Abstract
The nucleotide sequences of a segment of the Pneumocystis mitochondrial large-subunit (mt LSU) rRNA gene from rhesus macaques coinfected with simian immunodeficiency virus (SIV) and Pneumocystis carinii were examined. Of 12 isolates examined, 3 were found to be identical and the others showed substantial sequence variation, with up to 13% divergence among variants. We identified two general sequence types that differed at several sites, including a conserved 26-nucleotide insertion. Four monkeys had evidence of two Pneumocystis variants present simultaneously, indicative of a mixed infection. There was a high degree of variance between the rhesus macaque-derived Pneumocystis mt LSU rRNA gene sequence and the cognate sequences in Pneumocystis organisms derived from other hosts. Analysis of the mt LSU rRNA genes of Pneumocystis organisms derived from rhesus macaques and several other mammalian hosts supports the observation that rhesus macaque-derived Pneumocystis is most closely related to human-derived Pneumocystis. In addition, the data identify the mt LSU rRNA gene as an informative locus for transmission and epidemiological studies of the SIV-rhesus macaque model of Pneumocystis infection.
Pneumocystis carinii is a fungal organism that is a frequent opportunistic pulmonary pathogen in immunocompromised hosts with particular importance in human immunodeficiency virus (HIV)-infected individuals (16, 28). Despite improved treatment strategies for HIV infection, P. carinii pneumonia (PCP) remains a significant source of morbidity and mortality. The lack of a reliable in vitro culture system has hampered basic research on this organism; however, several immunocompromised animal models of PCP have been developed and have provided a source of organisms for molecular analysis (5, 22, 32). From these studies, it has become clear that Pneumocystis organisms derived from different mammalian hosts exhibit striking molecular and antigenic variation at a number of different genetic loci (14, 34, 36). In addition, it has been well documented that Pneumocystis derived from one mammalian host is not easily transmissible to other hosts, indicating a strong host-species restriction (13, 15; E. M. Aliouat, E. Mazars, E. Dei-Cas, P. Delcourt, P. Billaut, and D. Camus, abstr. Int. Workshop Protists, J. Eukaryot. Microbiol. 41:71S, 1994). As a consequence of host restriction and molecular analyses, a trinomial nomenclature for Pneumocystis has been used to describe variants derived from different host species (31). More recently, it has been proposed that the variants of Pneumocystis from different mammalian hosts are actually distinct species and that species names should be based upon the mammalian host from which the Pneumocystis organism is derived (35).
The principal PCP animal models currently in use are corticosteroid-treated rats and mice (9) and mice selectively depleted of specific immune cells (18). These studies have provided much insight regarding the central role of CD4+ T cells in resistance to infection by Pneumocystis. These models have been very important in advancing our understanding of the pathogenesis of PCP; however, there are limitations. For example, the use of corticosteroids compromises studies aimed at understanding immune effector mechanisms. Furthermore, the role of cytokines in control or pathology cannot be readily addressed in these models because of the broadly suppressive effects of drug treatment. The immune dysregulation associated with HIV infection involves alterations in B-cell and macrophage functions, as well as the primary defect of CD4 T-cell loss; the immunosuppressed rodent models of PCP cannot model these specific types of defects.
It has been established that infection of some Old World monkeys with simian immunodeficiency virus (SIV) produces a complex disease with many of the pathological aspects seen in AIDS (3, 6). SIV-induced immunodeficiency in macaques is a highly relevant model because macaques develop a constellation of opportunistic infections similar to that which occurs with AIDS. For these reasons, the SIV-infected macaque has become the principal animal model for the study of AIDS pathogenesis and vaccine development (20, 25). In addition, many human immunologic reagents are useful in these animals. Thus, biological and technical aspects of the model make it uniquely suitable for studies of AIDS.
There are several reasons to study PCP in the context of HIV infection, including differences between the progression and pathogenesis of PCP in HIV infection and in other immunosuppressed patients and the interaction of HIV with Pneumocystis in the lung. Studies have established that there are significant differences in the clinical features of AIDS-associated PCP and PCP in patients in other immunosuppressed states (21, 27, 29, 40). Thus, despite the similar losses of CD4+ T cells, the complexity of the immune dysfunction in both AIDS- and non-AIDS-associated PCP leads to very different pathogeneses of PCP in these two patient populations. There may also be important interactions of HIV and Pneumocystis in the lung that occur in HIV-infected individuals. These interactions may alter the resultant inflammatory responses and development of subsequent lung injury from those resulting from Pneumocystis infection alone.
