 4, 6, 24, and 48 h after the refeeding. The body weights were also measured at 24 and 48 h after the refeeding.
Orexigenic effect of ghrelin.
Mice (12 weeks old) were injected intraperitoneally first with 100 μl of saline and then 0.5 h later with 100 μl of saline containing 0.1, 1.0, or 10 μg of ghrelin (Phoenix Pharmaceuticals, Inc.). One hour after the first ghrelin injection, another dose of ghrelin was administered. Cumulative food intake was measured every 0.5 h.
Hormone assays.
The blood was collected by either retro-orbital bleeding or from the tail vein. Serum IGF-1 was measured by using a rat insulin-like growth factor 1 (IGF-1) radioimmunoassay (RIA) kit (Diagnostic Systems Laboratories, Inc.). Ghrelin in serum was measured by Phoenix Pharmaceuticals by using a rat ghrelin RIA kit. Leptin and insulin in serum were measured by Linco Research, Inc., by using a mouse leptin RIA kit and sensitive rat insulin RIA kit. The leptin and insulin samples were collected between 8 and 9 a.m. and at the same time after 48 h of fasting.
Special diet studies.
Twenty-week-old mice were fed either a high-fat diet (Teklad TD 02173; 35.1% fat and 22.9% protein) or a high-protein diet (Teklad TD 94266; 50.0% protein and 5.5% fat) for 10 weeks. Body weight was measured every week. At the end of week 10, the body composition of these animals was analyzed.
Statistical analysis.
The data were presented as mean ± the SEM unless stated otherwise. The data were analyzed by a two-tailed Student t test.
RESULTS
Mouse ghrelin genomic DNA clones were isolated from lambda KOS genomic library (39) with exon 4- and exon 5-specific primers. The LacZ/Neo selection cassette was inserted into the ghrelin locus to replace ghrelin exons 2 and 3 that encode ghrelin. The ES cell targeting vector consisted of 3.0- and 4.5-kb homologous regions of genomic DNA at 5′ and 3′ of the selection cassette, respectively (Fig. 1A). The targeted ES cells and mice were genotyped by Southern analysis. With the 5′ internal probe, EcoRI-digested genomic DNA produced a 6.5-kb fragment for the wild-type allele and 4.5-kb fragment for the mutant allele; with the 3′ external probe, NsiI-digested genomic DNA produced a 6.0-kb fragment for the wild-type allele and an 11-kb fragment for the mutant allele (Fig. 1B). The ghrelin exon-specific probe detected a signal in wild-type and heterozygotes but not in homozygotes. These results confirmed the specifically targeted recombination.
As additional confirmation for deletion of ghrelin, total RNA was isolated from stomach and brain of ghrelin−/− mice and wild-type littermates for RT-PCR analysis. Using primers designed to amplify the ghrelin transcript, the predicted 348-bp ghrelin mRNA RT-PCR product was identified in RNA from the stomachs and brains of wild-type mice but not in RNA from the stomachs and brains of ghrelin−/− mice (Fig. 2A). The amplified product could not be explained by DNA contamination because of the selected primers flanking ghrelin introns. Using primers designed to amplify lacZ transcript, as anticipated, the predicted 338-bp RT-PCR product was found only in RNA isolated from the stomachs and brains of ghrelin−/− mice but not in those of wild-type mice (Fig. 2A). The contrasting expression and appropriate lack of expression of ghrelin and lacZ transcripts in ghrelin+/+ and ghrelin−/− mice shows unambiguously that ghrelin is expressed in the brain. We also determined by semiquantitative RT-PCR data from Mouse Rapid-Scan Panels (OriGene) that the level of expression in the brain was about 100-fold lower than that in the stomach (data not shown).
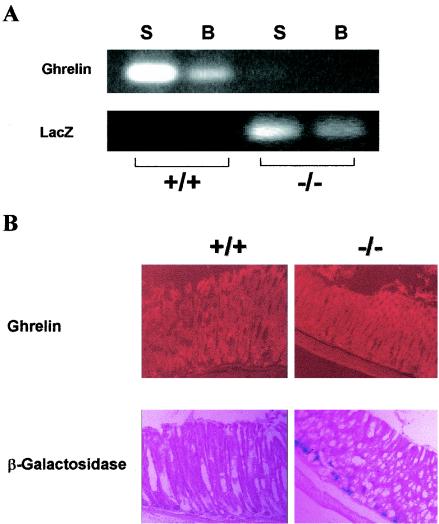
FIG. 2.
(A) RT-PCR analysis of ghrelin and lacZ mRNA expression. Lanes: S, stomach; B, brain. (B) Immunofluorescence analysis of ghrelin and expression of β-galactosidase activity in the stomach of 8-week-old mice. Magnification, ×8.
