Abstract
Motor axon projections are topographically ordered. Medial motor column axons project to axial muscles, whereas lateral motor column axons project to limb muscles and, along the rostrocaudal axis of the animal, the more rostral motor neuron pools project to more rostral muscle targets. We have shown that EphA3 is specifically expressed in the developing medial motor column and have postulated that EphA3 might be responsible for directing their axons to axial muscle targets. This hypothesis was supported by our demonstration that EphA3 can direct retinal ganglion cell axon targeting and by studies of ephrin-A5−/− mutants that show that EphA receptor signaling controls the topographic innervation of the acromiotrapezius. To test the role of EphA3 in motor axon guidance, we generated an EphA3 null mutant. Retrograde labeling studies in EphA3−/− embryos and adults indicate that, contrary to our predictions, EphA3 is not necessary to direct motor axons to axial muscle targets. Our results also demonstrate that ephrin A5's ability to direct topographic innervation of the acromiotrapezius must be mediated through EphA receptors other than, or in addition to, EphA3.
Processing sensory input and executing motor programs depends on the ordered connection of neurons that produce a spatially correct neural map of the body and environment. Such topographic maps develop when one set of neurons project onto their targets so that the order of the neurons is reflected in the order of their synaptic connections. Motor neurons are topographically organized in the spinal cord (13, 20, 28). First, motor neurons that innervate individual muscles form tightly clustered pools within the ventral horn of the spinal cord. Second, motor neurons at a given position in the rostrocaudal axis of the spinal cord tend to innervate skeletal muscles located proximate to that level. Third, in both the chick and the rodent, ventral motor neurons are readily subdivided into two discrete columns, the lateral motor column (LMC) and medial motor column (MMC) that, in turn, are subdivided into medial and lateral compartments, located at distinct positions within the axial and rostral-caudal planes of the spinal cord. Motor neurons within each column innervate a specific set of skeletal muscles; those within the MMC innervate axial and body wall musculature, while those within the LMC innervate limb musculature (25, 29, 42, 43).
Eph receptors are a family of receptor tyrosine kinases that have been shown to mediate axon guidance decisions during embryonic development (34). The Eph receptors have been subdivided into two classes, A and B, based on their structural properties and their ligand binding preferences (1, 7, 18, 35). All Eph receptors bind membrane-bound ligands designated as ephrins. In general, any EphA receptor can bind to any ephrin A ligand and any EphB receptor can bind to any ephrin B ligand. Eph receptors and ephrins have been extensively characterized as mediating axon-repelling (4, 8, 45) or -attracting (24, 36) events. The role of Eph receptors in topographic innervation was first investigated in the retinotectal system of the chick. The opposite but complementary patterns of EphA receptor expression in the retina and of ephrin A ligands in the tectum suggested that retinotectal mapping may be achieved through the repulsive interaction of EphA receptors and their ligands (3-5, 8, 30). More recently, experiments manipulating either EphA receptor or ephrin A ligand expression have demonstrated that retinal ganglion cell axon targeting depends on the relative levels of EphA receptor signaling (2, 10, 15, 21, 31). Whereas retinotectal mapping depends on the repulsive interactions between Ephs and ephrins, the mapping of vomeronasal axons on to their targets in the accessory olfactory bulb depends on attractive Eph/ephrin interactions (24). Eph receptors and ephrins have also been implicated in directing hippocamposetptal, corticospinal, and intercollicular axonal projections (6, 17, 37).
Like the visual system, topographic innervation of motor neurons was suggested to be dependent on EphA receptor-ligand interactions based on reciprocal patterns of Eph receptor expression in motor neurons and of ephrin ligands in muscle targets (5, 9, 22, 23). For example, a role for ephrin A5 in organizing the rostrocaudal projections of motor neurons was initially based on the observation that during development ephrin-A5 is expressed at higher levels in rostral muscle than in caudal muscle (5, 11). More recently, Feng et al. have shown that ephrin-A5−/− mice have a lengthening and caudalward shift in the motor pool innervating the acromiotrapezius (11). This is consistent with their demonstration that caudal motor neurons are more sensitive than rostral motor neurons to ephrin A-mediated repulsion (5, 11). Thus, the innervation of the acromiotrapezius in the ephrin-A5−/− mice by more caudally located motor neurons than in the wild type may be explained by a reduction in the level of repulsive ephrin A5 ligand in this muscle.
Our previous work has shown that EphA3 is expressed in a subset of mouse MMC motor neurons at embryonic day 13.5 (E13.5) and in a subset of their axial muscle targets (23). We found that EphA3 is expressed in motor neurons starting at E10 of mouse embryogenesis, during which time motor axons extend into the periphery and begin to make pathway choices (32). In view of the limb bud expression of ephrin A ligands (3, 14, 16, 33), and our own demonstration that ectopic EphA3 expression can direct retinal axon guidance decisions (2), we hypothesized that EphA3 directs axon guidance decisions in the MMC.