To study the interaction of Pneumocystis and HIV in the pathogenesis of lung injury and to define immune parameters associated with the control of PCP in the context of HIV infection, our laboratory has developed a nonhuman-primate model of PCP in SIV-infected rhesus macaques (4). In these studies, conditions for reproducible infection were established and the progression of Pneumocystis infection was characterized. A key feature of this model is the protracted course of infection, with a long period of asymptomatic carriage of Pneumocystis. This course is in contrast to the rapidly progressing, fulminant infection produced in immunosuppressed rodents. Pneumocystis-infected, SIV-immunosuppressed macaques develop respiratory symptoms associated with frank PCP 20 to 40 weeks after Pneumocystis infection; however, evidence of Pneumocystis-induced inflammatory response in the lungs, characterized by neutrophil and CD8+-T-cell infiltration, is evident early after inoculation. This extended colonization period and the indolent course of the infection are very similar to those of HIV-associated PCP. Naturally acquired and experimental PCP in SIV-infected macaques shows histopathologic features similar to those seen in HIV-associated PCP (1, 2, 4, 7, 13).
In addition to modeling mechanisms of lung injury and immune responses, we have begun studies to evaluate patterns of transmission of Pneumocystis. Little is known about the transmission and epidemiology of Pneumocystis infection, particularly among HIV-infected individuals. We therefore sought to examine at a molecular level Pneumocystis derived from the simian model of AIDS in order to develop genetic reagents to monitor the progression of infection in the monkey model and to evaluate natural transmission patterns. In the present study, we examined lung samples from 11 SIV-infected macaques that were part of ongoing studies of SIV vaccine efficacy and analyzed the nucleic acid sequence of a segment of the mitochondrial large subunit (mt LSU) rRNA gene. Significant sequence variability was observed among the macaque-derived Pneumocystis sequences, as well as between macaque-derived Pneumocystis and Pneumocystis sequences derived from other mammalian hosts. The results show that the mt LSU rRNA segment that was analyzed is a useful locus for the evaluation of transmission of Pneumocystis in SIV-infected rhesus macaques, as well as for epidemiological studies of this model.
MATERIALS AND METHODS
Pneumocystis nomenclature.
A trinomial system of nomenclature of P. carinii based upon the host of origin is used in this paper (31). Organisms obtained from rhesus macaques are called P. carinii f. sp. macaca. The prototype P. carinii variant first described for rats is referred to as P. carinii f. sp. carinii, and the rat-derived variant is P. carinii f. sp. ratti. Other P. carinii variants described in this study are P. carinii f. sp. hominis (human), P. carinii f. sp. muris (murine), P. carinii f. sp. mustelae (ferret), and P. carinii f. sp. oryctolagi (rabbit).
Animals.
Eleven male rhesus macaques of Indian origin (Macaca mulatta) were utilized for this study. The SIV infection status of each animal is indicated in Table 1. All animals were individually housed in a biosafety level 2+ primate facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care at the University of Pittsburgh School of Medicine. All animal manipulations were approved by the Institutional Animal Care and Use Committee of the School of Medicine prior to initiation of the study. Animals were chronically infected with pathogenic SIV DeltaB670 (animals 1799, 5199, 8397, 9297, 11897, 7097, 0398, 12097, and 11797) or mac239 (animals 1899 and 2099). Samples from SIV-infected and P. carinii-negative animals as well as SIV-naïve animals were also utilized as negative controls in PCR.
TABLE 1.
Animals used in this study
| Animal | Age (yr) | Date of SIV challenge | Date of sacrifice | No. of days after SIV infection |
|---|---|---|---|---|
| 1799 | 5.8 | 10/20/99 | 4/4/00 | 164 |
| 5199 | 7.8 | 10/20/99 | 3/21/00 | 181 |
| 8397 | 5.2 | 9/29/98 | 8/24/99 | 323 |
| 2099 | 5.4 | 5/19/99 | 1/27/00 | 248 |
| 1899 | 3.8 | 5/19/99 | 8/4/00 | 435 |
| 9297 | 7.7 | 6/21/95 | 12/23/99 | 1,622 |
| 11897 | 8.3 | 8/26/98 | 9/15/99 | 379 |
| 12097 | 8.3 | 8/26/98 | 9/17/99 | 381 |
| 7097 | 8.3 | 2/24/98 | 8/3/99 | 519 |
| 0398 | 3.6 | 9/28/98 | 8/4/99 | 306 |
| 11797 | 11.5 | 8/26/98 | 4/12/99 | 226 |
BAL.