The localization of ghrelin and β-galactosidase in ghrelin+/+ and ghrelin−/− mice was compared by immunofluorescence. Figure 2B illustrates localization of ghrelin from the neck to the base of the oxyntic gland in a stomach section from a wild-type mouse. In contrast, ghrelin immunoactivity was not observed in stomach sections from the ghrelin−/− mouse. Accordingly, β-galactosidase activity was detected in ghrelin−/− but not in ghrelin+/+ mice. We were unable to confirm expression of β-galactosidase activity in brain sections of ghrelin−/− mice. Based on the very low levels of expression of ghrelin mRNA in the brain (≤100-fold of that in the stomach), which could only be reproducibly detected by using RT-PCR, we conclude that the β-galactosidase activity produced in the ghrelin−/− mouse brain is below the sensitivity of our cytochemical assay.
We had confirmed deletion of the targeted ghrelin locus by DNA analyses, mRNA analyses, and by immunocytochemistry. To exclude the possibility of ghrelin being produced from a related gene, we collected sera from fasted (24 h) and nonfasted ghrelin−/− and ghrelin+/+ littermates for quantitation of ghrelin levels by RIA. Ghrelin levels were higher in fasted than in fed wild-type mice (fasted, 2,632.93 ± 120.97 pg/ml; fed, 813.175 ± 222.12 pg/ml; n = 6, P < 0.001), which was consistent with previously reported data in mice (33). In both fed and fasted ghrelin−/− mice, ghrelin levels were at the lower limit of the RIA detection range (no difference form the buffer control).
The ghrelin−/− mice were viable and of normal size. The activity and behavior of the ghrelin−/− and wild-type mice as observed under normal laboratory housing conditions were also indistinguishable. Breeding of the mutant mice was monitored over a 12-month period. Litter sizes were normal, and composition was consistent with normal Mendelian distribution according to genotype and gender (data not shown). Therefore, although it has been suggested that ghrelin plays a role in testicular and placental functions (10, 32), general reproductive functions appear normal in ghrelin−/− mice.
Because ghrelin−/− mice appear grossly normal, and published results from RT-PCR studies suggest that ghrelin is expressed in almost all tissues (9), we carried out a more detailed evaluation of the mice in order to detect some phenotypic change(s). We performed blood chemistry, organ weight, and complete tissue pathology analyses on 8-week-old littermate mice. No significant differences were detected in the levels of cholesterol, triglycerides, glucose, total protein, albumin, and alkaline phosphatase by blood chemistry analysis (data not shown). The organ weights of empty stomach, brain, heart, and liver were comparable between wild-type and −/− mice littermates (data not shown). Total necropsy and evaluation of individual tissues by hematoxylin and eosin staining of paraffin sections showed no pathological changes in ghrelin−/− mice compared to ghrelin+/+ mice; these tissues included heart, thymus, lymph nodes, lung, thyroid, kidneys, adrenals, parotid/salivary glands, submandibular/cervical lymph nodes, stomach, small and large intestines, pancreas, liver, spleen, brain, pituitary, eyes, harderian gland, ears, teeth, nose, sinuses, bone marrow, urinary ladder, testes, epididymis, seminal vesicle, and prostate.
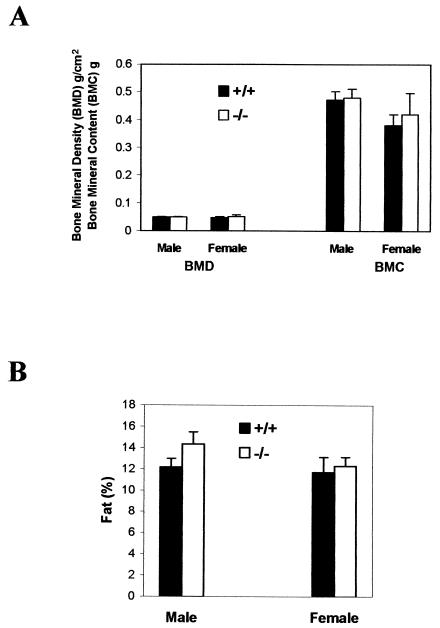
Since the detailed pathological studies did not reveal any specific changes, we decided to test some of the most documented pharmacological functions of ghrelin in ghrelin−/− mice. To address whether ghrelin is a functional antagonist on leptin's effect on bone metabolism (7), we analyzed the bone density of ghrelin−/− mice. The bone mineral density and bone mineral content of ghrelin−/− mice and wild-type littermates were identical (Fig. 3A), a finding which shows that ghrelin is not important in maintaining bone density. An overwhelming amount of pharmacological data suggests that ghrelin has significant impact on body fat content (12, 20, 21, 35, 40), but our body composition study showed that the fat contents as a percentage of body mass of ghrelin−/− and wild-type littermates were identical (Fig. 3B). Collectively, the data suggest that ghrelin is not a significant regulator for either bone density or fat deposition.
FIG. 3.
Bone density (A) and body composition (percent fat) (B) in 8-week-old mice (n = 6, P > 0.05 [ghrelin+/+ versus ghrelin−/− mice]).
