Abstract
The cytokine tumor necrosis factor alpha (TNF-α) stimulates the NF-κB, SAPK/JNK, and p38 mitogen-activated protein (MAP) kinase pathways by recruiting RIP1 and TRAF2 proteins to the tumor necrosis factor receptor 1 (TNFR1). Genetic studies have revealed that RIP1 links the TNFR1 to the IκB kinase (IKK) complex, whereas TRAF2 couples the TNFR1 to the SAPK/JNK cascade. In transfection studies, RIP1 and TRAF2 stimulate p38 MAP kinase activation, and dominant-negative forms of RIP1 and TRAF2 inhibit TNF-α-induced p38 MAP kinase activation. We found TNF-α-induced p38 MAP kinase activation and interleukin-6 (IL-6) production impaired in rip1−/− murine embryonic fibroblasts (MEF) but unaffected in traf2−/− MEF. Yet, both rip1−/− and traf2−/− MEF exhibit a normal p38 MAP kinase response to inducers of osmotic shock or IL-1α. Thus, RIP1 is a specific mediator of the p38 MAP kinase response to TNF-α. These studies suggest that TNF-α-induced activation of p38 MAP kinase and SAPK/JNK pathways bifurcate at the level of RIP1 and TRAF2. Moreover, endogenous RIP1 associates with the MAP kinase kinase kinase (MAP3K) MEKK3 in TNF-α-treated cells, and decreased TNF-α-induced p38 MAP kinase activation is observed in Mekk3−/− cells. Taken together, these studies suggest a mechanism whereby RIP1 may mediate the p38 MAP kinase response to TNF-α, by recruiting the MAP3K MEKK3.
Mitogen-activated protein kinase (MAPK) signal transduction pathways have been implicated in many physiological processes including growth, differentiation, cell death, and survival (reviewed in reference 13). The MAPK pathways employ a central three-tiered module of protein kinases, characterized by a MAPK kinase kinase (MAP3K) and a dual-specificity MAPK kinase (MAP2K). The MKKK phosphorylates and activates the MKK, which then activates the MAPK. The mammalian p38 MAPK family consists of at least four homologous proteins, p38α, -β, -γ, and -δ (19, 42). p38 MAPK is activated by at least two MAP2Ks, MKK3 and MKK6 (8, 35). Numerous MAP3Ks have been shown to activate MKK3-MKK6, and these include transforming growth factor β (TGF-β)-activating kinase 1 (TAK1) (22, 49), apoptosis signal-regulating kinase 1 (ASK1) (18), SPRK (31, 45), p21-activated kinase (PAK) (3), and MEKK3 (7). The activation of p38 MAPK through cellular stress or receptor activation results in the phosphorylation of a diverse array of substrates, suggesting that p38 MAPK contributes to many different biological processes. p38 MAPKs phosphorylate and activate the MAPK-activated protein (MAPKAP) kinases 2 and 3, which phosphorylate a small heat shock protein (hsp27) (36). Other p38 MAPK substrates include the transcription factors ATF2 (34, 35), Elk1 (52), Chop (50), MEF2C (14, 27), and SAP-1 (32, 52).
The pyridinylimidazole compounds such as SB203580 were prepared as anti-inflammatory agents that were subsequently found to inhibit the catalytic activity of p38 MAPK by binding in the ATP pocket (47, 53, 59). The p38 MAPK inhibitors are effective in several murine tumor necrosis factor alpha (TNF-α)-mediated disease models including those that induce inflammation, arthritis, septic shock, and myocardial injury. Studies using the p38 inhibitors indicate that these compounds regulate cytokine translation (24, 25, 33). Targeted disruption of components of the p38 MAPK pathway has confirmed and extended the inhibitor studies (23, 54). For example, mice deficient in MAPKAP-2, a p38 downstream kinase, have been shown to be resistant to endotoxic shock induced by lipopolysaccharide (LPS). The resistance appears to be due to a reduction in TNF-α translation and not to occur at the level of TNF mRNA stability or TNF-α secretion (23).
The proinflammatory cytokine TNF-α is a major mediator of apoptosis as well as inflammation and immunity. It has also been implicated in the pathogenesis of several human diseases including inflammatory bowel disease, arthritis, sepsis, diabetes, and cancer (reviewed in reference 12). TNF-α activates the transcription factors NF-κB and AP-1 and exerts its effects by binding to two receptors, the tumor necrosis factor receptor type 1 (TNFR1 or p55) and TNFR2 (or p75) (40, 51). Ligand binding displaces the inhibitory protein silencer of death domains (SODD) (20) and induces aggregation of the intracellular death domains and the subsequent recruitment of key adapter proteins. These adapter proteins include the TNFR-associated death domain protein (TRADD), the TNFR-associated factor 2 (TRAF2), and the death domain serine/threonine kinase RIP1 (15-17, 28). Overexpression of either RIP1 or TRAF2 results in SAPK/JNK, p38 MAPK, and NF-κB activation, suggesting that RIP1 and TRAF2 mediate these pathways (6, 16, 17, 46, 60). Genetic analysis in mice has revealed that the TNFR-associated adapter proteins, TRAF2 and RIP1, mediate discrete signaling pathways. RIP1 links the TNFR1 to the IκB kinase (IKK) complex whereas TRAF2 couples the TNFR1 to the SAPK/JNK cascade (21, 58).
To address the contribution of RIP1 and TRAF2 proteins to the TNF-α-induced p38 MAPK pathway, we examined p38 MAPK activity in rip1-deficient and traf2-deficient cells. Our study reveals an additional regulatory role for RIP1 in the TNF-α-induced p38 MAPK response and in TNF-α-induced interleukin-6 (IL-6) production. Importantly, this study also suggests that the TNF-α-induced p38 MAPK and SAPK/JNK pathways are independently regulated at the level of RIP1 and TRAF2, respectively.
MATERIALS AND METHODS
Preparation of MEF.
Targeted disruptions of the rip1, traf2, and Mekk3 loci have been described in detail elsewhere (21, 56, 58). Embryonic day 14 wild-type, rip1+/−, and rip1−/− embryos were equilibrated in trypsin overnight at 4°C. Trypsin was activated by incubation at 37°C for 15 min. The embryos were then dissociated in Dulbecco's modified Eagle's medium (BioWhittaker) containing 10% fetal calf serum. Murine embryonic fibroblasts (MEF) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (HyClone), 1% penicillin-streptomycin, and 1% l-glutamine (Gibco). Experiments were performed on cells between passage 2 and passage 5. Immortalized wild-type and rip1−/− 3T3 cell lines were prepared according to established protocols. traf2−/− MEF were provided by Wen-chen Yeh (Ontario Cancer Institute, Ontario, Calif.) and cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (HyClone). Two wild-type and three Mekk3−/− MEF lines were prepared by Bing Su (M. D. Anderson Cancer Center, Houston, Tex.).
Plasmids and constructs.