Bronchoalveolar lavage (BAL) was performed as described previously (7). Lavage fluid was centrifuged onto glass slides and stained with modified Giemsa reagents (Diff-Quik; Baxter Scientific Products, McGraw Park, Ill.) according to the manufacturer's protocol. Slides were examined microscopically for the presence of Pneumocystis. An aliquot from each BAL sample was utilized for Pneumocystis-specific PCR.
DNA preparation and PCR.
Approximately 3 ml of lavage fluid was microcentrifuged for 5 min, and the pellet was resuspended in 100 μl of PCR buffer (20 mM Tris-HCl [pH 8.4], 50 mM KCl, 3 mM MgCl2) and boiled for 15 min. Cellular debris was pelleted, and the DNA was quantified by UV spectroscopy at a wavelength of 260 nm. PCR was performed using primers PAZ 102-E (5′ GAT GGC TGT TTC CAA GCC CA 3′) and PAZ 102-H (5′ GTG TAC GTT GCA AAG TAC TC 3′), which amplify an approximately 350-bp segment of the Pneumocystis mt LSU rRNA gene (38). The PCR was performed in a 50-μl reaction mixture containing a 0.2 mM concentration of each deoxynucleoside triphosphate, 1 μM (each) primer, 1 μg of template DNA, and 2.5 U of Taq polymerase (GIBCO-BRL, Grand Island, N.Y.) in PCR buffer. Thirty-five cycles of amplification were performed with denaturation at 94°C for 90 s, annealing at 54°C for 90 s, and extension at 72°C for 2 min. DNA samples were also subjected to PCR with β-globin primers to assess the quality of the DNA preparation, as described previously (8). PCRs were performed with DNA samples of archival lung homogenates from SIV-infected rhesus macaques with no clinical or microscopic evidence of Pneumocystis infection, as well as lung samples from SIV-naïve monkeys. PCR mixtures containing no template DNA were used to evaluate cross contamination of tubes. PCR products were electrophoresed in 1.5% agarose gels and visualized by ethidium bromide staining.
DNA cloning and sequencing.
PCR products were gel purified using GeneClean (Bio 101, La Jolla, Calif.) and cloned directly into plasmid pCR2-TOPO (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions. Plasmids were prepared for sequences using a Wizard mini-prep kit (Promega, Madison, Wis.). Sequencing reactions were performed by the University of Pittsburgh Department of Molecular Genetics and Biochemistry Core Facility using a Dye Deoxy Terminator cycle sequencing kit (Applied Biosystems, Inc., Foster City, Calif.). Sequencing reaction mixtures contained 0.5 μg of template DNA and 3.4 pmol of primer. Both strands of the sequences of multiple clones of each PCR product were confirmed. Automated sequencing was carried out using a PRISM DNA sequencer (Applied Biosystems, Inc.). Analysis of the DNA sequence was performed with Gene Inspector version 1.5 software (Textco, Inc., Lebanon, N.H.), and sequence similarity was confirmed with LALIGN (http://www.ch.embnet.org/software/LALIGN_form.html).
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study were submitted to GenBank and assigned the following accession numbers: AF402683, AF402684, AF402685, AF402686, AF402687, AF402688, AF402689, AF402690, AF402691, AF402692, AF402693, and AF402694. Other sequences used for comparison were obtained from GenBank and had the following accession numbers: M58605 (P. carinii f. sp. hominis), S42915 (P. carinii f. sp. oryctolagi), S42921 (P. carinii f. sp. mustelae), U20169 (P. carinii f. sp. carinii), U20173 (P. carinii f. sp. ratti), L36903 (Saccharomyces cerevisiae), and AY011165 (M. mulatta).
RESULTS
BAL samples from 11 SIV-infected rhesus macaques were collected and analyzed. Four animals had severe PCP at the time of necropsy (animals 0398, 8397, 11797, and 2099), and the remaining animals had microscopic and/or histologic evidence of Pneumocystis infection (data not shown).