To investigate the role of EphA3 in motor axon guidance, we generated a mouse mutant with a targeted deletion in the EphA3 gene. Using the EphA3 null mutant two hypotheses were tested. The first hypothesis was that EphA3 mediates the ephrin A5 signal involved in establishing the rostrocaudal position of the motor pool innervating the acromiotrapezius. This hypothesis rests on the observations that EphA3 is expressed in the cervical MMC and that there is a caudal shift in the position of the motor pool innervating the acroiotrapezius in ephrin-A5−/− mice (11). The second hypothesis tested was that EphA3 signaling directs MMC axons away from the limb musculature and toward axial and body wall muscle targets. This hypothesis rests on the MMC-specific expression pattern of EphA3. We report here that EphA3 is not necessary for the axial muscle targeting of MMC axons and that another EphA receptor must be responsible for mediating the ephrin A5 signal that directs the topographic innervation of the acromiotrapezius.
MATERIALS AND METHODS
Generation of the EphA3−/− mouse line.
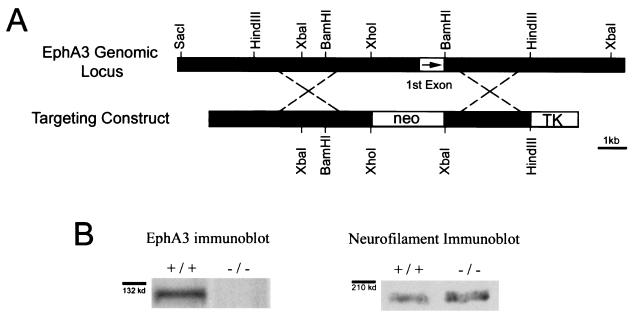
The EphA3 null mice were generated through homologous recombination of the targeting vector shown in Fig. 1. The targeting vector was electroporated into W9.5 embryonic stem (ES) cells. Clones that had undergone homologous recombination were identified by Southern blotting (38) and microinjected into C57BL/6 blastocysts. Chimeric founder mice that transmitted the targeted allele were bred to 129S3Svimj mice (the mouse strain from which the W9.5 ES cells were derived). This mating scheme allowed us to analyze the mutants on a pure genetic background. All wild-type mice and embryos used in the experiments detailed below were derived from 129S3Svimj mice (hereafter referred to as 129Sv mice).
FIG. 1.
Generation and characterization of the EphA3−/− mutant. (A) The top line represents the wild-type EphA3 genomic locus. The line below is a sketch of the targeting construct used to generate the EphA3 null mutant. The portion of the EphA3 gene removed by homologous recombination includes EphA3's first exon (indicted by a box with an arrow in it) that encodes its signal sequence. (B) Western blots of E13.5 spinal cord protein extracts from wild-type and EphA3−/− mutants with an anti-EphA3 antibody demonstrate that no EphA3 protein is produced in EphA3−/− E13.5 spinal cords (n = 6). The same samples were analyzed by Western blotting with an anti-neurofilament antibody (right panel) as a loading control.
Assay of grip strength in EphA3−/− adults.
EphA3−/− mice were tested for evaluated for forelimb grip strength. Male wild-type (n = 8) and EphA3−/− (n = 8) mice between 3 and 4 months of age were timed for how long they could support their weight holding onto a pencil suspended in mid-air. Each mouse was subjected to three trials with at least a half hour rest period between each test. The results were tabulated and compared for statistical significance by using the Student t test (P < 0.5 considered statistically significant).
Western blotting.
Proteins were extracted from E12.5 (the day of the vaginal plug was considered E0.5) mouse embryo spinal cords and separated by electrophoresis on an sodium dodecyl sulfate-8% polyacrylamide electrophoresis gel. The protein gels were blotted onto a nitrocellulose membrane (Bio-Rad) and blocked in 5% powdered milk in Tris-buffered saline-0.5% Tween 20. The protein blots were subsequently probed with a goat anti-mouse EphA3 antibody (R&D Systems) at a dilution of 1:200 and a mouse anti-neurofilament antibody at a dilution of 1:500. A horseradish peroxidase-conjugated donkey anti-goat antibody (Santa Cruz) was used for EphA3 detection, and a horseradish peroxidase-conjugated donkey anti-mouse antibody (Pierce Immunochemicals) was used for neurofilament detection. Signal was visualized through enhanced chemiluminescence (Amersham Pharmacia Biotech).
Immunohistochemistry.
Immunohistochemistry to detect Lim3, Islet-1, and Islet-2 were carried out on 12-μm serial sections. Briefly, E13.5 mouse embryos were fixed for 2 h in 4% paraformaldehyde, washed in phosphate-buffered saline (PBS) and processed through 10, 20, and 30% sucrose solutions in PBS. The processed embryos were sectioned by using a cryostat and thaw mounted onto positively charged glass slides. The slides were washed in immunobuffer (PBS containing 0.3% Triton X-100). Blocking was performed with 10% horse serum in immunobuffer overnight. Anti-Lim3 and anti-Islet1/2 primary antibodies (generous gifts from Sam Pfaff) were diluted in immunobuffer and placed on slides for 3 days. Incubation with a biotin-labeled donkey anti-rabbit antibody (Jackson Laboratories), was followed by incubation with ExtrAvidin (Sigma). Antigen detection was carried out through a nickel-intensified diaminobenzidine reaction (Sigma).