MEKK3-specific primers (5′-GCC CTC GAG ATG GAT GAA CAA GAG GCA TTA GAC TCG-3′ and 5′-GAT GGT ACC TCA GTA CAC TAG CTG TGC AAA GTG G-3′) were used in a PCR to generate full-length MEKK3 cDNA by using first-strand cDNA generated from polyadenylated mouse spleen RNA. The 1.8-kb PCR fragment was cloned and sequenced. Mouse MEKK3 was epitope tagged at the NH2 terminus with a hemagglutinin (HA) tag sequence by a PCR strategy. For inserting the NH2-terminal epitope tag in MEKK3, sense oligonucleotide was synthesized with a KpnI restriction site, a methionine codon (ATG), 27 bases coding for the HA epitope, and 24 bases of MEKK3 sequence. The sense oligonucleotide was 5′-GCC GGT ACC GCC ACC ATG TAC CCA TAC GAC GTA CCT GAT TAC GCA GAT GAA CAA GAG GCA TTA GAC TCG-3′. The antisense oligonucleotide was 5′-GGG CTC GAG TCA GTA CAC TAG CTG TGC AAA G-3′, encoding a XhoI site. The PCR product was purified and cloned into pcDNA3. The full-length RIP-myc, ΔKIN-myc, and ΔDD-myc constructs and RIP-KD have been described previously (41, 46). MEKK1-HA, ASK1-HA, MEKK2-HA, and Tpl2-HA constructs were provided by Gary Johnson, H. Ichijo, Richard Vaillancourt, and Philip Tsichlis, respectively.
In vitro kinase assay.
Wild-type, rip1−/−, or traf2−/− cells were plated at 106 cells/10-cm plate. The cells were left untreated or treated with 10 ng of TNF-α/ml or 10 ng of IL-1α/ml or with increasing concentrations of sorbitol (100, 200, or 400 mM) for the time periods indicated. Cells were washed once with cold phosphate-buffered saline, and an in vitro kinase assay was performed as described previously (29).
IL-6 production assays.
Wild-type, rip1−/−, or traf2−/− cells were plated at 3 × 104 cells/well on 24-well plates. The cells were left untreated or treated with 10 ng of TNF-α/ml for 20 h. The supernatants were collected, and the levels of IL-6 produced from each cell line were assayed with the OptEIA mouse IL-6 enzyme-linked immunosorbent assay (ELISA) kit (PharMingen). RNase protection assays of total RNA isolated from wild-type and rip1−/− MEF were performed using the Riboquant kit (PharMingen).
Coimmunoprecipitation and Western blot analysis.
For all transfection studies, 293T cells were plated at 3 × 105 cells/plate on six-well plates. To determine the specificity of RIP1-MEKK3 interactions, 293T cells were transfected with HA-ASK1, HA-MEKK3, HA-MEKK1, HA-MEKK2, and HA-Tpl2 by using Fugene 6 (Roche). The transfected cells were lysed in 0.5 ml of E1A buffer (50 mM HEPES [pH 7.6], 250 mM NaCl, 0.1% NP-40, 5 mM EDTA), immunoprecipitated with anti-RIP antibody (Ab; PharMingen), and immunoblotted with anti-HA Ab. Expression of transfected constructs was confirmed by immunoblotting with an anti-HA Ab. To map the RIP1-MEKK3 interaction, 293T cells were transfected with FLAG-MEKK3 and RIP deletion constructs by using Fugene 6 (Roche). The transfected cells were lysed in E1A lysis buffer (50 mM HEPES [pH 7.6], 250 mM NaCl, 0.1% NP-40, 5 mM EDTA), the lysates were immunoprecipitated with anti-MYC Ab, and the associated MEKK3 proteins were detected by immunoblotting with anti-FLAG Ab. Expression of the MEKK3-FLAG and various RIP-MYC deletion constructs was determined by immunoblotting with anti-FLAG or anti-MYC Abs, respectively. To examine whether the endogenous RIP1 and MEKK3 proteins interact and whether the interaction is TNF-α dependent, three 10-cm-diameter plates of 293T cells were left untreated or treated with 100 ng of human TNF-α/ml for the time periods indicated. Cells were lysed in 20 mM Tris (pH 7.5)-150 mM NaCl-1% Triton-1 mM EDTA-30 mM NaF-2 mM sodium pyrophosphate-10 μg of aprotinin/ml-10 μg of leupeptin/ml and immunoprecipitated with anti-MEKK3 Ab. The immune complexes were fractionated by gel electrophoresis and transferred to an Immobilon P membrane (Millipore). RIP1 proteins were detected by immunoblotting with an anti-RIP Ab (PharMingen). To determine relative expression levels of p38α MAPK, wild-type, rip1−/−, traf2−/−, or Mekk3−/− MEF were lysed in cell lysis buffer and 30 μg of total protein was immunoblotted with anti-p38α Ab (Santa Cruz). To determine p38α MAPK activity, 30 μg of total protein was immunoblotted with an anti-phospho-p38 Ab (Cell Signaling Technology).
RESULTS
RIP1 participates in TNF-α-induced p38 MAPK activation.
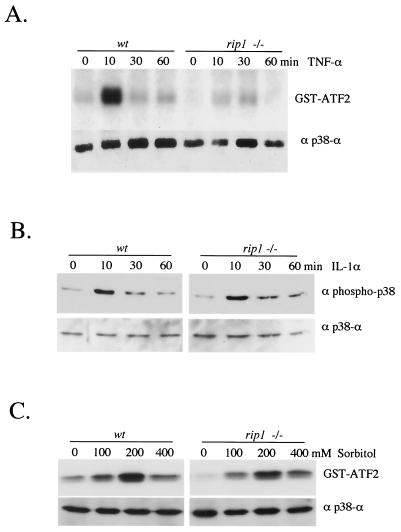
To examine the role of the death domain kinase RIP1 in TNF-α-induced p38 MAPK activation, we treated wild-type and rip1−/− MEF with TNF-α and performed a p38α immune complex kinase assay. In wild-type cells, we observed an increase in p38 MAPK activity in response to TNF-α. However, we failed to observe any TNF-α-induced p38 MAPK activity in the TNF-α-treated rip1−/− cells (Fig. 1A). Similar results were obtained when TNF-α-treated rip1−/− lysates were probed with an anti-phospho-p38 Ab (data not shown). Failure to activate p38 MAPK was not due to down regulation of the p38α isoform in rip1−/− cells, as wild-type and rip1−/− cells expressed similar amounts of p38α. In contrast to the TNF-α response, rip1−/− cells exhibit normal IL-1α-induced and sorbitol-induced p38α MAPK activation (data not shown). These studies suggest that RIP1 is a specific mediator of the p38α MAPK response to TNF-α.
FIG. 1.
TNF-α-induced p38 MAPK activity is impaired in rip1−/− cells. (A) Wild-type and rip−/− MEF were grown to confluence on 10-cm-diameter plates and treated with 10 ng of murine TNF-α/ml for 0, 10, 30, and 60 min. Cells were subsequently lysed and immunoprecipitated with anti-p38α Ab (a gift from R. Davis), and the kinase activity of p38 MAPK was measured by an in vitro kinase assay with GST-ATF2 as substrate. The p38α level in each cell type was determined by immunoblotting with anti-p38α (Santa Cruz). (B) Normal IL-1α-induced p38 MAPK activity in rip−/− cells. Wild-type and rip−/− MEF were treated with 10 ng of IL-1α/ml for various times (0, 10, 30, and 60 min), and the p38 MAPK activity was measured by immunoblotting with an anti-phospho-p38 Ab (Cell Signaling Technology catalog no. 9211S). (C) Normal sorbitol-induced p38 MAPK activity in rip−/− cells. Wild-type and rip−/− MEF were treated with 0, 100, 200, and 400 mM sorbitol for 15 min, and the kinase activity of p38 was measured by in vitro kinase assay with GST-ATF2 as substrate.
p38 MAPK activation in traf2−/− cells.