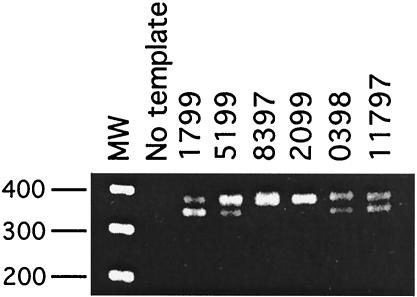
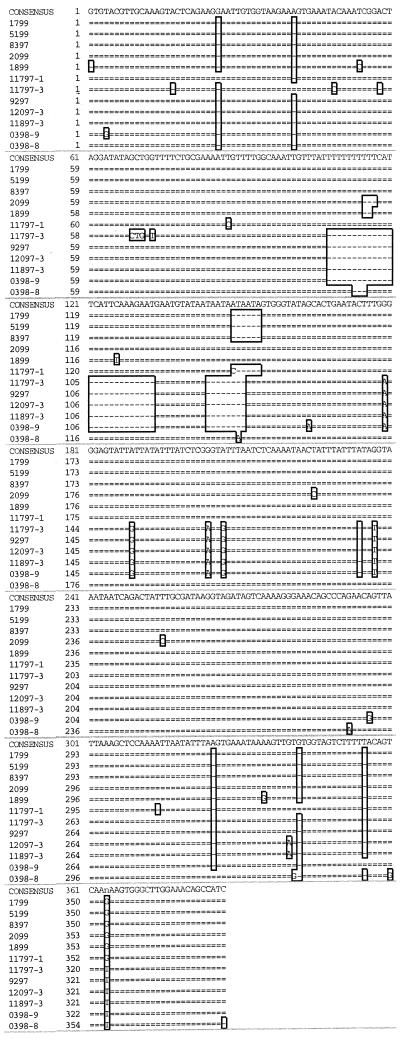
PCR of BAL samples using mt LSU primers PAZ 102-E and PAZ 102-H produced a product of approximately 350 bp in all monkey samples with microscopically confirmed Pneumocystis infection. PCR products from six samples are shown in Fig. 1. Size variations were observed on agarose electrophoresis of the PCR products, and in four cases (samples 1799, 5199, 0398, and 11797), two separate but closely migrating bands were observed (Fig. 1). PCR products were cloned in Escherichia coli, and plasmids containing the PCR products were sequenced in both directions. The DNA from two to three clones from each PCR product was sequenced. The PCR products from monkeys 0398 and 11797, which yielded a doublet on electrophoresis, were cloned and analyzed separately. Based on DNA sequence analysis, the clones ranged in size from 345 to 379 bp, which was consistent with the electrophoretic mobility of the PCR products (Fig. 1 and Table 2). Multiple sequence alignment of the macaque-derived Pneumocystis mt LSU sequences revealed a 26-bp insert starting at nucleotide 107 in the higher-molecular-weight amplicon that was not present in Pneumocystis organisms derived from other hosts (not shown). This insertion was found in isolates from 7 of the 11 animals studied (Fig. 2). The sequence and position of the insertion are similar to those reported for a single isolate from an SIV-infected macaque (12). In addition, an 8-bp nucleotide deletion (relative to sequences of Pneumocystis mt LSU rRNA genes derived from other species) was found at nucleotide position 143 in 5 of the 11 isolates (Fig. 2). The presence of these insertions and deletions in the cloned PCR products is consistent with the variations in the electrophoretic mobilities of the PCR products, as shown in Fig. 1. In addition, single-nucleotide differences were observed at 33 sites within the amplicon (Fig. 2). The percent divergence among the macaque-derived isolates was calculated, and the highest difference (13.1%) was found in samples from monkeys 11797 and 0398. This level of divergence has not been observed in Pneumocystis derived from other hosts, except in the case of the two different rat variants, P. carinii f. sp. carinii and P. carinii f. sp. ratti.
FIG. 1.
Agarose gel electrophoresis of PCR products from SIV-infected rhesus macaque BAL samples. DNA from BAL samples was amplified with P. carinii mt LSU rRNA primers, and products were resolved on 1.5% agarose gels. The expected product of approximately 350 nucleotides was visualized by ethidium bromide staining. Two separate bands were observed in samples from monkeys 1799, 5199, 0398, and 11797. Molecular weight standards (MW) in 100-nucleotide increments are at the left. A PCR with no template added was used as a negative control.