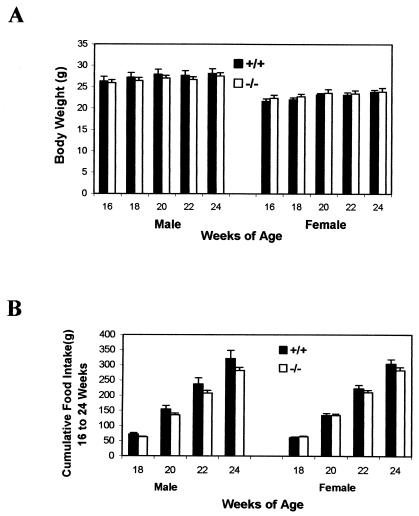
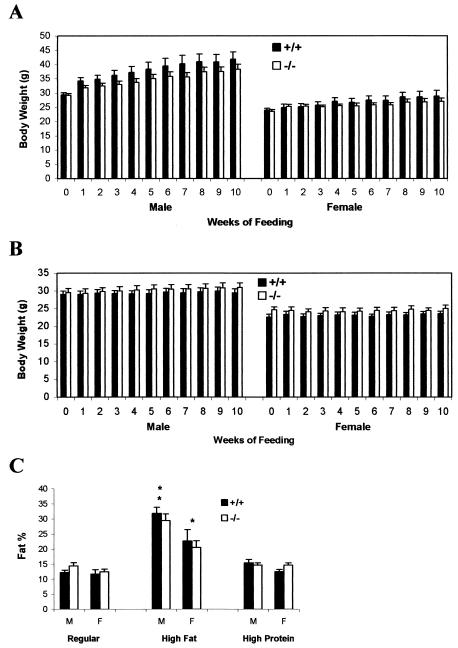
Because it is believed that ghrelin plays an important regulatory role on appetite, we compared the weight gain and food intake in ghrelin−/− mice with their wild-type littermates. There was no significant difference in either body weight (Fig. 4A) or food intake (Fig. 4B) in 16- to 24-week-old ghrelin−/− mice compared to their wild-type littermates. There was also no difference in body weight and food intake in younger mice (i.e., 8 to 16 weeks of age; data not shown). The IGF-1 concentration in serum tended to be slightly lower in ghrelin−/− mice, but these differences did not reach statistical significance (445.28 ± 34.56 ng/ml in ghrelin+/+ mice and 426.27 ± 28.66 ng/ml in ghrelin−/− mice; n = 7, P > 0.05). In summary, there were no significant differences in body weight or food intake between ghrelin−/− mice and their wild-type littermate controls up to at least 24 weeks of age.
FIG. 4.
ghrelin−/− mice exhibit normal growth rate and normal food intake. (A) Body weight; (B) cumulative food intake. Mice were 16 weeks old at the beginning of the study, and data were collected every other week (n = 8, P > 0.05 [ghrelin+/+ versus ghrelin−/− mice]).
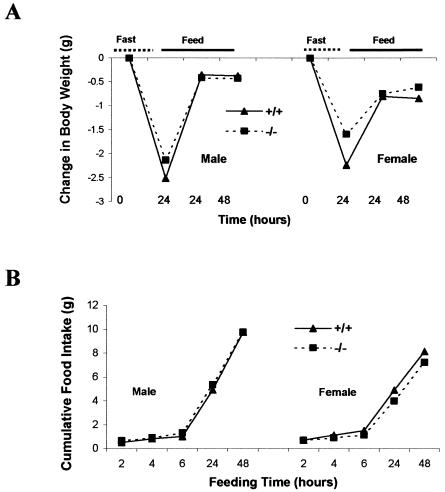
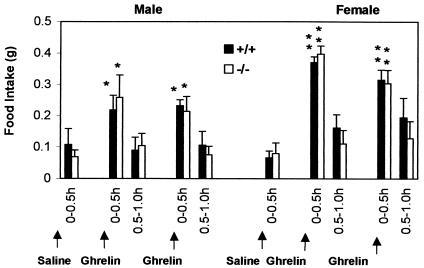
Ghrelin administration causes an acute increase in appetite and serum ghrelin is upregulated during fasting (33, 40). Our data showed that fasting increased the ghrelin levels in serum in wild-type control mice, suggesting that ghrelin might be involved in fasting-induced hyperphagia. Therefore, we evaluated reflex hyperphagia after fasting in ghrelin−/− mice. The changes in body weight and food intake were identical in ghrelin−/− and wild-type littermates after 24 h of food deprivation (Fig. 5). We also observed that there was no significant difference over a short period (0.5, 1.0, and 2 h) of food consumption after either 24 or 48 h of fasting (data not shown). These data show that ghrelin is not a required orexigenic factor and that other mechanism(s) compensate for the lack of ghrelin in appetite control. If ghrelin normally has a prominent role in initiating food intake, deletion of ghrelin might result in activation of compensatory pathway(s). To determine whether the ghrelin/GHS-R pathway was still functional in the absence of ghrelin, the responses to different doses of ghrelin (0.1, 1, and 10 μg of ghrelin per mouse injected intraperitoneally) were compared in ghrelin−/− mice and wild-type littermates. Figure 6 illustrates that in both genotypes food intake was unchanged at 0 to 0.5 h after saline injection but increased at 0 to 0.5 h after 10-μg ghrelin treatment (P < 0.05 in males and P < 0.001 in females compared to saline administration). During the next 30 min (0.5 to 1 h), food intake returned to control levels. After a second injection of ghrelin, food intake was again stimulated. Injection of 1 μg of ghrelin produced a modest but significant increase in food intake, but at the 0.1-μg dose of ghrelin neither genotype showed stimulation in food intake (data not shown). The duration and level of response to different doses of ghrelin were similar to the results which were previously reported in mice (38). We conclude that ghrelin−/− and wild-type mice are equally responsive to ghrelin, which suggests that the ghrelin orexigenic signaling pathway remains fully functional in the absence of ghrelin.