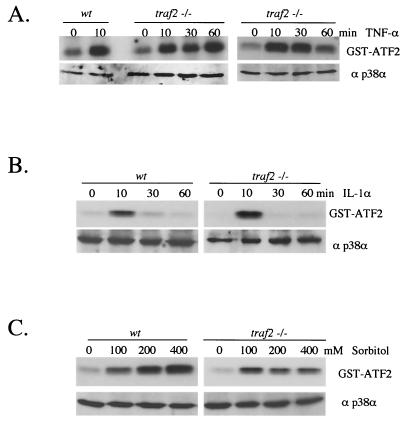
The death domain kinase RIP1 interacts with TRAF2, and together these proteins mediate signals from the activated TNFR1. Moreover, expression of a dominant-negative form of TRAF2 inhibits TNF-α-induced p38 MAPK activity in 293T cells (60). Hence, we reasoned that the p38 MAPK response to TNF-α may also be affected in traf2−/− cells. To test this possibility, two immortalized traf2−/− cell lines (A7 and A4) and three primary traf2−/− MEF lines were examined for their ability to respond to TNF-α. In contrast to rip-deficient cells, MEF from traf2−/− mice exhibit normal TNF-α-induced p38 MAPK activation (Fig. 2A). traf2-deficient cells also exhibit a normal p38 MAPK response to treatment with IL-1α or to osmotic shock induced by sorbitol treatment by either immune complex kinase assays or by immunoblotting with a phosphospecific p38 MAPK Ab (Fig. 2B and C). No significant differences in the p38 MAPK responses to TNF-α were observed between wild-type and traf2−/− cells examined. This study suggests that TRAF2 is not required to mediate TNF-α-stimulated p38 MAPK activation in MEF. Alternatively, other TNF-α-responsive TRAF family members may substitute for the traf2 deficiency.
FIG. 2.
TNF-α-induced p38 MAPK activity is unaffected in traf2−/− cells. (A) Wild-type and traf2−/− MEF were grown to confluence on 10-cm-diameter plates and treated with 10 ng of TNF-α/ml for 0, 10, 30, and 60 min. Cells were lysed and immunoprecipitated with anti-p38α Ab (a gift from R. Davis), and the kinase activity of p38 MAPK was measured by an in vitro kinase assay using GST-ATF2 as substrate. The p38α level in each cell type was determined by immunoblotting with anti-p38α (Santa Cruz). (B) IL-1α-induced p38 MAPK activity is unaffected in traf2−/− cells. Wild-type and traf2−/− MEF were treated with 10 ng of IL-1α/ml and measured for p38 activity with anti-phospho-p38 Ab. (C) Sorbitol-induced p38 MAPK activity is unaffected in traf2−/− cells. Wild-type and traf2−/− MEF were also treated with 0, 100, 200, and 400 mM sorbitol for 15 min, and p38 MAPK activity was measured by an in vitro kinase assay with GST-ATF2 as substrate.
Decreased TNF-α-induced IL-6 production in rip1−/− cells.
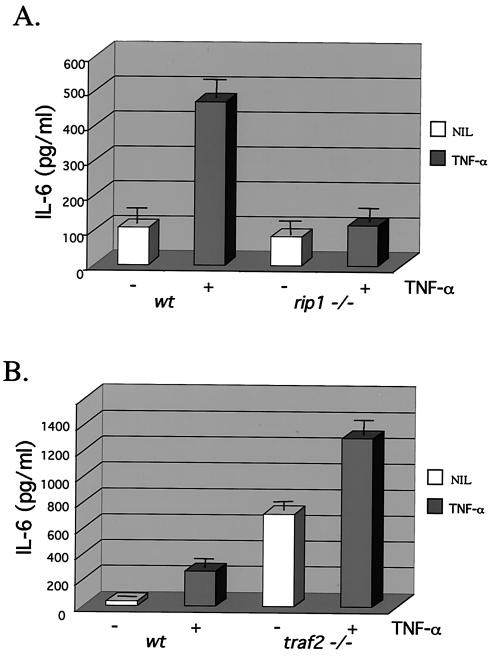
The NF-κB and p38 MAPK pathways have been implicated in stress-induced cytokine production (4, 25, 26, 39, 54, 62). We therefore examined cytokine production in rip1−/− and traf2−/− MEF in response to TNF-α. Treatment of wild-type cells with TNF-α resulted in increased production of IL-6, whereas little to no TNF-α-induced IL-6 production was observed in rip1−/− cells (Fig. 3A). These data suggest that RIP1 kinase contributes to TNF-α-induced IL-6 production potentially by regulating both the IKK and p38 MAPK activities.
FIG. 3.
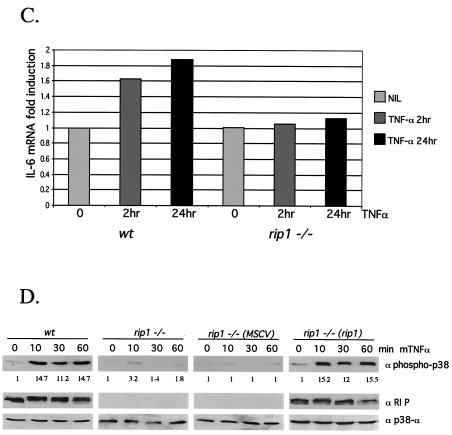
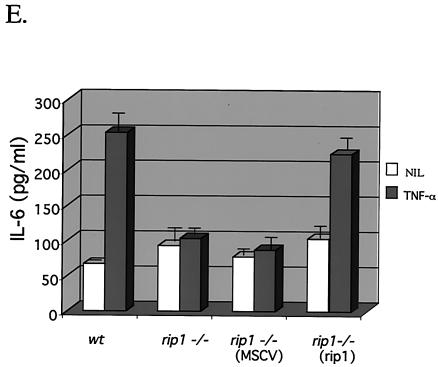
Decreased TNF-α-induced IL-6 production in rip1−/− cells. (A) Wild-type and rip1−/− MEF were plated at 3 × 104 cells per well on 24-well plates and left untreated or treated with 10 ng of TNF-α/ml for 24 h. The supernatants were then analyzed for IL-6 levels with the OptEIA mouse IL-6 ELISA kit (PharMingen catalog no. 2653KI). (B) TNF-α-induced IL-6 production in traf2−/− cells. Wild-type and traf2−/− cells were plated on 24-well plates at 3 × 104 cells per well and left untreated or treated with 10 ng of TNF-α/ml for 24 h. The supernatants were then analyzed for IL-6 levels with the OptEIA mouse IL-6 ELISA kit. The amount of IL-6 is presented as the mean ± standard deviation of triplicate observations. Similar data were obtained in six independent experiments with six independent rip−/− MEF lines and five traf2−/− MEF lines. (C) Decreased TNF-α-induced IL-6 mRNA in rip1−/− MEF. Wild-type and rip1−/− MEF were left untreated or treated with TNF-α (10 ng/ml) for 2 and 24 h. The amount of IL-6 mRNA and GAPDH mRNA was measured by RNase protection assay. The protected RNA was detected by autoradiography after denaturing polyacrylamide gel electrophoresis and was quantitated by PhosphorImager analysis. For clarity, exposure time varied for autoradiography for IL-6 and GAPDH mRNAs. Similar data were obtained in three separate experiments. (D) TNF-α-induced p38 MAPK activation in rip1−/− MEF infected with RIP1 retrovirus. rip1−/− MEF were infected with vector alone (MSCV) or with a RIP1 retrovirus [rip1−/− (rip1)]. Wild-type, rip1−/−, rip1−/− (MSCV), and rip1−/− (rip1) cells were left untreated or stimulated with TNF-α for the time periods indicated, and p38 MAPK activity was measured by immunoblotting with an anti-phospho-p38 Ab. The RIP1 and p38α expression was determined by immunoblotting with anti-RIP and anti-p38α Abs. The percentage of green fluorescent protein-positive cells in the infected populations was determined by flow cytometry. Results from one of three independent infection experiments are shown. (E) TNF-α-induced IL-6 production is increased in rip1−/− MEF infected with a RIP1 retrovirus. Wild-type, rip1−/−, and rip1−/− MEF infected with vector (MSCV) or with a RIP1 retrovirus [rip1−/− (rip1)] were plated at 3 × 104 cells per well on 24-well plates and left untreated or treated with 10 ng of TNF-α/ml for 24 h. The supernatants were then analyzed for IL-6 levels with the OptEIA mouse IL-6 ELISA kit. The amount of IL-6 is presented as the mean ± standard deviation of triplicate observations.
