Abstract
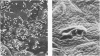
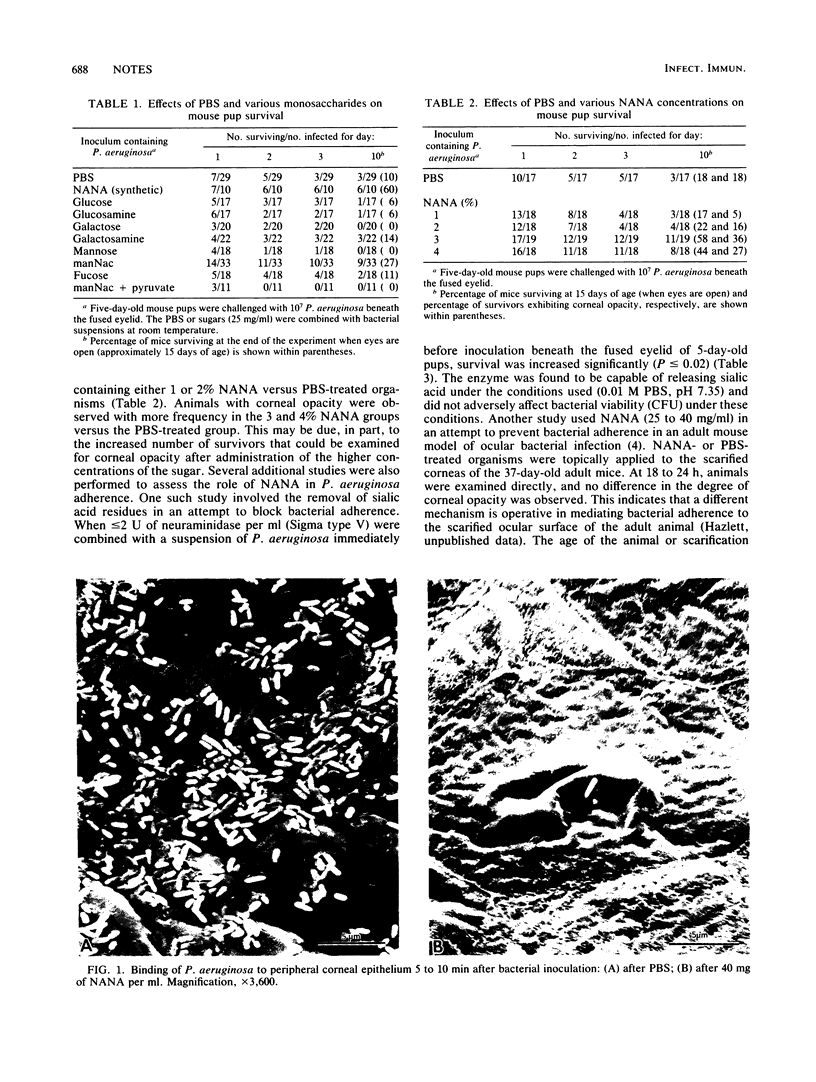
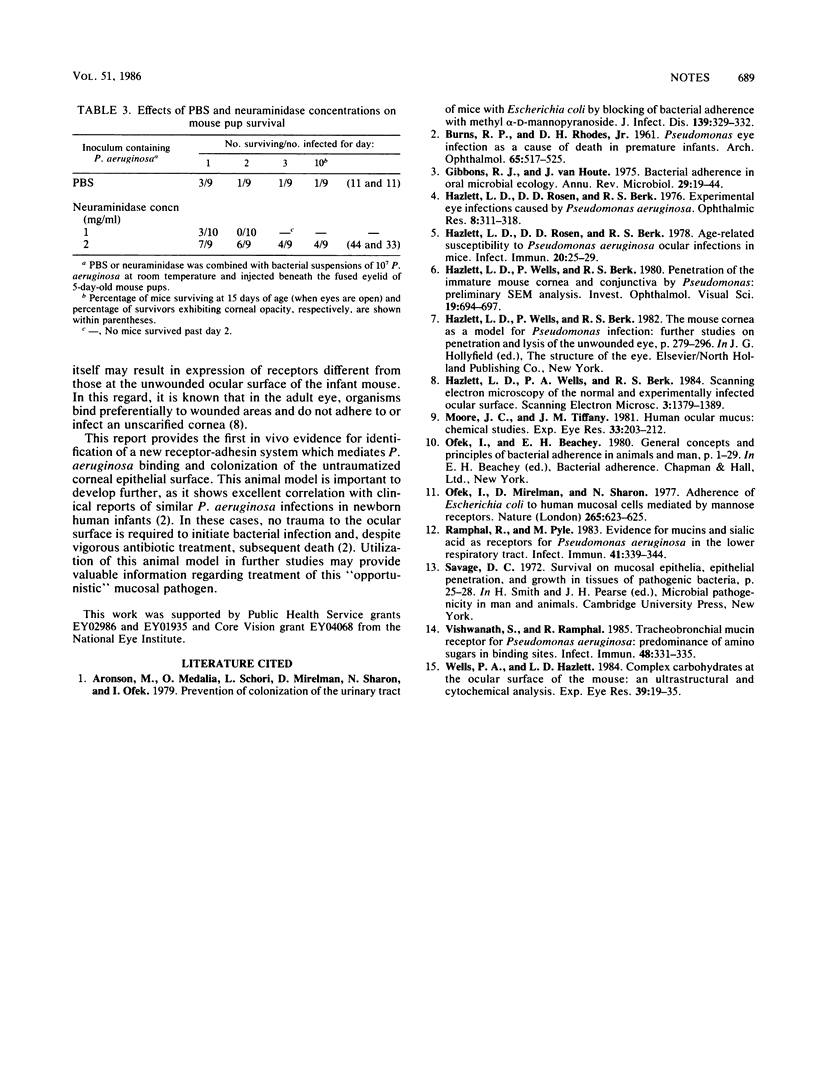
In vivo bacterial adherence of Pseudomonas aeruginosa to the immature ocular epithelium was mediated by a sialic (N-acetylneuraminic) acid (NANA) receptor. Saturation of binding sites on the bacterial surface by NANA prevented attachment of the organism to the epithelial cell membrane receptor. Additionally, a significant number of animals receiving NANA-treated organisms were protected from septicemia and death. In vivo protection studies showed excellent correlation with scanning electron microscopy, in that the number of adherent organisms at the corneal surface decreased dramatically in the presence of NANA. These studies exhibit a strong correlation with clinical cases of human infant ocular infection.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson M., Medalia O., Schori L., Mirelman D., Sharon N., Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J Infect Dis. 1979 Mar;139(3):329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- BURNS R. P., RHODES D. H., Jr Pseudomonas eye infection as a cause of death in premature infants. Arch Ophthalmol. 1961 Apr;65:517–525. doi: 10.1001/archopht.1961.01840020519010. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Hazlett L. D., Rosen D. D., Berk R. S. Age-related susceptibility to Pseudomonas aeruginosa ocular infections in mice. Infect Immun. 