Abstract
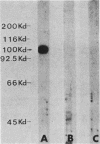
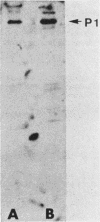
In previous studies with hyperimmune rabbit antisera, we found evidence of serologic cross-reactivity among Mycoplasma pneumoniae, Mycoplasma genitalium, and Mycoplasma gallisepticum. Because of certain biologic and morphologic similarities of these species, attempts were made to determine if this cross-reactivity related to the attachment protein (P1) of M. pneumoniae. Monoclonal and monospecific antibodies against P1 were used to probe proteins of the other species by immunoblotting. One of the P1 monoclonal antibodies was reactive with a smaller protein of M. genitalium; rabbit antiserum raised by immunization with P1 excised from a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel was found to react with a similar-sized protein of M. gallisepticum. These preliminary findings suggest antigenic sharing among the species examined; however, limitations of the methods used are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Drouillard D. L., Leith D. K., Tully J. G. Absence of Mycoplasma pneumoniae cytadsorption protein P1 in Mycoplasma genitalium and Mycoplasma gallisepticum. Infect Immun. 1984 Mar;43(3):1103–1105. doi: 10.1128/iai.43.3.1103-1105.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbrink P., Van Bussel F. J., Warnaar S. O. The antigen spot test (AST): a highly sensitive assay for the detection of antibodies. J Immunol Methods. 1982;48(3):293–298. doi: 10.1016/0022-1759(82)90330-1. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Clyde W. A., Jr, Collier A. M. Conservation of pathogenic mycoplasma antigens. Isr J Med Sci. 1984 Oct;20(10):916–919. [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Interaction of virulent Mycoplasma pneumoniae with hamster tracheal organ cultures. Infect Immun. 1976 Jul;14(1):217–224. doi: 10.1128/iai.14.1.217-224.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J Exp Med. 1977 May 1;145(5):1328–1343. doi: 10.1084/jem.145.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Huang C. H., Huang Y. S., Collier A. M., Clyde W. A., Jr Demonstration of multiple antigenic determinants on Mycoplasma pneumoniae attachment protein by monoclonal antibodies. Infect Immun. 1985 Oct;50(1):292–296. doi: 10.1128/iai.50.1.292-296.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Huang Y. S., Graham J. A., Gardner D. E. Identification of immunogens of Mycoplasma pneumoniae by protein blotting. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1363–1370. doi: 10.1016/0006-291x(81)90273-4. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Powell D. A., Albright F., Gardner D. E., Collier A. M., Clyde W. A., Jr A solid-phase radioimmunoassay for detection of antibodies against Mycoplasma pneumoniae. J Clin Lab Immunol. 1983 Aug;11(4):209–213. [PubMed] [Google Scholar]
- Lipman R. P., Clyde W. A., Jr, Denny F. W. Characteristics of virulent, attenuated, and avirulent Mycoplasma pneumoniae strains. J Bacteriol. 1969 Nov;100(2):1037–1043. doi: 10.1128/jb.100.2.1037-1043.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniloff J., Morowitz H. J., Barrnett R. J. Ultrastructure and Ribosomes of Mycoplasma gallisepticum. J Bacteriol. 1965 Jul;90(1):193–204. doi: 10.1128/jb.90.1.193-204.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. A., Clyde W. A., Jr Opsonin-reversible resistance of Mycoplasma pneumoniae to in vitro phagocytosis by alveolar macrophages. Infect Immun. 1975 Mar;11(3):540–550. doi: 10.1128/iai.11.3.540-550.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Tully J. G., Rose D. L., Whitcomb R. F., Wenzel R. P. Enhanced isolation of Mycoplasma pneumoniae from throat washings with a newly-modified culture medium. J Infect Dis. 1979 Apr;139(4):478–482. doi: 10.1093/infdis/139.4.478. [DOI] [PubMed] [Google Scholar]