TABLE 2.
Size range of mt LSU rRNA PCR products from P. carinii- and SIV-coinfected macaques
| Animal | GenBank accession no. | Size of PCR product (bp) | Multiple PCR products in lung samplea |
|---|---|---|---|
| 1799 | AF402692 | 376 | + |
| 5199 | AF402691 | 376 | + |
| 8397 | AF402693 | 376 | − |
| 2099 | AF402689 | 379 | − |
| 1899 | AF402690 | 380 | − |
| 7097 | AF402685 | 347 | − |
| 11897 | AF402687 | 347 | − |
| 9297 | AF402687 | 347 | − |
| 12097 | AF402686 | 345 | + |
| AF402687 | 347 | + | |
| 0398 | AF402683 | 348 | + |
| AF402684 | 379 | + | |
| 11797 | AF402688 | 347 | + |
| AF402694 | 376 | + |
+, present; −, absent.
FIG. 2.
Multiple sequence alignment of mt LSU rRNA fragments of rhesus macaque-derived P. carinii organisms. DNA sequence alignment of a portion of the Pneumocystis carinii mt LSU rRNA genes amplified from SIV-immunosuppressed rhesus macaque BAL fluid samples was performed with Gene Inspector version 1.5 software. In samples with mixed infections, PCR products were cloned and sequenced separately (0398-8, 0398-9, 11797-1, and 11797-3).
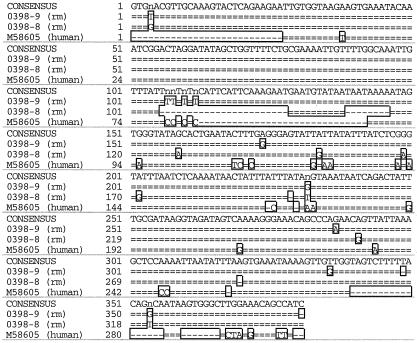
A comparison of the nucleic acid sequences of this locus to the Pneumocystis mt LSU rRNA sequences derived from other mammalian hosts was carried out, and the percent divergence was calculated (Table 3). The sequence from monkey 0398 showed the closest similarity to human-derived Pneumocystis (12.2% divergence compared to 29.2% divergence between macaque- and rat-derived Pneumocystis organisms) (Fig. 3). The divergence between rat- and macaque-derived Pneumocystis variants is nearly as great as that between P. carinii f. sp. carinii, the rat prototypic strain, and S. cerevisiae, another member of the Ascomycota (Table 3).
TABLE 3.
Percentages of divergence of mt LSU rRNA PCR products of P. carinii organisms from different host species
| Species (accession no.) | % Divergence
|
|||||
|---|---|---|---|---|---|---|
| P. carinii f. sp. hominis | P. carinii f. sp. oryctolagi | P. carinii f. sp. mustelae | P. carinii f. sp. carinii | P. carinii f. sp. ratti | S. cerevisiae L36903 | |
| P. carinii f. sp. macaca (AF402684) | 12.2 | 20.8 | 26.8 | 28.3 | 29.2 | 37.6 |
| P. carinii f. sp. hominis (M58605) | 14 | 15.7 | 27.5 | 29.2 | 33.4 | |
| P. carinii f. sp. oryctolagi (S42915) | 20.7 | 23.8 | 25 | 35.2 | ||
| P. carinii f. sp. mustelae (S42921) | 21.8 | 21.7 | 32.7 | |||
| P. carinii f. sp. carinii (U20169) | 14.1 | 31.6 | ||||
| P. carinii f. sp. ratti (U20173) | 36.6 | |||||
FIG. 3.
Multiple sequence alignment of human- and rhesus macaque-derived P. carinii mt LSU rRNA genes. DNA sequences of a portion of the P. carinii mt LSU rRNAs from P. carinii f. sp. macaca (rm) from monkey 0398 and P. carinii f. sp. hominis (human) were aligned as described in the text.