Whole-mount neurofilament immunohistochemistry.
Whole-mount immunohistochemistry was carried out as follows. E11.5, E12.5, and E13.5 mouse embryos were harvested and collected in PBS. Embryos were fixed overnight in 4% paraformaldehyde and then incubated overnight in Dent's Fix (80% methanol, 20% dimethyl sulfoxide). After a wash in Tris-buffered saline containing 1% Tween 20 for 2 days, the embryos were incubated with primary antibody 2H3 diluted 1:100 in 5% skim milk, 0.5% dimethyl sulfoxide, and 0.01% sodium azide in Tris-buffered saline containing 1% Tween 20 for 3 days. The 2H3 monoclonal antibody was developed by T. Jessell and J. Dodd and obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences, Iowa City. Visualization was performed by using a rhodamine red-conjugated goat anti-mouse secondary antibody diluted 1:100.
Embryonic retrograde labeling.
All protocols for these experiments were approved by the University of Western Ontario Animal Care Committee in accordance with the policies established in the Guide to the Care and Use of Experimental Animals prepared by the Canadian Council on Animal Care. E13.5 embryos were dissected from extraembryonic tissues, decapitated, and immersed in cold PBS. The internal organs of each embryo were removed. Each embryo was then pinned onto a Sylguard-coated dish. A constant flow of oxygenated incubation buffer (124 mM NaCl, 5 mM KCl, 1.2 mM KH2PO4, 1.3 mM MgSO4, 2.4 mM CaCl2, 26 mM NaHCO3, 10 mM d-glucose) was maintained through the dish to preserve the neural tissue. A total of 1 to 2 μl of a 5% dextran-tetramethylrhodamine conjugate (Molecular Probes) in saline containing 1% lysolecithin was injected into the paravertebral and limb muscles of wild-type and EphA3 knockout embryos. After 12 h of incubation, the embryos were fixed in 4% paraformaldehyde for 90 min and then washed in several changes of PBS. The embryos were then sectioned at 150 μm on a Vibratomed (Leica) and observed under epifluorescence.
Adult retrograde labeling.
The animals were anesthetized with a 2:1 mixture of ketamine and xylazine (0.01 ml/10 g), and a 2-cm incision made above the scapula. A blunt dissection was performed to expose the acromiotrapezius. Approximately 2 μl of 4% Fluorogold (Fluorochrome, Inc.) in saline was injected into the muscle by using a pulled glass micropipette and mouth pipettor. Excess dye was absorbed with cotton swabs. The muscle was then sealed with Nexaband (Veterinary Products Laboratories) to prevent leakage. The incision was sutured, and the dye was allowed to transport for 3 days, at which time the mice were again anesthetized and transcardially perfused with 50 ml of Dulbecco modified Eagle medium (Gibco-BRL), followed by 100 ml of 4% paraformaldehyde. Each spinal cord was dissected from the vertebral column and the C1 to C8 region was isolated. Cryostat sections (16 μm) were cut and thaw mounted onto positively charged glass slides. The slides were coverslipped with 2% β-mercaptoethanol in DePeX mounting medium (BDH, Inc.). Thaw-mounted sections were viewed under fluorescence to reveal labeled motor neurons. The spinal segment location of retrogradely labeled motor neurons was determined by counting dorsal roots.
RNA in situ hybridization.
RNA in situ hybridizations with 35S-labeled riboprobes were performed as previously described (39). The 900-base EphA3 riboprobe was generated from the 5′ end of the EphA3 gene. The EphA4 riboprobe was generated from nucleotides 3439 to 3867 and the Islet-2 riboprobe was generated from nucleotides 301 to 829.
RESULTS
Generating the EphA3−/− mutant.
The EphA3−/− mouse line was constructed to have a 2-kb deletion at the 5′ end of the EphA3 gene, including its first exon, which encodes its signal sequence (Fig. 1A). Any aberrant receptor that might be produced from this allele would (i) encounter the neo stop signal, (ii) not be localized to the membrane, and (iii) not be in frame if the neo cassette were spliced over to either the next or the following exon. To verify that the EphA3 knockouts were indeed null mutants, we performed Western blot analysis on wild-type and EphA3−/− E12.5 spinal cord protein extracts with an anti-EphA3 polyclonal antibody. To ensure equal loading, the protein blots were also probed with an anti-neurofilament antibody. As predicted by the construct design, EphA3−/− animals do not produce any EphA3 protein (n = 6) (Fig. 1B).
Partially penetrant perinatal mortality in EphA3−/− mice.
Early in the breeding of the EphA3 null mutants it became obvious that EphA3−/− homozygotes were not being produced in the expected numbers. Of 194 pups from EphA3+/− matings, 68 wild types, 110 heterozygotes, and 16 homozygotes were obtained. The observed number of EphA3−/− mice from these matings was significantly lower than the expected 25% (chi-square test, P < 0.0001). Closer observation of newborn pups demonstrated that ca. 70% EphA3−/− mice die within the first 48 h of birth. Postmortem examination of the tissues taken from the EphA3−/− neonates indicates that they die of pulmonary edema secondary to cardiac failure, since the lungs are only poorly inflated and the atria are engorged with blood. EphA3−/− mice that survive the perinatal period develop normally and have no obvious cardiac or other abnormalities.