FIG. 5.
Changes in body weight (A) and food intake (B) during fasting and refeeding. Twenty-week-old mice were fasted for 24 h and then allowed to eat. Body weight was measured before and after fasting and at 24 and 48 h after food was given. Cumulative food intake was measured 2, 4, 6, 24, and 48 h after food was given. The results were plotted according to the mean of each datum point (n = 5, P > 0.05 (ghrelin+/+ versus ghrelin−/− mice]).
FIG. 6.
ghrelin−/− mice exhibit a normal orexigenic response to exogenous ghrelin. Cumulative food intake was measured in 12-week-old mice every 30 min for the duration of the experiment. Saline alone (100 μl) or saline containing 10 μg of ghrelin (Phoenix Pharmaceuticals, Inc.) was injected intraperitoneally into each mouse. Mice were first treated with saline and then 0.5 and 1.5 h later with saline containing ghrelin. For each gender group (n = 5), P < 0.05 (✽) in males and P < 0.001 in females (✽✽) (saline versus ghrelin).
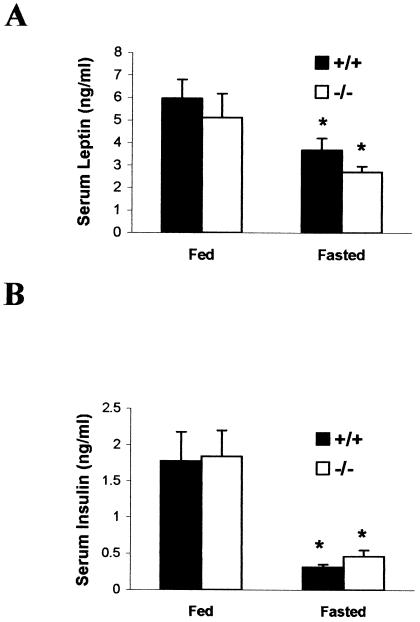
We next tested whether the reported reciprocal actions of ghrelin and leptin on appetite were independent or interdependent. Studies in rats show that ghrelin and leptin are secreted episodically during ad libitum feeding and that fasting increases the amplitude of ghrelin secretion but diminishes leptin pulse amplitude (1). Based on the reciprocal relationships between these hormones, leptin levels in the absence of ghrelin might be less sensitive to feeding and fasting. We found that leptin was high during feeding and low after a 48-h fast (P < 0.05 [fed versus fasted] in both +/+ and −/− groups) and that leptin concentrations and the magnitude of the changes were not significantly different in ghrelin−/− and ghrelin+/+ mice (Fig. 7A). These results challenge the contention that ghrelin has a primary role in regulating energy balance by antagonizing leptin production. They also illustrate that the effects of ghrelin and leptin on appetite are distinct, and show unequivocally that ghrelin and leptin are regulated independently.
FIG. 7.
Serum leptin and insulin concentrations in 18-week-old fed or fasted male mice (n = 5). Blood was collected between 8 and 9 a.m. Mice were fasted for 48 h, which produced a fall in leptin and insulin in each genotype (✽, P < 0.05 [fed versus fasted] for both ghrelin+/+ and ghrelin−/− mice). There was no significant difference in the levels of leptin and insulin between each genotype whether they were fed or fasted (P > 0.05 [ghrelin+/+ versus ghrelin−/− mice]).
A reciprocal relationship has also been identified for ghrelin and insulin in rats and humans (24, 33). Therefore, in mice that cannot produce ghrelin, changes in insulin levels might be less sensitive to feeding and fasting. We measured fed and fasted serum insulin. As with leptin, we found that insulin was high during feeding and low after a 48-h fast (P < 0.05 [fed versus fasted] in both +/+ and −/− groups) but that insulin concentrations and the magnitude of the changes were not significantly different in ghrelin−/− and ghrelin+/+ mice (Fig. 7B). Hence, ghrelin does not appear to have a dominant role in regulating insulin secretion.