In contrast, TNF-α-induced IL-6 production was observed in traf2-deficient MEF. Surprisingly, high basal levels of IL-6 were detected in the traf2−/− cells, which may reflect the fact that traf2 deficiency results in increased levels of TNF-α mRNA and circulating TNF-α (30). Yet, consistent with the p38 MAPK activity, treatment of traf2−/− cells with TNF-α stimulated IL-6 production above basal levels to approximately 1,200 pg of culture supernatant/ml (Fig. 3B). Therefore, TNF-α-induced IL-6 production does not appear to require the adapter protein TRAF2.
TNF-α-induced IL-6 expression may be regulated at several levels including increased IL-6 mRNA expression and increased IL-6 mRNA translation (4, 25, 26, 39, 54, 62). The defect in TNF-α-induced IL-6 production in the rip1−/− MEF may result from alterations in either or both of these processes. To examine the mechanism whereby IL-6 production is impaired in rip1−/− MEF, we examined the ability of TNF-α to induce IL-6 mRNA expression by an RNase protection assay. Control experiments demonstrated that equal amounts of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA were detected in wild-type and rip1−/− MEF and that TNF-α treatment did not affect GAPDH mRNA levels (data not shown). Treatment of wild-type MEF with TNF-α increased the amount of IL-6 mRNA, whereas rip1−/− MEF were defective in the TNF-α-stimulated accumulation of IL-6 mRNA (Fig. 3C). Together, these data indicate that impaired cytokine production in rip1−/− MEF may reflect in part the failure of TNF-α to induce expression of IL-6 mRNA. However, in wild-type MEF, TNF-α stimulates a twofold induction of IL-6 mRNA, yet five- to eightfold increases in IL-6 protein are observed in the supernatants of TNF-α-treated MEF. Thus, it seems likely that TNF-α-induced synthesis of IL-6 involves both a transcriptional and a posttranscriptional mechanism(s).
The defect(s) in TNF-α-induced IL-6 production observed in rip1−/− MEF may reflect differences in MEF populations or the presence of additional mutations that affect the p38 MAPK pathway. To rule out this possibility, we infected rip1−/− MEF with MSCV2.2-IRES-GFP retrovirus that expresses RIP1 or with MSCV2.2-IRES-GFP retrovirus vector alone. Wild-type, rip1−/−, and rip1−/− MEF infected with vector alone or with RIP1 retrovirus were left untreated or treated with murine TNF-α for 10, 30, and 60 min. To detect TNF-α-induced p38 MAPK activity, cell lysates were probed with a phosphospecific p38 MAPK Ab. TNF-induced p38 MAPK activity was observed in treated wild-type MEF and in rip1−/− MEF infected with the RIP1 retrovirus [rip1−/− (rip1), Fig. 3D]. Moreover, TNF-α-induced IL-6 production was also restored in the infected rip1−/− (rip1) MEF (Fig. 3E). Taken together, these experiments suggest that the defect in TNF-α-induced p38 MAPK activation and IL-6 production observed in rip1−/− MEF is due to an absence of RIP1.
RIP1 interacts with p38 MAP3K MEKK3.
The death domain kinase RIP1 associates with a MAP3K activity in vivo, which activates MKK6 and p38 MAPK in an in vitro kinase assay (60). The p38 MAPKs can be regulated by a variety of MAP3Ks including ASK1, MEKK2, MEKK3, and Tpl2, whereas MEKK1 preferentially activates the SAPK/JNK pathway (7, 18, 37, 60). ASK1 is activated in response to TNF-α, and expression of a kinase-inactive ASK1 inhibits TNF-α-induced cell death (18). The MAP3K MEKK3 activates the p38 MAPK, SAPK/JNK, and NF-κB pathways by an as yet undefined mechanism(s) (5, 7, 11, 63).
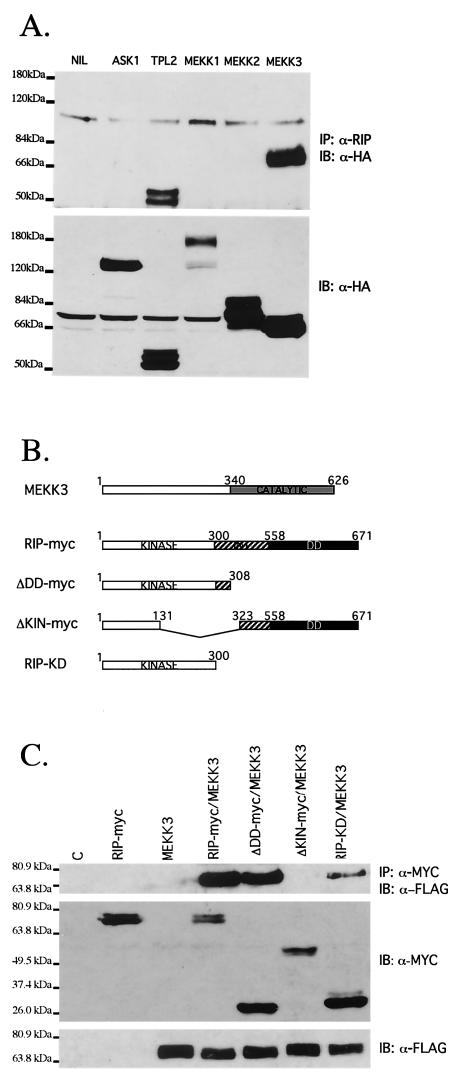
To investigate whether RIP1 contributes to TNF-α-induced p38 MAPK activation by recruiting a MAP3K, we transfected 293T cells with HA-tagged ASK1, MEKK3, MEKK2, MEKK1, or Tpl2. Although equivalent expression levels were achieved, we were unable to demonstrate interaction between RIP1 and MEKK1, MEKK2, or ASK1, suggesting that TNF-α-induced p38 MAPK activation involves the recruitment of another TNF-α-responsive p38 MAPK. We did, however, observe interaction between endogenous RIP1 and transfected MEKK3 and Tpl2 (Fig. 4A). Both the RIP1-MEKK3 and RIP1-Tpl2 interactions were stable throughout washes in lysis buffer containing 1 M NaCl, suggesting that both interactions may be specific. Although clearly implicated in LPS-induced extracellular signal-regulated kinase (ERK) activation, TNF-α-induced p38 MAPK activation is not affected in tpl2-deficient cells (10; P. Tsichlis, personal communication), suggesting to us that RIP1 may mediate p38 MAPK activation by recruiting MEKK3.
FIG. 4.