1978 Apr;20(1):25–29. doi: 10.1128/iai.20.1.25-29.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett L. D., Wells P. A., Berk R. S. Scanning electron microscopy of the normal and experimentally infected ocular surface. Scan Electron Microsc. 1984;(Pt 3):1379–1389. [PubMed] [Google Scholar]
- Hazlett L. D., Wells P., Spann B., Berk R. S. Penetration of the unwounded immature mouse cornea and conjunctiva by Pseudomonas: SEM-TEM analysis. Invest Ophthalmol Vis Sci. 1980 Jun;19(6):694–697. [PubMed] [Google Scholar]
- Moore J. C., Tiffany J. M. Human ocular mucus. Chemical studies. Exp Eye Res. 1981 Aug;33(2):203–212. doi: 10.1016/s0014-4835(81)80069-3. [DOI] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Pyle M. Evidence for mucins and sialic acid as receptors for Pseudomonas aeruginosa in the lower respiratory tract. Infect Immun. 1983 Jul;41(1):339–344. doi: 10.1128/iai.41.1.339-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Tracheobronchial mucin receptor for Pseudomonas aeruginosa: predominance of amino sugars in binding sites. Infect Immun. 1985 May;48(2):331–335. doi: 10.1128/iai.48.2.331-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells P. A., Hazlett L. D. Complex carbohydrates at the ocular surface of the mouse: an ultrastructural and cytochemical analysis. Exp Eye Res. 1984 Jul;39(1):19–35. doi: 10.1016/0014-4835(84)90111-8. [DOI] [PubMed] [Google Scholar]