Samples from monkeys 1799, 5199, and 8397 had PCR products with identical sequences; however, 1799 and 5199 had an additional unique mt LSU rRNA amplicon. These results are consistent with the electrophoretic pattern observed (Fig. 1) and a mixed infection. Animals 1799 and 5199 were housed in the same room, and the SIV infection was initiated at the same time (Table 1). Both animals rapidly progressed to AIDS, and they were sacrificed 164 and 181 days after SIV infection. Interestingly, monkey 8397 had no contact with either 1799 or 5199 and was sacrificed prior to their arrival at the facility. A second group of animals, 12097 and 11897, also had identical sequences, were part of the same SIV infection cohort (Table 1), and were housed in the facility for several months during the same time period.
DISCUSSION
P. carinii was originally considered a single species capable of infecting a wide range of mammalian hosts. Molecular analyses have demonstrated that the taxonomy of this organism is very complex (11, 24, 30, 33, 39). There is a high level of diversity at a number of unlinked loci in Pneumocystis organisms derived from different hosts. In addition, Pneumocystis is not readily transmissible between mammalian hosts, suggesting that Pneumocystis is host species specific (15). Genetic heterogeneity has been examined at several loci, including the mt LSU rRNA (37), the nuclear rRNA operon (26), and the folic acid synthase gene (23). In addition to the genetic diversity seen in these loci in Pneumocystis organisms derived from different hosts, there is also significant diversity in some loci in Pneumocystis organisms derived from the same hosts. These studies have demonstrated that Pneumocystis is comprised of many different types of organisms with specific host tropisms, and accumulating evidence supports the concept that organisms derived from different mammalian hosts are distinct species (35).
In this study, we analyzed DNA samples from BAL fluid of SIV-infected rhesus macaques that were infected with Pneumocystis. PCR of the mt LSU locus of Pneumocystis revealed the presence of two distinct products, and the DNA sequence analysis confirmed that both of these were mt LSU rRNA amplicons. PCR and nucleic acid sequence analysis also provided evidence of a mixed infection in several of the animals (Fig. 1 and 2). These results are consistent with those showing the presence of mixed infections in humans and rats (30).
Sequences of the mt LSU locus in rat- and macaque-derived Pneumocystis organisms show nearly 30% divergence (Table 3). This striking level of diversity in the mt LSU loci among members of the same species was nearly as great as that between P. carinii f. sp. carinii and S. cerevisiae. Other studies have indicated that human and nonhuman-primate-derived Pneumocystis organisms are most closely related (10, 17, 39), and our results confirm this conclusion (Table 3). In addition to the high level of heterogeneity observed at the mt LSU rRNA site between macaque-derived Pneumocystis and Pneumocystis organisms derived from other hosts, we observed up to 13% divergence among macaque-derived sequences. Most of the difference was due to the presence of a 26-nucleotide insert present in some of the isolates (Fig. 2). This insert is thus far unique to primate-derived Pneumocystis, although it was not present in all of the isolates. Two apparently distinct genotypes were reported for the internal transcribed spacer locus from macaque-derived Pneumocystis DNA, suggestive of two strains in this host; however, no mixed infections were observed (19). Our results at the mt LSU site support the distinction of two macaque-derived Pneumocystis variants, and the presence of both types was readily detectable in individual animals.
The identification of distinct variants of Pneumocystis in the macaque model should facilitate studies of transmission in this host, since strains can be easily tracked by mt LSU rRNA sequence. Recently, our laboratory has developed an experimental model of Pneumocystis infection in rhesus macaques (4). In these studies, Pneumocystis was introduced intrabronchially, and the progression of infection was monitored by Pneumocystis-specific PCR of BAL fluid (4). In addition to its usefulness as a diagnostic probe, the sequence variability at the mt LSU locus allows for the distinction between the presence of the inoculating strain and a naturally acquired infection in this model. In addition, the sequence variability at the mt LSU site makes transmission studies feasible with the primate model.
In summary, these results support the concept that Pneumocystis organisms derived from different mammalian hosts represent different species and that these organisms likely coevolved with their mammalian hosts, as has been suggested by Demanche and colleagues (10). In addition, the genetic heterogeneity observed at the mt LSU locus of primate-derived Pneumocystis provides a useful marker for transmission and epidemiological studies of this animal model of PCP.
Acknowledgments
This work was supported by National Institutes of Health Heart Lung Blood Institute grant HL064563 (K.A.N.).
We thank Irina Lebedeva for technical assistance, Anita Trichel for veterinary services, and Michael Murphey-Corb for kindly providing BAL samples.