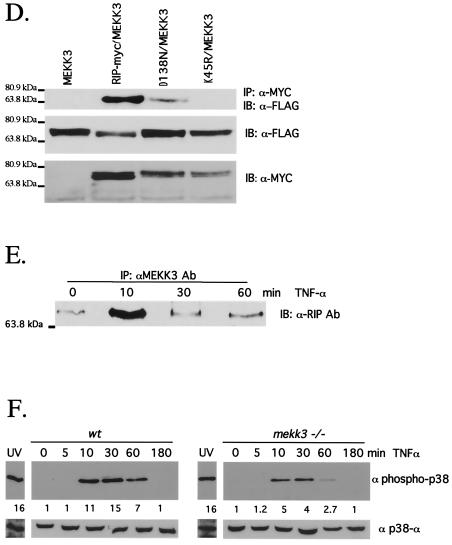
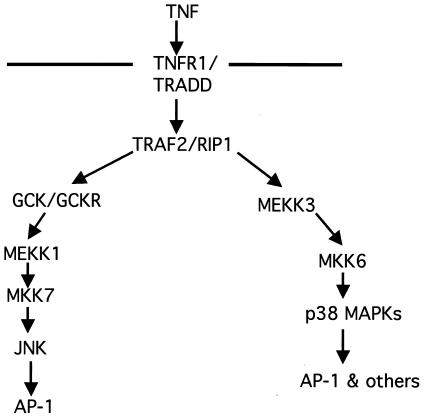
RIP1 associates with the MAP3K MEKK3 in TNF-α-stimulated cells. (A) RIP1 interacts with MEKK3 and Tpl2 kinases. 293T cells were left untransfected (NIL) or transfected with HA-ASK1, HA-Tpl2, HA-MEKK1, HA-MEKK2, or HA-MEKK3. The cells were lysed and immunoprecipitated with anti-RIP Ab and immunoblotted with anti-HA Ab. Expression of transfected constructs was confirmed by immunoblotting with anti-HA. (B) Schematic representation of MEKK3, RIP1, and the RIP1 deletion constructs used to map interactions. (C) The kinase domain of RIP1 binds MEKK3. 293T cells were transfected with FLAG-tagged MEKK3 and various MYC-tagged RIP1 deletion constructs. The transfected cells were immunoprecipitated with anti-MYC Ab, and the associated MEKK3 proteins were detected by immunoblotting with anti-FLAG Ab. Expression of various RIP1-MYC deletion constructs was determined by immunoblotting with anti-MYC Ab. (D) Kinase-inactive versions of RIP1 fail to interact with MEKK3. 293T cells were transfected with a FLAG-tagged MEKK3 and either wild-type, MYC-tagged RIP1, or MYC-tagged kinase-inactive versions of RIP1 (RIP1D138N and RIP1K45R). Cells were lysed and immunoprecipitated with anti-MYC Ab, and MEKK3 protein was detected by immunoblotting with an anti-FLAG Ab. Expression levels of various constructs were determined by immunoblotting with anti-MYC and anti-FLAG Abs. (E) Endogenous RIP1-MEKK3 interaction is enhanced in TNF-α-stimulated cells. 293T cells were left untreated or treated with 100 ng of human TNF-α/ml for the time periods indicated and then immunoprecipitated with anti-MEKK3 Ab. RIP1 proteins were detected by immunoblotting with anti-RIP Ab (PharMingen). (F) TNF-α-induced p38 MAPK activation is impaired in Mekk3−/− MEF. Two wild-type and three Mekk3−/− MEF lines were left untreated or stimulated with TNF-α for the time periods indicated, and p38 MAPK activity was measured by immunoblotting with an anti-phospho-p38 Ab. The RIP1 and p38α expression was determined by immunoblotting with anti-RIP and anti-p38α Abs.
To further examine how RIP1 interacts with MEKK3, we expressed a FLAG-tagged MEKK3 and various MYC-tagged RIP1 constructs in 293T cells (Fig. 4B). Transfected cells were immunoprecipitated with an anti-MYC Ab, and MEKK3 was detected by immunoblotting with an anti-FLAG Ab. We observed interaction between MEKK3 and full-length RIP1 and expression constructs which expressed primarily the RIP1 kinase domain and part of the intermediate domain (Fig. 4C). In contrast, deletion of part of the kinase and intermediate regions (Δ132-322 amino acids) eliminated association with MEKK3. Expression of the intermediate domain without the kinase domain was also capable of resulting in binding to MEKK3. Taken together, these studies suggest that MEKK3 interacts with both the kinase and intermediate regions of RIP1.
To determine whether the kinase activity of RIP1 was required for interaction with MEKK3, we transfected cells with MEKK3 and kinase-inactive versions of RIP1 (RIP1D138N and RIP1K45R). The RIP1-MEKK3 interaction was observed when kinase-active versions of RIP1 were expressed (Fig. 4D). Kinase-inactive versions of RIP1 (RIP1K45R and RIP1D138N) failed to form stable associations with MEKK3, although similar levels of expression were achieved. These studies suggest that the kinase activity of RIP1 may be important for the stable interaction and potential activation of MEKK3.
Thus, RIP1 may mediate TNF-α-induced p38 MAPK activation by recruiting MEKK3 to the activated TNFR1. To determine whether the endogenous RIP1 and MEKK3 proteins interact and whether this interaction is enhanced in TNF-α-treated cells, we stimulated 293T cells with TNF-α for the time periods indicated. MEKK3 was then immunoprecipitated with an anti-MEKK3 Ab, and associated RIP1 proteins were detected by immunoblotting. Interaction between the endogenous RIP1 and MEKK3 proteins was stimulated in cells treated with TNF-α for 10 min (Fig. 4E). Consistent with the kinetics of TNF-α-induced p38 MAPK activation observed in wild-type MEF (Fig. 1A), the RIP1-MEKK3 interaction is enhanced at 10 min but returns to uninduced levels by 30 min. Thus, TNF-α-induced p38 MAPK activation may involve the RIP1-mediated recruitment of the MAP3K MEKK3.
To provide genetic evidence for a role for MEKK3 in the TNF-α-induced p38 MAPK pathway, we examined p38 MAPK activity in TNF-α-treated Mekk3−/− MEF. We observed a two- to fourfold decrease in the p38 MAPK response to TNF-α in Mekk3−/− MEF compared to wild-type cells, suggesting that MEKK3 participates in the p38 MAPK response to TNF-α (Fig. 4F). In contrast, no difference in p38 MAPK activity was observed between control cells and Mekk3−/− MEF exposed to 20 J of UV irradiation/m2. Although TNF-α-induced p38 MAPK activity was significantly decreased in the Mekk3−/− cells at all time points examined, it was not completely abolished. This study suggests that redundant factors exist that also mediate the TNF-α-induced p38 MAPK pathway. Impaired p38 MAPK responses to the cytokine TNF-α were observed in Mekk3−/− and rip1−/− MEF, thereby providing genetic evidence of one mechanism whereby TNF-α stimulates p38 MAPK activity.
DISCUSSION
Previous studies of rip1-deficient and traf2-deficient cells have revealed defects in the TNF-α-induced NF-κB and SAPK/JNK activation, respectively (9, 21, 46, 58). In this study, we demonstrate further bifurcation of the TNF signal transduction pathway at the level of RIP1 and TRAF2. In cells deficient in RIP1 we observe an impaired TNF-α-induced p38 MAPK response and an inability to produce the cytokine IL-6 in response to TNF-α. In contrast, in traf2-deficient cells, TNF-α-induced p38 MAPK activation is observed and the cytokine IL-6 is produced in response to TNF-α. Although both RIP1 and TRAF2 proteins are recruited to the activated TNFR1 and are capable of activating p38 MAPK in transfected cells (60), genetic analysis reveals distinct signaling functions for RIP1 and TRAF2. RIP1 plays a central role in TNF-induced NF-κB activation (9, 21, 46), and herein we demonstrate an additional function for the death domain kinase RIP1 in TNF-α-induced p38 MAPK activation. In contrast, TRAF2 principally regulates TNF-α-induced SAPK/JNK activation (28, 58) and does not appear to play a major role in the p38 MAPK response to TNF-α (this study).