Ghrelin administration is reported to increase fat deposition in rodents (35, 40). In rats, the intake of a high fat diet decreases ghrelin levels, and fat-preferring rats have lower levels of ghrelin in plasma, suggesting that ghrelin may be involved in the development of diet-induced obesity (3). Because of the increased susceptibility to obesity in mature adults, we compared the effects of a high-fat (35%) and high-protein (50%) diets in 20-week-old ghrelin−/− and wild-type littermate mice for 10 weeks (Fig. 8A and B). As anticipated in wild-type mice, body weight gain was significantly higher with the high-fat diet (P < 0.001, high fat versus high protein). However, most importantly, there was no statistically significant difference in weight gain between the two genotypes that were fed the same diet. When younger animals (8 and 12 weeks old) were fed a high fat diet for 8 weeks, no significant differences in weight gain were observed between ghrelin−/− and wild-type littermates either (data not shown). Body composition measurements confirmed that fat content was significantly higher in animals fed the high fat compared to those fed either a normal or high-protein diet (Fig. 8C). The increased fat content was accompanied by increased leptin levels in serum, but there was no significant difference between the two genotypes (P > 0.05; data not shown). Collectively, these data show that ghrelin−/− animals are not resistant to diet-induced obesity.
FIG. 8.
ghrelin−/− mice exhibit normal susceptibility to diet-induced obesity. Twenty-week-old mice were fed either a high-fat (A) or a high-protein (B) diet for 10 weeks. M, male (n = 7); F, female (n = 7). Body weight, P > 0.05 for +/+ versus −/− animals of the same sex on each diet. (C) Body composition after 10 weeks on normal, high-fat, and high-protein diets. Males, **, P < 0.001 high-fat versus normal and high-protein diets; females, *, P < 0.05 high-fat versus normal and high-protein diets. P > 0.05 for +/+ versus −/− animals within each diet.
DISCUSSION
RT-PCR studies with human tissues demonstrated expression of ghrelin in stomach and other parts of the gut, adrenal gland, atrium, breast, buccal mucosa, esophagus, fallopian tube, fat tissue, gallbladder, lymphocytes, ileum, kidney, left colon, liver, lung, lymph node, muscle, myocardium, ovary, pancreas, pituitary, placenta, prostate, right colon, skin, spleen, testis, thyroid, and vein (9). Using ghrelin−/− mice as controls, we now demonstrate conclusively that ghrelin is also expressed in the brain.
Ghrelin has been detected in human and rat placenta with a pregnancy-related time course of expression (10) and is also speculated to modulate rat testicular function (32) and inhibit luteinizing hormone secretion (8). However, our ghrelin−/− mice are fertile and produce normal-sized litters, suggesting that ghrelin is not critical for reproductive functions. Ghrelin is reported to inhibit gastric acid secretion (27) and has been suggested to be involved in fetal lung development (37), but our gross observation suggested that ghrelin−/− mice do not have severe digestive and respiratory problems. Furthermore, there were no abnormalities identified during our routine pathological evaluation of these tissues.
A series of studies show that acute administration of pharmacological doses of ghrelin stimulates food intake and adiposity (20, 35, 40), which suggests that ghrelin is involved in the hypothalamic regulation of energy homeostasis. However, ghrelin−/− mice have normal body composition and show a normal growth rate, appetite, and feeding behavior (Fig. 3 to 5), indicating that ghrelin is not a critically required orexigenic factor or that other mechanism(s) can compensate for ghrelin's effect on appetite. ghrelin−/− mice are still capable of producing an orexigenic response to exogenous ghrelin (Fig. 6), showing that the ghrelin-signaling pathway remains functional and has sensitivity indistinguishable from wild-type mice. It has been suggested that ghrelin signals through neuropeptide Y and agouti-related protein in arcuate hypothalamic nuclei (5, 13). A recent study has shown that there is no impairment of growth and appetite in neuropeptide Y and agouti-related protein double-knockout mice (22), a determination that is consistent with our findings in ghrelin−/− mice.
The effects of feeding and fasting on leptin and insulin levels were identical in ghrelin+/+ and ghrelin−/− mice, which suggests that ghrelin is not a direct regulator of leptin and insulin (Fig. 7). We have also shown that ghrelin−/− mice are not resistant to diet-induced obesity (Fig. 8). Together with our observations that ghrelin−/− mice lack significant differences in phenotype regarding growth and appetite, it appears that ghrelin is unlikely to be a dominant and critical factor involved in the hypothalamic regulation of energy homeostasis and that antagonists of ghrelin are unlikely to have broad efficacy as antiobesity agents.