Assay of grip strength in EphA3−/− adult mice.
To assay the EphA3−/− adult mice for deficits in motor function, their forelimb grip strength was assessed by timing how long they could support their body weight by holding onto a pencil suspended in the air. The average time wild-type mice could support their weight by forelimb strength was 10 ± 2 s, while the average time for EphA3−/− mice 12 ± 4 s. These differences were not statistically significant.
Motor column organization.
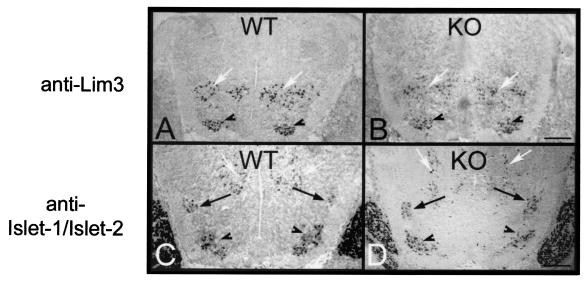
To be able to interpret retrograde labeling studies of motor axons, we first assessed whether the EphA3 mutation perturbed motor column organization patterns in the EphA3−/− spinal cords. To assess spinal cord organization, we evaluated the expression patterns of Lim-homeodomain genes that delineate different motor column populations in E13.5 wild-type and EphA3−/− embryos (44). E13.5 spinal cords were analyzed for their motor neuron organization since by this developmental time point motor neurons have assumed their characteristic positions in the spinal cord. The organization of motor neuron columns, as revealed by immunohistochemistry with an anti-Islet1/Islet2 antibody (to delineate MMC and LMC motor neurons) and an anti-Lim3 antibody (to delineate only MMC motor neurons), are identical in wild-type (n = 5) and EphA3−/− (n = 10) embryos (Fig. 2).
FIG. 2.
Motor column organization in the EphA3 mutants. To determine whether the EphA3 null mutant has the normal columnar organization of motor neurons, immunohistochemistry was carried out on cross sections through wild-type (WT) (n = 5) and EphA3−/− (KO) (n = 10) E13.5 spinal cords. (A and B) Sections probed with an anti-Lim3 antibody to delineate the MMC; (C and D) sections probed with an antibody that recognizes both Islet-1 and Islet-2 and therefore delineates both the MMC (arrowheads) and the LMC (black arrows). Interneurons are also immunoreactive for these markers, and their positions are indicated by white arrows. This analysis demonstrates that the motor column organization in EphA3−/− mice appears to be unaffected by their mutation. Bar, 0.2 mm.
Motor axon targeting in the EphA3−/− embryos.
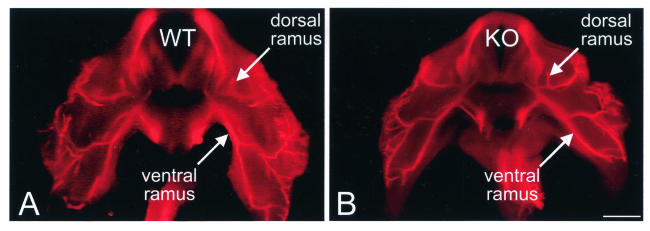
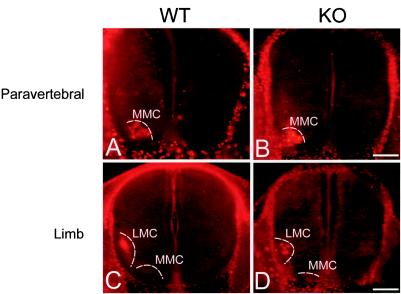
The specific expression of EphA3 in a subset of MMC motor neurons and in a subset of MMC muscle targets, led us to the hypothesis that EphA3 directs MMC axons to these targets (23). Specifically, we predicted that if EphA3 is necessary for MMC axon targeting, then MMC axons in the EphA3−/− mutant would innervate abnormal targets such as the limb musculature. To analyze the general pattern of nerve projection patterns in the EphA3−/− mutants, we performed whole-mount immunohistochemistry on E12.5 embryos with an anti-neurofilament antibody (Fig. 3). No significant differences in the patterns of spinal nerve projections were observed between wild-type (n = 12) and EphA3−/− (n = 21) embryos when they were analyzed by whole-mount anti-neurofilament antibody staining. For a more detailed analysis of the MMC projection patterns in these mutants, we injected a rhodamine-dextran conjugate into the paravertebral and limb muscles of E13.5 wild-type and EphA3−/− embryos. If, as we predicted, EphA3 signaling is necessary to prevent MMC axons from innervating the limb musculature, then we would have observed some fluorescently labeled motor neurons in the MMC of EphA3−/− spinal cords after retrograde labeling of the limb. Examination of the retrogradely labeled wild-type and EphA3−/− spinal cords revealed that, in all cases, the fluorescently labeled motor neurons were confined to the MMC after paravertebral muscle injection (n = 12, wild type; n = 12, EphA3−/−) (Fig. 4A and B) and to the LMC after limb muscle injections (n = 8, wild type; n = 12, EphA3−/−) (Fig. 4C and D).