The p38 MAPK and SAPK/JNK kinases are both activated by environmental stresses such as UV radiation, osmotic shock, and heat shock and by treatment with proinflammatory cytokines TNF-α and IL-1α (51). Moreover, expression of several MAP3Ks stimulates both p38 MAPK and SAPK/JNK activity, suggesting that these stress-induced kinase pathways may be coregulated (18, 43, 45, 48, 55). Yet, our analysis of the p38 MAPK response to TNF-α in rip1-deficient or traf2-deficient cells reveals that the p38 MAPK and SAPK/JNK pathways may be independently regulated. In rip−/− MEF, TNF-α-induced SAPK/JNK activation is observed (20), whereas the p38 MAPK response is impaired (this study). In contrast, JNK activation is impaired in traf2-deficient MEF (58), and the p38 MAPK response to TNF-α appears unaffected (this study).
The precise mechanism by which RIP1 mediates p38 MAPK activation is unclear. In transfection studies, the kinase activity of RIP1 does not appear to be required for TNF-α-induced NF-κB activation or TNF-α-induced p38 MAPK activation (46, 60). Consistent with this idea, RIP1 associates with an endogenous MAP3K activity in vivo that is capable of activating MKK6 and p38 in vitro (60). Yet, the identity of the RIP1-associated MAP3K is not known. Our studies suggest that the kinase and intermediate domains of RIP1 interact with the MAP3K MEKK3. We demonstrate that the association of endogenous RIP1 and MEKK3 is enhanced in TNF-α-treated cells and that the kinetics of association correlate with TNF-α-induced p38 MAPK activity.
Thus, the kinase activity of RIP1 may contribute to TNF-α-induced p38 MAPK activation by directly phosphorylating-activating MEKK3. Consistent with this idea, TNF-α-induced p38 MAPK activation is not restored when rip1−/− MEF are infected with a kinase-inactive version of RIP1 (RIP1D138N) (unpublished data). Alternatively, the kinase activity of RIP1 may be important in the recruitment of MEKK3 but may not be required for its activation. Consistent with this idea, kinase-inactive versions of RIP1 do not form stable associations with MEKK3 in transfected cells (Fig. 4D).
Support for the role of MEKK3 in the p38 MAPK pathway comes from transfection studies and genetic analyses of these kinases in mice (5, 7, 57). Mice deficient in mekk3 die at E11.5, whereas targeted disruption of p38α results in embryonic lethality beginning at E10.5 and persisting for variable periods depending on the genetic background (1, 2, 29, 44, 56). Both the mekk3- and p38α-deficient mice exhibit angiogenic defects in both the placenta and embryo, consistent with a regulatory role for MEKK3 upstream of p38 MAPK (1, 29, 56). Moreover, we find TNF-α-induced p38 MAPK activation impaired in Mekk3−/− MEF (Fig. 4F). Taking the evidence together, we provide genetic evidence for roles for RIP1 and MEKK3 in the TNF-induced p38 MAPK pathway. A previous study found MEKK3 to be required for TNF-α-induced NF-κB activation (56). Thus, RIP1 may mediate both TNF-α-induced p38 MAPK and IKK-β activation by recruiting-activating the MAP3K MEKK3.
Accumulating biochemical and genetic data suggest that both the NF-κB and the p38 MAPK pathways are required for efficient induction of IL-6 by the proinflammatory cytokines TNF and IL-1 (4, 47, 60). Because RIP1 has been previously implicated in the regulation of the IKK (9, 21, 45) and herein the p38 MAPK pathway, it is difficult to discern the relative contribution of the NF-κB and p38 MAPK pathways to the decreased IL-6 production in rip1−/− MEF. Both NF-κB and p38 MAPK have been implicated in the transcriptional regulation of the IL-6 gene, and studies with a p38 MAPK inhibitor, SB203580, suggest that p38 MAPK may be required for transcriptional induction of IL-6 by NF-κB (4, 26, 38, 53, 61). Thus, the failure to produce IL-6 in response to TNF in rip1−/− MEF most likely reflects defects in both the NF-κB and p38 MAPK pathways.
The p38 MAPK regulates the activity of several transcription factors; defects in the activity of one or more of these transcription factors in rip1−/− cells may contribute to the reduced TNF-α-stimulated expression of IL-6. Further studies are required to identify the specific defect(s) in rip1−/− MEF.
The cytokine TNF-α and the p38 MAPK pathway are important mediators of the inflammatory process. The anti-inflammatory effects of the pyridinylimidazole derivatives (SB203580) are the result of inhibition of the expression of IL-1α, TNF-α, and IL-6 (23). We show that the death domain kinase RIP1 is required in MEF for the specific activation of the p38 MAPK pathway in response to TNF-α. Targeted disruption of the rip1 gene prevents IL-6 production in response to TNF-α, whereas traf2 deficiency has no apparent effect on TNF-α-induced p38 MAPK activation or IL-6 production. These data provide further genetic evidence that RIP1 and TRAF2 have discrete functions in TNF-α signaling. In addition, we provide genetic evidence that MEKK3 participates in the TNF-α-induced p38 MAPK pathway. Taken together, these studies suggest that the death domain kinase RIP1 may mediate TNF-α-induced p38 MAPK activation by recruiting the MAP3K MEKK3 (Fig. 5). Finally RIP1 may represent a novel target for anti-inflammatory drugs that would be highly specific for inhibiting TNF-α-induced cytokine production.
FIG. 5.
Proposed model for JNK and p38 MAPK activation in response to the cytokine TNF-α. TNF-α-induced JNK activation is mediated by TRAF2-dependent recruitment of GCK-GCKR and subsequent activation of the MAP3K MEKK1 (6), whereas TNF-α-induced p38 MAPK activation may be mediated by the RIP1-dependent recruitment of the MAP3K MEKK3 and subsequent activation of MKK3-MKK6. The precise mechanism(s) of RIP1-mediated activation of MEKK3 remains unclear.
Acknowledgments
This work was supported by an American Cancer Society Research Project grant and National Institutes of Health grant GM61298 to M.A.K. M.A.K. is also the recipient of a Sidney Kimmel Cancer Scholar Award.