In spite of all of the different functions that have been recently proposed for ghrelin, ghrelin−/− mice are visibly normal, healthy, and reproduce normally. Of course, this could readily be explained by activation of a compensatory pathway(s) in the mutant mice. However, it seems unlikely that such a pathway(s) would accommodate all of the suggested actions of ghrelin. Our earlier studies involving ghrelin mimetics show that GHS-R agonists modulate function by increasing neuronal activity in the hypothalamus (2, 30). In elderly human subjects, activation of this pathway with a ghrelin mimetic restores the amplitude of GH pulsatility to that observed in young adults, and the stimulatory effect of the ghrelin mimetic on the GH/IGF-1 axis is more pronounced in elderly subjects than in young subjects (4). In mice and humans, treatment with ghrelin mimetics improves immune function and increases bone density (16, 19). Recently, it has been reported that ghrelin production declines during aging (18, 23). Therefore, we anticipate that the phenotype of ghrelin−/− mice may become more apparent during aging.
Acknowledgments
The ghrelin−/− mice were generated in collaboration with Lexicon Genetics, Inc. (Woodlands, Tex.). We thank Adelina Gunawan for excellent technical assistance. We thank Fred Pereira, Mark Asnicar, Hui Zheng, Hong Jiang, and Lorena Betancourt for stimulating discussions during the study. We thank Michael R. Honig for help with the manuscript.
We gratefully acknowledge the support of National Institutes of Health grants RO1AG18895 and RO1AG19230, the Hankamer Foundation, and a postdoctoral fellowship for Yuxiang Sun from the Canadian Institutes of Health Research.
REFERENCES
- 1.Bagnasco, M., P. S. Kalra, and S. P. Kalra. 2002. Ghrelin and leptin pulse discharge in fed and fasted rats. Endocrinology 143:726-729. [DOI] [PubMed] [Google Scholar]
- 2.Bailey, A. R. T., R. G. Smith, and G. Leng. 1998. The non-peptide growth hormone secretagogue, MK-0677, activates hypothalamic neurones in vivo. J. Neuroendocrinol. 10:111-118. [DOI] [PubMed] [Google Scholar]
- 3.Beck, B., N. Musse, and A. Stricker-Krongrad. 2002. Ghrelin, macronutrient intake and dietary preferences in long-evans rats. Biochem. Biophys. Res. Commun. 292:1031-1035. [DOI] [PubMed] [Google Scholar]
- 4.Chapman, I. M., M. A. Bach, C. Van, E., M. Farmer, D. A. Krupa, A. M. Taylor, L. M. Schilling, K. Y. Cole, E. H. Skiles, S. S. Pezzoli, M. L. Hartman, J. D. Veldhuis, G. J. Gormley, and M. O. Thorner. 1996. Stimulation of the growth hormone (GH)-insulin-like growth factor-I axis by daily oral administration of a GH secretagogue (MK-0677) in healthy elderly subjects. J. Clin. Endocrinol. Metab. 81:4249-4257. [DOI] [PubMed] [Google Scholar]
- 5.Cowley, M., R. G. Smith, S. Diano, M. Tschop, N. Pronchuk, K. Grove, C. Strasburger, M. Bidlingmaier, M. Esterman, M. Heiman, L. Garcia-Segura, E. Nillni, P. Mendez, M. Low, P. Sotonyi, J. Friedman, H. Liu, S. Pinto, W. Colmers, R. Cone, and T. Horvath. 2003. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37:649-661. [DOI] [PubMed] [Google Scholar]
- 6.Cummings, D. E., D. S. Weigle, R. S. Frayo, P. A. Breen, M. K. Ma, E. P. Dellinger, and J. Q. Purnell. 2002. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med. 346:1623-1630. [DOI] [PubMed] [Google Scholar]
- 7.Ducy, P., M. Amling, S. Takeda, M. Priemel, A. F. Schilling, F. T. Beil, J. Shen, C. Vinson, J. M. Rueger, and G. Karsenty. 2000. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197-207. [DOI] [PubMed] [Google Scholar]
- 8.Furuta, M., T. Funabashi, and F. Kimura. 2001. Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem. Biophys. Res. Commun. 288:780-785. [DOI] [PubMed] [Google Scholar]
- 9.Gnanapavan, S., B. Kola, S. A. Bustin, D. G. Morris, P. McGee, P. Fairclough, S. Bhattacharya, R. Carpenter, A. B. Grossman, and M. Korbonits. 2002. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 87:2988. [DOI] [PubMed] [Google Scholar]