FIG. 3.
Spinal nerve projection patterns in EphA3−/− mutants. To analyze the EphA3−/− mutants for gross abnormalities in the patterns of their spinal nerve projections, E12.5 wild-type (n = 12) and EphA3−/− (n = 21) embryos were subjected to whole-mount immunohistochemistry by using an anti-neurofilament antibody. The embryos were subsequently sectioned on a Vibratome at 150 μm and viewed under epifluorescence. No significant differences between the mutants and wild-type were detected at this level of analysis. Bar, 0.5 mm.
FIG. 4.
Retrograde labeling studies in EphA3−/− mutant embryos. Wild-type (A and C) and EphA3−/− (B and D) E13.5 embryos were retrogradely labeled by injecting a rhodamine-dextran conjugate into their paravertebral muscles (A and B) or limb muscles (C and D). The positions of the MMC and of the LMC (indicated by the white dashed curves) were observed by overexposing the image. In both wild-type and EphA3−/− mutants, tracer injection of paravertebral muscles consistently retrogradely labeled the MMC, while tracer injection of the limb muscles consistently labeled the LMC. Bar, 0.2 mm.
Motor axon targeting in the EphA3−/− adults.
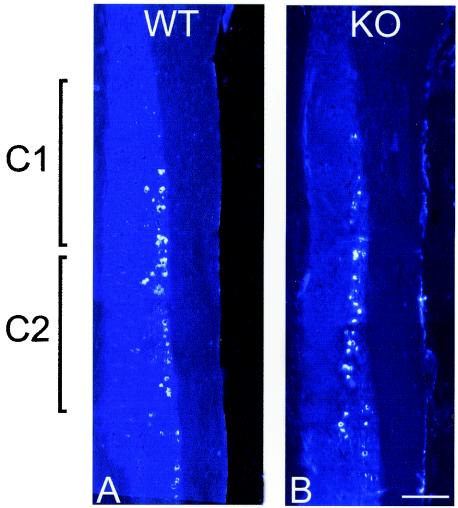
The motor neuron pool innervating the acromiotrapezius is shifted caudally in ephrin-A5−/− mice (11). Since EphA3 is expressed in cervical motor neurons, we postulated that a similar caudalward shift would be found in this same motor neuron pool in EphA3−/− mice. Retrograde labeling studies were performed by injecting the acromiotrapezius with Fluorogold in order to compare the location and extent of the acromiotrapezius motor pool in EphA3−/− and wild-type mice. We were particularly careful to inject the caudal half of the muscle because we wanted to examine the caudalmost extent of this motor pool. Examination of the labeled motor neurons in the spinal cords indicated that the majority of motor neurons innervating the acromiotrapezius are in C1 and C2 in both wild-type (n = 6) and EphA3−/− (n = 6) mice. Some retrogradely labeled motor neurons were in C3, but none were caudal to C3. These experiments revealed no shift in the rostro-caudal location of motor neurons innervating the acromiotrapezius in the EphA3−/− mutants (Fig. 5).
FIG. 5.
Retrograde labeling studies in EphA3 mutant adults. The acromiotrapezius muscles of wild-type (n = 6) (A) and EphA3−/− (n = 6) (B) mice were injected with Fluorogold. After we allowed time for the tracer to be transported to the innervating motor neurons, the spinal cords were sectioned and analyzed for the position of labeled motor neurons. Labeled motor neurons fell almost exclusively in spinal segments C1 to C3. Thus, no shift in the motor neuron pool innervating the acromiotrapezius in EphA3−/− mutants could be detected. Bar, 0.5 mm.
EphA4 expression in motor neurons.
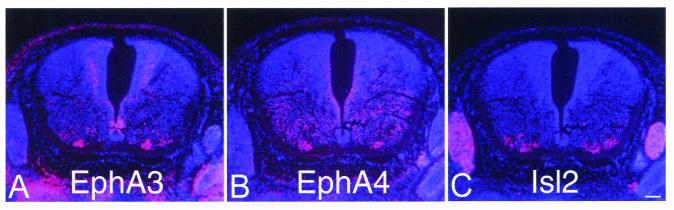
One possible explanation for the absence of an axon guidance defect in the EphA3−/− mice is that another EphA receptor compensates for the EphA3 mutation. We therefore carried out RNA in situ hybridizations on cross sections of E11.5 spinal cords to analyze the expression of all EphA receptors (EphA1 to EphA8) in developing motor neurons. Only EphA4 and EphA3 were observed to be expressed in developing motor neurons. Furthermore, the RNA in situ hybridizations carried out on serial sections of E11.5 embryos reveal that EphA3 and EphA4 are both expressed in developing MMC motor neurons (Fig. 6).
FIG. 6.
The EphA3 and EphA4 genes are coexpressed in motor neurons during development. RNA in situ hybridizations for EphA3 (A), EphA4 (B), and (C) Islet-2 were carried out on 7-μm serial sections of E11.5 wild-type embryos. The sections were counterstained with DAPI (4′,6′-diamidino-2-phenylindole), and the silver grains were viewed with a red filter under dark-field illumination. Islet-2 expression delineates the motor neurons in the field. Note that EphA3 and EphA4 are coexpressed in a subset of MMC motor neurons at this developmental time point. Bar, 0.1 mm.