REFERENCES
- 1.Adams, R. H., A. Porras, G. Alonso, M. Jones, K. Vintersten, S. Panelli, A. Valladares, L. Perez, R. Klein, and A. Nebreda. 2000. Essential role of p38 alpha MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell 6:109-116. [PubMed] [Google Scholar]
- 2.Allen, M., L. Svensson, M. Roach, J. Hambor, J. McNeish, and C. A. Gabel. 2000. Deficiency of the stress kinase p38α results in embryonic lethality: characterization of kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J. Exp. Med. 191:859-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagrodia, S., B. Derijard, R. J. Davis, and R. A. Cerione. 1995. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 270:27995-27998. [DOI] [PubMed] [Google Scholar]
- 4.Beyaert, R., A. Cuenda, W. Vanden Berghe, S. Plaisance, J. C. Lee, G. Haegmann, P. Cohen, and W. Fiers. 1996. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. 15:1914-1923. [PMC free article] [PubMed] [Google Scholar]
- 5.Blank, J. L., et al. 1996. Molecular cloning of mitogen-activated protein/ERK kinase kinases (MEKK) 2 and 3. Regulation of sequential phosphorylation pathways involving mitogen-activated protein kinase and c-Jun kinase. J. Biol. Chem. 271:5361-5368. [DOI] [PubMed] [Google Scholar]
- 6.Chadee, D., T. Yuasa, and J. M. Kyriakis. 2002. Direct activation of mitogen-activated protein kinase kinase kinase MEKK1 by the Ste20p homologue GCK and the adapter protein TRAF2. Mol. Cell. Biol. 22:737-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deacon, K., and J. L. Blanc. 1999. MEK kinase 3 directly activates MKK6 and MKK7, specific activators of the p38 and c-Jun NH2-terminal kinases. J. Biol. Chem. 274:16604-16610. [DOI] [PubMed] [Google Scholar]
- 8.Derijard, B., J. Raingeaud, T. Barrett, L.-H. Wu, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267:682-685. [DOI] [PubMed] [Google Scholar]
- 9.Devin, A., A. Cook, Y. Lin, Y. Rodriguez, M. Kelliher, and Z. Liu. 2000. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity 12:419-429. [DOI] [PubMed] [Google Scholar]
- 10.Dumitru, C. D., J. D. Ceci, C. Tsatsanis, D. Kontoyiannis, C. Patriotis, N. A. Jenkins, N. G. Copeland, G. Kollias, and P. Tsichlis. 2000. TNF-α induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103:1071-1083. [DOI] [PubMed] [Google Scholar]
- 11.Ellinger-Zieglebauer, H., et al. 1997. Direct activation of the stress-activated protein kinase (SAPK) and extracellular signal-regulated protein kinase ERK pathways by an inducible mitogen-activated protein kinase/ERK kinase kinase 3 (MEKK) derivative. J. Biol. Chem. 272:2668-2674. [DOI] [PubMed] [Google Scholar]
- 12.Feldmann, M., and R. N. Maini. 2001. Anti-TNF-α therapy of RA: what have we learned? Annu. Rev. Immunol. 19:163-196. [DOI] [PubMed] [Google Scholar]
- 13.Garrington, T. P., and G. L. Johnson. 1999. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 11:211-218. [DOI] [PubMed] [Google Scholar]
- 14.Han, J., Y. Jiang, Z. Li, V. V. Kravenchko, and R. J. Ulevitch. 1997. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature 386:296-299. [DOI] [PubMed] [Google Scholar]
- 15.Hsu, H., J. Xiong, and D. V. Goeddel. 1995. The TNF receptor-1-associated protein TRADD signals cell death and NF-κB activation. Cell 81:495-504. [DOI] [PubMed] [Google Scholar]
- 16.Hsu, H., H. B. Shu, M. G. Pan, and D. V. Goeddel. 1996. TRAD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84:299-308. [DOI] [PubMed] [Google Scholar]
- 17.Hsu, H., J. Huang, H. B. Shu, V. Baichwal, and D. V. Goeddel. 1996. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor 1 signalling complex. Immunity 4:387-396. [DOI] [PubMed] [Google Scholar]
- 18.Ichijo, H., E. Nishida, K. Irie, P. ten Dijke, M. Saitoh, T. Moriguchi, M. Takagi, K. Matsumoto, K. Miyazono, and Y. Gotoh. 1997. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates the SAPK/JNK and p38 signaling pathways. Science 275:90-94. [DOI] [PubMed] [Google Scholar]
- 19.Jiang, Y., H. Gram, M. Zhao, L. New, J. Gu, L. Feng, F. DiPadova, R. J. Ulevitch, and J. Han. 1997. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38δ. J. Biol. Chem. 272:30122-30128. [DOI] [PubMed] [Google Scholar]
- 20.Jiang, Y., J. D. Woronicz, W. Liu, and D. V. Goeddel. 1999. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science 283:543-546. [DOI] [PubMed] [Google Scholar]
- 21.Kelliher, M., S. Grimm, Y. Ishida, F. Kuo, B. Z. Stanger, and P. Leder. 1998. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity 8:297-303. [DOI] [PubMed] [Google Scholar]
- 22.Kishimoto, K., K. Matsumoto, and J. Ninomiya-Tsuji. 2000. TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J. Biol. Chem. 275:7359-7364. [DOI] [PubMed] [Google Scholar]
- 23.Kotyarov, A., A. Neininger, C. Schubert, R. Eckert, C. Birchmeler, H. D. Volk, and M. Gaestel. 1999. MAPKAP kinase 2 is essential for LPS-induced TNF-α biosynthesis. Nat. Cell Biol. 1:94-99. [DOI] [PubMed] [Google Scholar]
- 24.Kruys, V., B. Beutler, and G. Huez. 1990. Translational control mediated by UA-rich sequences. Enzyme 44:193-202. [DOI] [PubMed] [Google Scholar]
- 25.Lee, J. C., J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, T. F. Gallagher, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, et al. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 327:739-746. [DOI] [PubMed] [Google Scholar]
- 26.Libermann, T. A., and D. Baltimore. 1990. Activation of interleukin-6 gene expression through the NF-κB transcription factor. Mol. Cell. Biol. 10:2327-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin, Q., J. Schwarz, C. Bucana, and E. N. Olsen. 1997. Control of cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 276:1404-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, Z., H. Hsu, D. V. Goeddel, and M. Karin. 1996. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 87:565-576. [DOI] [PubMed] [Google Scholar]
- 29.Mudgett, J. S., J. Ding, L. Guh-Siesel, N. Chartrain, L. Yang, S. Gopal, and M. M. Shen. 2000. Essential role for p38-α mitogen-activated protein kinase in placental angiogenesis. Proc. Natl. Acad. Sci. USA 97:10454-10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen, T., G. S. Duncan, C. Mirtsos, M. Ng, D. E. Speiser, A. Shahinian, M. W. Marino, T. W. Mak, P. Ohashi, and W. C. Yeh. 1999. Traf 2 deficiency results in hyperactivity of certain TNFR1 signals and impairment of CD40-mediated responses. Immunity 11:379-389. [DOI] [PubMed] [Google Scholar]
- 31.Nihalani, D., S. Merritt, and L. B. Holzman. 2000. Identification of structural and functional domains in mixed lineage kinase dual leucine zipper-bearing kinase required for complex formation and stress-activated protein kinase activation. J. Biol. Chem. 275:7273-7279. [DOI] [PubMed] [Google Scholar]
- 32.Price, M. A., F. Cruzalegui, and R. Treisman. 1996. The p38 and ERK MAP kinase pathways cooperate to activate ternary complex factors and c-fos transcription in response to UV light. EMBO J. 15:6552-6563. [PMC free article] [PubMed] [Google Scholar]
- 33.Prichett, W., A. Hand, J. Sheilds, and D. Dunnington. 1995. Mechanism of action of bicyclic imidazoles defines a translational regulatory pathway for tumor necrosis factor α. J. Inflamm. 45:97-105. [PubMed] [Google Scholar]