- 10.Gualillo, O., J. Caminos, M. Blanco, T. Garcia-Caballero, M. Kojima, K. Kangawa, C. Dieguez, and F. Casanueva. 2001. Ghrelin, a novel placental-derived hormone. Endocrinology 142:788-794. [DOI] [PubMed] [Google Scholar]
- 11.Guan, X. M., H. Yu, O. C. Palyha, K. K. McKee, S. D. Feighner, D. J. Sirinathsinghji, R. G. Smith, L. H. Van der Ploeg, and A. D. Howard. 1997. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res. Mol. Brain Res. 48:23-29. [DOI] [PubMed] [Google Scholar]
- 12.Hataya, Y., T. Akamizu, K. Takaya, N. Kanamoto, H. Ariyasu, M. Saijo, K. Moriyama, A. Shimatsu, M. Kojima, K. Kangawa, and K. Nakao. 2001. A low dose of ghrelin stimulates growth hormone (GH) release synergistically with GH-releasing hormone in humans. J. Clin. Endocrinol. Metab. 86:4552. [DOI] [PubMed] [Google Scholar]
- 13.Horvath, T. L., S. Diano, P. Sotonyi, M. Heiman, and M. Tschop. 2001. Minireview: ghrelin and the regulation of energy balance-a hypothalamic perspective. Endocrinology 142:4163-4169. [DOI] [PubMed] [Google Scholar]
- 14.Howard, A. D., S. D. Feighner, D. F. Cully, J. P. Arena, P. A. Liberator, C. I. Rosenblum, M. Hamelin, D. L. Hreniuk, O. C. Palyha, J. Anderson, P. S. Paress, C. Diaz, M. Chou, K. K. Liu, K. K. McKee, S.-S. Pong, L.-Y. P. Chaung, A. Elbrecht, M. Dashkevicz, R. Heavens, M. Rigby, D. J. S. Sirinathsinghji, D. C. Dean, D. G. Melillo, A. A. Patchett, R. P. Nargund, P. R. Griffin, J. A. DeMartino, S. K. Gupta, J. M. Schaeffer, R. G. Smith, and L. H. T. Van der Ploeg. 1996. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273:974-977. [DOI] [PubMed] [Google Scholar]
- 15.Kojima, M., H. Hosoda, Y. Date, M. Nakazato, H. Matsuo, and K. Kangawa. 1999. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656-660. [DOI] [PubMed] [Google Scholar]
- 16.Koo, G. C., C. Huang, R. Camacho, C. Trainor, J. T. Blake, A. Sirotina-Meisher, K. D. Schleim, T. J. Wu, K. Cheng, R. Nargund, and G. McKissick. 2001. Immune enhancing effect of a growth hormone secretagogue. J. Immunol. 166:4195-4201. [DOI] [PubMed] [Google Scholar]
- 17.Levine, A. S., M. Grace, D. D. Krahn, and C. J. Billington. 1989. The adenosine agonist N6-R-phenylisopropyladenosine (R-PIA) stimulates feeding in rats. Brain Res. 477:280-285. [DOI] [PubMed] [Google Scholar]
- 18.Liu, Y. L., S. Yakar, V. Otero-Corchon, M. J. Low, and J. L. Liu. 2002. Ghrelin gene expression is age-dependent and influenced by gender and the level of circulating IGF-I. Mol. Cell Endocrinol. 189:97-103. [DOI] [PubMed] [Google Scholar]
- 19.Murphy, M. G., S. Weiss, M. McClung, T. Schnitzer, K. Cerchio, J. Connor, D. Krupa, and B. J. Gertz. 2001. Effect of alendronate and MK-677 (a growth hormone secretagogue), individually and in combination, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J. Clin. Endocrinol. Metab. 86:1116-1125. [DOI] [PubMed] [Google Scholar]
- 20.Nakazato, M., N. Murakami, Y. Date, M. Kojima, H. Matsuo, K. Kangawa, and S. Matsukura. 2001. A role for ghrelin in the central regulation of feeding. Nature 409:194-198. [DOI] [PubMed] [Google Scholar]
- 21.Peino, R., R. Baldelli, J. Rodriguez-Garcia, S. Rodriguez-Segade, M. Kojima, K. Kangawa, E. Arvat, E. Ghigo, C. Dieguez, and F. F. Casanueva. 2000. Ghrelin-induced growth hormone secretion in humans. Eur. J. Endocrinol. 143:R11-R14. [DOI] [PubMed] [Google Scholar]
- 22.Qian, S., H. Chen, D. Weingarth, M. E. Trumbauer, D. E. Novi, X. Guan, H. Yu, Z. Shen, Y. Feng, E. Frazier, A. Chen, R. E. Camacho, L. P. Shearman, S. Gopal-Truter, D. J. MacNeil, L. H. Van der Ploeg, and D. J. Marsh. 2002. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol. Cell. Biol. 22:5027-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rigamonti, A. E., A. I. Pincelli, B. Corra, R. Viarengo, S. M. Bonomo, D. Galimberti, M. Scacchi, E. Scarpini, F. Cavagnini, and E. E. Muller. 2002. Plasma ghrelin concentrations in elderly subjects: comparison with anorexic and obese patients. J. Endocrinol. 175:R1-R5. [DOI] [PubMed] [Google Scholar]