DISCUSSION
Studies in the development of motor neuron projections in the chick demonstrate that motor neurons are predestined to innervate particular muscles before their axons extend into the periphery. This has been demonstrated by the ability of motor neurons to correct their trajectories and project to appropriate targets despite early spinal cord segment reversals (26), limb bud reversals (12), and limb shifts (27). Accumulated evidence suggests that members of the LIM homeodomain (LIM-HD) family of transcription factors could be responsible for this early specification of motor neuron targets. Support for this assertion originally came from the observation that motor neurons with similar targets express the same combination of LIM homeodomain genes (44). The ability of LIM-HD genes to specify motor neuron targets has been more recently demonstrated by gene targeting studies in which targeted disruptions of specific LIM-HD genes or their ectopic expression have been shown to respecify motor neuron targets according to the motor neuron's new combinatorial code of LIM-HD genes (40, 41). The ability of LIM-HD genes to determine motor neuron target specificity suggests that these transcription factors must regulate the expression of proteins, such as Eph receptors and their ligands that can regulate axon guidance decisions (2, 10, 11, 15, 21, 31, 37).
The motor pool that innervates the acromiotrapezius is shifted caudally in ephrin-A5−/− mice (11). We hypothesized that the ephrin-A5 sensitivity of cervical motor axons is mediated by EphA3 and, that in EphA3−/− mice, we would observe a caudalward shift in the motor neuron pool innervating the acromiotrapezius. The absence of any shift in the position of the motor pool innervating the acromiotrapezius in the EphA3−/− mutants suggests that EphA3 alone is not responsible for mediating the ephrin A5 signal that organizes these projections. The early (E11.5) coexpression of EphA3 and EphA4 in the cervical MMC suggests that EphA4 alone, or in conjunction with EphA3, is responsible for mediating the ephrin A5 signal that organizes the topographic innervation of the acromiotrapezius.
Based on the expression of EphA3 in the MMC and on the expression of ephrin A's in the limb bud, we hypothesized that EphA3 would be necessary for the appropriate axial and body wall targeting of MMC axons. We also predicted that, in the absence of EphA3, some MMC axons would inappropriately innervate limb musculature. Our analysis demonstrated that MMC axons in the EphA3−/− mutants project normally despite the absence of EphA3 expression. Thus, it appears that EphA3 is not necessary for MMC axon guidance. One explanation for the correct targeting of MMC axons in the EphA3−/− mutant is that the early expression of EphA4 in MMC neurons may compensate for the absence of EphA3. Indeed, EphA4 has been shown to be involved in the formation of corticospinal projections (6) and in the correct targeting of LMC motor axons (19). However, this explanation is only partially satisfactory since we have previously demonstrated that the topographic projections of retinal ganglion cells onto the superior colliculus depends on the relative levels of EphA receptor expression on axons competing for synaptic targets (2). Thus, if motor axon projections are also dependent on the relative levels of EphA receptor signaling, then MMC axons should project abnormally in the EphA3−/− mutant because in this mutant the relative levels of EphA receptor expression between different motor neuron columns has been altered. Our inability to demonstrate abnormalities in motor axon targeting in the EphA3 mutants indicates that either EphA3 controls only minor, subtle aspects of motor axon guidance or that other molecular pathways may be rescuing the mutants from axon guidance defects and suggests that these pathways may play a dominant role in motor axon guidance.
Acknowledgments
This work was supported by grants from The Canadian institutes of Health Research (A.B.) and from the NIH of the United States (G.L.). A.B. is a Research Scholar of the Heart and Stroke Foundation of Canada.