- 34.Raingeaud, J., S. Gupta, J. Rogers, M. Dickens, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause the p38 mitogen-activated kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270:7420-7426. [DOI] [PubMed] [Google Scholar]
- 35.Raingeaud, J., A. J. Whitmarsh, T. Barrett, B. Derijard, and R. J. Davis. 1996. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16:1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rouse, J. P. Cohen, S. Trigon, M. Morange, A. Alonso-Llamazares, D. Zamanillo, T. Hunt, and A. R. Nebreda. 1994. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78:1027-1037. [DOI] [PubMed] [Google Scholar]
- 37.Salmeron, A., T. B. Ahmad, G. W. Carlile, D. Pappin, R. P. Narsimhan, and S. C. Ley. 1996. Activation of MEK-1 and SEK-1 by Tpl-2 proto-oncoprotein, a novel MAP kinase kinase kinase. EMBO J. 15:817-826. [PMC free article] [PubMed] [Google Scholar]
- 38.Shi, C.-S., A. Leonardi, J. Kyriakis, U. Siebenlist, and J. H. Kehrl. 1999. TNF-mediated activation of the stress-activated protein kinase pathway: TNF receptor-associated factor 2 recruits and activates germinal center kinase related. J. Immunol. 163:3279-3285. [PubMed] [Google Scholar]
- 39.Shimizu, H., K. Mitomo, T. Watanabe, S. Okamoto, and K. Yamamoto. 1990. Involvement of a NF-κB-like transcription factor in the activation of the interleukin-6 gene by inflammatory lymphokines. Mol. Cell. Biol. 10:561-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith, C. A., T. Farrah, and R. G. Goodwin. 1994. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation and death. Cell 76:959-962. [DOI] [PubMed] [Google Scholar]
- 41.Stanger, B. Z., P. Leder, T.-H. Lee, E. Kim, and B. Seed. 1995. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 in yeast and causes cell death. Cell 81:513-523. [DOI] [PubMed] [Google Scholar]
- 42.Stein, B., H. Brady, M. X. Yang, D. B. Young, and M. S. Barbosa. 1997. P38-2, a novel mitogen-activated protein kinase with distinct properties. J. Biol. Chem. 272:19509-19517. [DOI] [PubMed] [Google Scholar]
- 43.Takekawa, M., F. Posas, and H. Saito. 1997. A human homologue of the yeast ssk2/ssk22 MAP kinase kinase kinases, MTK1, mediates stress-induced activation of the p38 and JNK pathways. EMBO J. 16:4973-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamura, K., T. Sudo, U. Senftleben, A. M. Dadak, R. Johnson, and M. Karin. 2000. Requirement for p38α in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell 102:221-231. [DOI] [PubMed] [Google Scholar]
- 45.Tibbles, L. A., Y. L. Ing, F. Kiefer, J. Chan, N. Iscove, J. R. Woodgett, and N. J. Lassam. MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J. 15:7026-7035. [PMC free article] [PubMed]
- 46.Ting, A. T., F. X. Pimentel-Muinos, and B. Seed. 1996. RIP mediates tumor necrosis factor receptor 1 activation of NF-κB but not Fas/APO-1-initiated apoptosis. EMBO J. 15:6189-6196. [PMC free article] [PubMed] [Google Scholar]
- 47.Tong, L., S. Pav, D. M. White, S. Rogers, K. M. Crane, C. L. Cywin, M. L. Brown, and C. A. Pargellis. 1997. A highly specific inhibitor of human p38 MAP kinase binds in the ATP pocket. Nat. Struct. Biol. 4:311-316. [DOI] [PubMed] [Google Scholar]
- 48.Vanden Berghe, W., S. Plaisance, E. Boone, K. De Bosscher, M. L. Schmitz, W. Fiers, and G. Haegemann. 1998. p38 and extracellular signal-related kinase mitogen-activated protein kinase pathways are required for nuclear factor-κB p65 transactivation. J. Biol. Chem. 273:3285-3290. [DOI] [PubMed] [Google Scholar]
- 49.Wang, W., G. Zhou, M. C. T. Hu, Z. Yao, and T. H. Han. 1997. Activation of the hematopoietic progenitor kinase-1 (HPK1)-dependent, stress-activated c-Jun N-terminal kinase (JNK) pathway by transforming growth factor beta (TGF-β)-activated kinase (TAK1), a kinase mediator of TGF-β signal transduction. J. Biol. Chem. 272:22771-22775. [DOI] [PubMed] [Google Scholar]
- 50.Wang, X., and D. Ron. 1996. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science 272:1347-1349. [DOI] [PubMed] [Google Scholar]
- 51.Whitmarsh, A., and R. J. Davis. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction. J. Mol. Med. 74:589-607. [DOI] [PubMed] [Google Scholar]
- 52.Whitmarsh, A. J., S.-H. Yang, M. S.-S. Su, A. D. Sharrocks, and R. J. Davis. 1997. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol. Cell. Biol. 17:2360-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson, K., P. G. McCaffrey, K. Hsiao, S. Pazhanisamy, V. Galullo, G. W. Blemis, M. J. Fitzgibbons, P. R. Caron, M. A. Murco, and M. S. Su. 1997. The structural basis for the specificity of pyridinylimidazole inhibitors of p38 MAP kinase. Pyridinylimidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. Chem. Biol. 4:423-431. [DOI] [PubMed] [Google Scholar]
- 54.Wysk, M., D. D. Yang, H. T. Lu, R. Flavell, and R. J. Davis. 1999. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc. Natl. Acad. Sci. USA 96:3763-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu, S., D. J. Robbins, L. B. Christerson, J. M. English, C. Vanderbilt, and M. H. Cobb. 1996. Cloning of rat MEK kinase 1 cDNA reveals an endogenous membrane-associated 195-kDa protein with a large regulatory domain. Proc. Natl. Acad. Sci. USA 93:5291-5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang, J., M. Boerm, M. McCarty, C. Bucana, I. Fidler, Y. Zhuang, and B. Su. 2000. Mekk3 is essential for early embryonic cardiovascular development. Nat. Genet. 24:309-313. [DOI] [PubMed] [Google Scholar]
- 57.Yang, J., Y. Lin, Z. Guo, J. Cheng, J. Huang, L. Deng, W. Liao, Z. Chen, Z. Liu, and B. Su. 2001. The essential role of MEKK3 in TNF-induced NF-κB activation. Nat. Immunol. 2:620-624. [DOI] [PubMed] [Google Scholar]
- 58.Yeh, W. C., A. Shahinian, D. Speiser, J. Kraunus, F. Billia, A. Wakeham, J. L. de la Pompa, D. Ferrick, B. Hum, N. Iscove, et al. 1997. Early lethality, functional NF-κB activation and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 7:715-725. [DOI] [PubMed] [Google Scholar]
- 59.Young, P. R., M. M. McLaughlin, S. Kumar, S. Kassis, M. L. Doyle, D. McNulty, T. F. Gallagher, S. Fisher, P. C. McDonnell, S. A. Carr, et al. 1997. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J. Biol. Chem. 272:12116-12121. [DOI] [PubMed] [Google Scholar]
- 60.Yuasa, T., S. Ohno, J. H. Kerl, and J. M. Kyriakis. 1998. Tumor necrosis factor signaling to stress-activated protein kinase (SAPK)/Jun NH2-terminal kinase (JNK) and p38. J. Biol. Chem. 273:22681-22692. [DOI] [PubMed] [Google Scholar]
- 61.Zechner, D., R. Craig, D. S. Hanford, P. M. McDonough, R. A. Sabbadini, and C. C. Glembotski. 1998. MKK6 activates myocardial cell NF-κB and inhibits apoptosis in a p38 mitogen-activated protein kinase-dependent manner. J. Biol. Chem. 273:8232-8239. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, Y., J.-X. Lin, and J. Vilèek. 1990. Interleukin-6 induction by tumor necrosis factor and interleukin-1 in human fibroblasts involves activation of a nuclear factor binding to a κB-like sequence. Mol. Cell. Biol. 10:3818-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao, Q., and F. S. Lee. 1999. Mitogen-activated protein kinase/ERK kinase kinases 2 and 3 activate nuclear factor-κB through IκB kinase-α and IκB kinase-β. J. Biol. Chem. 274:8355-8358. [DOI] [PubMed] [Google Scholar]