- 24.Saad, M. F., B. Bernaba, C. M. Hwu, S. Jinagouda, S. Fahmi, E. Kogosov, and R. Boyadjian. 2002. Insulin regulates plasma ghrelin concentration. J. Clin. Endocrinol. Metab. 87:3997-4000. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Shintani, M., Y. Ogawa, K. Ebihara, M. Aizawa-Abe, F. Miyanaga, K. Takaya, T. Hayashi, G. Inoue, K. Hosoda, M. Kojima, K. Kangawa, and K. Nakao. 2001. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes 50:227-232. [DOI] [PubMed] [Google Scholar]
- 27.Sibilia, V., F. Pagani, F. Guidobono, V. Locatelli, A. Torsello, R. Deghenghi, and C. Netti. 2002. Evidence for a central inhibitory role of growth hormone secretagogues and ghrelin on gastric Acid secretion in conscious rats. Neuroendocrinology 75:92-97. [DOI] [PubMed] [Google Scholar]
- 28.Smith, R. G., P. R. Griffin, Y. Xu, A. G. Smith, K. Liu, J. Calacay, S. D. Feighner, C. Pong, D. Leong, A. Pomes, K. Cheng, L. H. Van der Ploeg, A. D. Howard, J. Schaeffer, and R. J. Leonard. 2000. Adenosine: a partial agonist of the growth hormone secretagogue receptor. Biochem. Biophys. Res. Commun. 276:1306-1313. [DOI] [PubMed] [Google Scholar]
- 29.Smith, R. G., S.-S. Pong, G. J. Hickey, T. M. Jacks, K. Cheng, R. J. Leonard, C. J. Cohen, J. P. Arena, C. H. Chang, J. E. Drisko, M. J. Wyvratt, Jr., M. H. Fisher, R. P. Nargund, and A. A. Patchett. 1996. Modulation of pulsatile GH release through a novel receptor in hypothalamus and pituitary gland. Rec. Prog. Horm. Res. 51:261-286. [PubMed] [Google Scholar]
- 30.Smith, R. G., L. H. Van der Ploeg, A. D. Howard, S. D. Feighner, K. Cheng, G. J. Hickey, M. J. Wyvratt, Jr., M. H. Fisher, R. P. Nargund, and A. A. Patchett. 1997. Peptidomimetic regulation of growth hormone secretion. Endocrine Rev. 18:621-645. [DOI] [PubMed] [Google Scholar]
- 31.Svensson, J., L. Lonn, J.-O. Jansson, G. Murphy, D. Wyss, D. Krupa, K. Cerchio, W. Polvino, B. Gertz, I. Boseaus, L. Sjostrom, and B.-A. Bengtsson. 1998. Two-Month treatment of obese subjects with the oral growth hormone (GH) secretagogue MK-677 increases GH secretion, fat-free mass, and energy expenditure. J. Clin. Endocrinol. Metab. 83:362-369. [DOI] [PubMed] [Google Scholar]
- 32.Tena-Sempere, M., M. L. Barreiro, L. C. Gonzalez, F. Gaytan, F. P. Zhang, J. E. Caminos, L. Pinilla, F. F. Casanueva, C. Dieguez, and E. Aguilar. 2002. Novel expression and functional role of ghrelin in rat testis. Endocrinology 143:717-725. [DOI] [PubMed] [Google Scholar]
- 33.Toshinai, K., M. S. Mondal, M. Nakazato, Y. Date, N. Murakami, M. Kojima, K. Kangawa, and S. Matsukura. 2001. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem. Biophys. Res. Commun. 281:1220-1225. [DOI] [PubMed] [Google Scholar]
- 34.Traebert, M., T. Riediger, S. Whitebread, E. Scharrer, and H. A. Schmid. 2002. Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. J. Neuroendocrinol. 14:580-586. [DOI] [PubMed] [Google Scholar]
- 35.Tschop, M., D. L. Smiley, and M. L. Heiman. 2000. Ghrelin induces adiposity in rodents. Nature 407:908-913. [DOI] [PubMed] [Google Scholar]
- 36.Tullin, S., B. S. Hansen, M. Ankersen, J. Moller, K. A. Von Cappelen, and L. Thim. 2000. Adenosine is an agonist of the growth hormone secretagogue receptor. Endocrinology 141:3397-3402. [DOI] [PubMed] [Google Scholar]
- 37.Volante, M., E. Fulcheri, E. Allia, M. Cerrato, A. Pucci, and M. Papotti. 2002. Ghrelin expression in fetal, infant, and adult human lung. J. Histochem. Cytochem. 50:1013-1021. [DOI] [PubMed] [Google Scholar]
- 38.Wang, L., D. H. Saint-Pierre, and Y. Tache. 2002. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y-synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci. Lett. 325:47-51. [DOI] [PubMed] [Google Scholar]
- 39.Wattler, S., M. Kelly, and M. Nehls. 1999. Construction of gene targeting vectors from lambda KOS genomic libraries. BioTechniques 26:1150-1156. [DOI] [PubMed] [Google Scholar]
- 40.Wren, A. M., C. J. Small, C. R. Abbott, W. S. Dhillo, L. J. Seal, M. A. Cohen, R. L. Batterham, S. Taheri, S. A. Stanley, M. A. Ghatei, and S. R. Bloom. 2001. Ghrelin causes hyperphagia and obesity in rats. Diabetes 50:2540-2547. [DOI] [PubMed] [Google Scholar]