REFERENCES
- 1.Brambilla, R., and R. Klein. 1995. Telling axons where to grow: a role for eph receptor tyrosine kinases in guidance. Mol. Cell. Neurosci. 6:487-495. [DOI] [PubMed] [Google Scholar]
- 2.Brown, A., P. A. Yates, P. Burrola, D. Ortuno, A. Vaidya, T. M. Jessell, S. L. Pfaff, D. D. O'Leary, and G. Lemke. 2000. Topographic mapping from the retina to the midbrain is controlled by relative but not absolute levels of EphA receptor signaling. Cell 102:77-88. [DOI] [PubMed] [Google Scholar]
- 3.Cheng, H., and J. Flanagan. 1994. Identification and cloning of Elf-1, a developmentally expressed ligand for the Mek4 and Sek receptor kinases. Cell 79:157-168. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, H., M. Nakamoto, A. Bergmann, and J. Flanagan. 1995. Complementary gradients in expression and binding of Elf-1 and Mek4 in development of the topographic retinotectal projection map. Cell 82:371-381. [DOI] [PubMed] [Google Scholar]
- 5.Donoghue, M., R. Lewis, J. Merlie, and J. Sanes. 1996. The Eph kinase ligand AL-1 is expressed by rostral muscles and inhibits outgrowth from caudal neurons. Mol. Cell. Neurosci. 8:185-198. [DOI] [PubMed] [Google Scholar]
- 6.Dottori, M., L. Hartley, M. Galea, G. Paxinos, M. Polizzotto, T. Kilpatrick, P. Bartlett, M. Murphy, F. Kontgen, and A. Boyd. 1998. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proc. Natl. Acad. Sci. USA 95:13248-13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drescher, U., F. Bonhoeffer, and B. Muller. 1997. The Eph family in retinal axon guidance. Curr. Opin. Neurobiol. 7:75-80. [DOI] [PubMed] [Google Scholar]
- 8.Drescher, U., C. Kremoser, C. Handwerker, J. Loschinger, M. Noda, and F. Bonhoeffer. 1995. In vitro guidance of retinal ganglion cell axons by RAGS, a 25-kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell 82:359-370. [DOI] [PubMed] [Google Scholar]
- 9.Eberhart, J., M. Swartz, S. A. Koblar, E. B. Pasquale, H. Tanaka, and C. E. Krull. 2000. Expression of EphA4, ephrin-A2 and ephrin-A5 during axon outgrowth to the hindlimb indicates potential roles in pathfinding. Dev. Neurosci. 22:237-250. [DOI] [PubMed] [Google Scholar]
- 10.Feldheim, D. A., Y. I. Kim, A. D. Bergemann, J. Frisen, M. Barbacid, and J. G. Flanagan. 2000. Genetic analysis of ephrin-A2 and ephrin-A5 shows their requirement in multiple aspects of retinocollicular mapping. Neuron 25:563-574. [DOI] [PubMed] [Google Scholar]
- 11.Feng, G., M. B. Laskowski, D. A. Feldheim, H. Wang, R. Lewis, J. Frisen, J. G. Flanagan, and J. R. Sanes. 2000. Roles for ephrins in positionally selective synaptogenesis between motor neurons and muscle fibers. Neuron 25:295-306. [DOI] [PubMed] [Google Scholar]
- 12.Ferns, M. J., and M. Hollyday. 1993. Motor innervation of dorsoventrally reversed wings in chick/quail chimeric embryos. J. Neurosci. 13:2463-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fetcho, J. R. 1987. A review of the organization and evolution of motoneurons innervating the axial musculature of vertebrates. Brain Res. 434:243-280. [DOI] [PubMed] [Google Scholar]
- 14.Flenniken, A. M., N. W. Gale, G. D. Yancopoulos, and D. G. Wilkinson. 1996. Distinct and overlapping expression patterns of ligands for Eph-related receptor tyrosine kinases during mouse embryogenesis. Dev. Biol. 179:382-401. [DOI] [PubMed] [Google Scholar]
- 15.Frisen, J., P. Yates, T. McLaughlin, G. Friedman, D. O'Leary, and M. Barbacid. 1998. Ephrin A5 (AL-1/RAGS) is essential for proper retinal axon guidance and topographic mapping in the mammalian visual system. Neuron 20:235-243. [DOI] [PubMed] [Google Scholar]
- 16.Gale, N., D. Valenzuela, A. Flenniken, L. Pan, T. Ryan, M. Henkemeyer, K. Strebhardt, H. Hirai, D. Wilkinson, T. Pawson, and G. Yancopoulos. 1996. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron 17:9-19. [DOI] [PubMed] [Google Scholar]
- 17.Gao, P., J. Xhang, M. Yokoyama, B. Racey, C. Dreyfus, I. Black, and R. Zhou. 1996. Regulation of topographic projection in the brain: Elf-1 in the hippocamposeptal system. Proc. Natl. Acad. Sci. USA 93:11161-11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris, W., and C. Holt. 1995. From tags to RAGS: chemoaffinity finally has receptors and ligands.Neuron 15:241-244. [DOI] [PubMed] [Google Scholar]
- 19.Helmbacher, F., S. Schneider-Maunoury, P. Topilko, L. Tiret, and P. Charnay. 2000. Targeting of the EphA4 tyrosine kinase receptor affects dorsal/ventral pathfinding of limb motor axons. Development 127:3313-3324. [DOI] [PubMed] [Google Scholar]
- 20.Hollyday, M. 1980. Organization of motor pools in the chick lumbar lateral motor column. J. Comp. Neurol. 194:143-170. [DOI] [PubMed] [Google Scholar]
- 21.Hornberger, M., D. Dutting, T. Ciossek, T. Yamada, C. Hendwerker, S. Lang, F. Weth, J. Huf, R. Webel, C. Logan, H. Tanaka, and U. Drescher. 1999. Modulation of ephA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron 22:731-742. [DOI] [PubMed] [Google Scholar]
- 22.Iwamasa, H., K. Ohta, T. Yamada, K. Ushijima, H. Terasaki, and H. Tanaka. 1999. Expression of Eph receptor tyrosine kinases and their ligands in chick embryonic motor neurons and hindlimb muscles. Dev. Growth Differ. 41:685-698. [DOI] [PubMed] [Google Scholar]
- 23.Kilpatrick, T. J., A. Brown, C. Lai, M. Gassmann, M. Goulding, and G. Lemke. 1996. Expression of the Tyro4/Mek4/Cek4 gene specifically marks a subset of embryonic motor neurons and their muscle targets. Mol. Cell Neurosci. 7:62-74. [DOI] [PubMed] [Google Scholar]
- 24.Knoll, B., K. Zarbalis, W. Wurst, and U. Drescher. 2001. A role for the EphA family in the topographic targeting of vomeronasal axons. Development 128:895-906. [DOI] [PubMed] [Google Scholar]
- 25.Lance-Jones, C., and M. Dias. 1991. The influence of presumptive limb connective tissue on motoneuron axon guidance. Dev. Biol. 143:93-110. [DOI] [PubMed] [Google Scholar]
- 26.Lance-Jones, C., and L. Landmesser. 1980. Motoneurone projection patterns in the chick hind limb following early partial reversals of the spinal cord. J. Physiol. 302:581-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lance-Jones, C., and L. Landmesser. 1981. Pathway selection by embryonic chick motoneurons in an experimentally altered environment. Proc. R. Soc. Lond. B Biol. Sci. 214:19-52. [DOI] [PubMed] [Google Scholar]
- 28.Landmesser, L. 1978. The development of motor projection patterns in the chick hind limb. J. Physiol. 284:391-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laskowski, M. B., and J. R. Sanes. 1987. Topographic mapping of motor pools onto skeletal muscles. J. Neurosci. 7:252-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcus, R., N. Gale, M. Morrison, C. Mason, and G. Yancopoulos. 1996. Eph family receptors and their ligands distribute in opposing gradients in the developing mouse retina. Dev. Biol. 180:786-789. [DOI] [PubMed] [Google Scholar]
- 31.Nakamoto, et al. 1996. Topographically specific effects of Elf-1 on retinal axon guidance in vitro and retinal axon guidance in vivo. Cell 86:755-766. [DOI] [PubMed] [Google Scholar]
- 32.Nakao, T., and A. Ishizawa. 1994. Development of the spinal nerves in the mouse with special reference to innervation of the axial musculature. Anat. Embryol. 89:115-138. [DOI] [PubMed] [Google Scholar]
- 33.Ohta, K., H. Iwamasa, U. Drescher, H. Terasaki, and H. Tanaka. 1997. The inhibitory effect on neurite outgrowth of motoneurons exerted by the ligands ELF-1 and RAGS. Mech. Dev. 64:127-135. [DOI] [PubMed] [Google Scholar]
- 34.O'Leary, D., and D. Wilkinson. 1999. Eph receptors and ephrins in neural development. Curr. Opin. Neurobiol. 9:65-73. [DOI] [PubMed] [Google Scholar]
- 35.Orioli, D., and R. Klein. 1997. The Eph receptor family: axonal guidance by contact repulsion. Trends Genet. 13:354-359. [DOI] [PubMed] [Google Scholar]
- 36.Pandey, A., H. Shao, R. M. Marks, P. J. Polverini, and V. M. Dixit. 1995. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis. Science 268:567-569. [DOI] [PubMed] [Google Scholar]
- 37.Park, S., J. Frisen, and M. Barbacid. 1997. Aberrant axonal projections in mice lacking EphA8 (Eek) tyrosine protein kinase receptors. EMBO J. 16:3106-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, New York, N.Y.
- 39.Schaeren-Wiemers, N., and A. Gerfin-Moser. 1993. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labeled cRNA probes. Histochemistry 100:431-440. [DOI] [PubMed] [Google Scholar]
- 40.Sharma, K., A. E. Leonard, K. Lettieri, and S. L. Pfaff. 2000. Genetic and epigenetic mechanisms contribute to motor neuron pathfinding. Nature 406:515-519. [DOI] [PubMed] [Google Scholar]
- 41.Sharma, K., H. Z. Sheng, K. Lettieri, H. Li, A. Karavanov, S. Potter, H. Westphal, and S. L. Pfaff. 1998. LIM homeodomain factors Lhx3 and Lhx4 assign subtype identities for motor neurons. Cell 95:817-828. [DOI] [PubMed] [Google Scholar]
- 42.Tosney, K. 1991. Cells and cell interactions that guide motor axons in the developing chick embryo. Bioessays 13:17-23. [DOI] [PubMed] [Google Scholar]
- 43.Tosney, K. W. 1987. Proximal tissues and patterned neurite outgrowth at the lumbosacral level of the chick embryo: deletion of the dermamyotome. Dev. Biol. 122:540-558. [DOI] [PubMed] [Google Scholar]
- 44.Tsuchida, T., M. Ensini, S. Morton, M. Baldassare, T. Edlund, T. Jessell, and S. Pfaff. 1994. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell 79:957-970. [DOI] [PubMed] [Google Scholar]
- 45.Winslow, J., P. Moran, J. Valverde, A. Shih, J. Yuan, S. Wong, S. Tsai, A. Goddard, W. Henzel, F. Hefti, K. Beck, and I. Caras. 1995. Cloning of AL-1, a ligand for an Eph-related tyrosine kinase receptor involved in axon bundle formation. Neuron 14:973-981. [DOI] [PubMed] [Google Scholar]