REFERENCES
- 1.Baskerville, A., A. B. Dowsett, R. W. Cook, M. J. Dennis, M. P. Cranage, and P. J. Greenaway. 1991. Pneumocystis carinii pneumonia in simian immunodeficiency virus infection: immunohistological and scanning and transmission electron microscopical studies. J. Pathol. 164:175-184. [DOI] [PubMed] [Google Scholar]
- 2.Baskerville, A., A. Ramsay, M. P. Cranage, N. Cook, R. W. Cook, M. J. Dennis, P. J. Greenaway, P. A. Kitchin, and E. J. Stott. 1990. Histopathological changes in simian immunodeficiency virus infection. J. Pathol. 162:67-85. [DOI] [PubMed] [Google Scholar]
- 3.Baskin, G. B., M. Murphey-Corb, E. A. Watson, and L. N. Martin. 1988. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/delta. Vet. Pathol. 25:456-467. [DOI] [PubMed] [Google Scholar]
- 4.Board, K. F., S. Patil, I. Lebedeva, S. Capuano III, A. M. Trichel, M. Murphey-Corb, P. A. Rajakumar, J. L. Flynn, C. G. Haidaris, and K. A. Norris. 2003. Experimental Pneumocystis carinii pneumonia in SIV-infected rhesus macaques. J. Infect. Dis. 187:576-587. [DOI] [PubMed] [Google Scholar]
- 5.Boylan, C. J., and W. L. Current. 1992. Improved rat model of Pneumocystis carinii pneumonia: induced laboratory infections in Pneumocystis-free animals. Infect. Immun. 60:1589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalifoux, L. V., D. J. Ringler, N. W. King, P. K. Sehgal, R. C. Desrosiers, M. D. Danial, and N. L. Letvin. 1987. Lymphadenopathy in macaques experimentally infected with the simian immunodeficiency virus. Am. J. Pathol. 128:104-110. [PMC free article] [PubMed] [Google Scholar]
- 7.Croix, D. A., K. Board, S. Capuano III, M. Murphey-Corb, C. G. Haidaris, J. L. Flynn, T. Reinhart, and K. A. Norris. 2002. Alterations in T lymphocyte profiles of bronchoalveolar lavage fluid from SIV- and Pneumocystis carinii-coinfected rhesus macaques. AIDS Res. Hum. Retrovir. 18:391-401. [DOI] [PubMed] [Google Scholar]
- 8.Das, B., J. Sharma, V. Gopalakrishna, and U. Luthra. 1992. Analysis by polymerase chain reaction of the physical state of human papillomavirus type 16 DNA in cervical preneoplastic and neoplastic lesions. J. Gen. Virol. 73:2327-2336. [DOI] [PubMed] [Google Scholar]
- 9.Dei-Cas, E., M. Brun-Pascaud, V. Bille-Hansen, A. Allaert, and E. A. Aliouat. 1998. Animal models of pneumocystosis. FEMS Immunol. Med. Microbiol. 22:163-168. [DOI] [PubMed] [Google Scholar]
- 10.Demanche, C., M. Berthelemy, T. Petit, B. Polack, A. E. Wakefield, E. Dei-Cas, and J. Guillot. 2001. Phylogeny of Pneumocystis carinii from 18 primate species confirms host specificity and suggests coevolution. J. Clin. Microbiol. 39:2126-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denis, C., E. Mazars, K. Guyot, C. Oderberg-Ferragut, E. Viscoglios, E. Dei-Cas, and A. E. Wakefield. 2000. Genetic divergence at the SODA locus of six different forma speciales of Pneumocystis carinii. Med. Mycol. 38:289-300. [DOI] [PubMed] [Google Scholar]
- 12.Durand-Joly, I., A. Wakefield, R. Palmer, C. Denis, C. Creusy, L. Fleurisse, I. Ricard, J. Gut, and E. Dei-Cas. 2000. Ultrastructural and molecular characterization of Pneumocystis carinii isolated from a rhesus monkey (Macaca mulatta). Med. Mycol. 38:61-72. [DOI] [PubMed] [Google Scholar]
- 13.Furuta, T., M. Fujita, R. Mukai, I. Sakakibara, T. Sata, K. Miki, M. Hayami, S. Kojima, and Y. Yoshikawa. 1993. Severe pulmonary pneumocystosis in simian acquired immunodeficiency syndrome induced by simian immunodeficiency virus: its characterization by the polymerase-chain-reaction method and failure of experimental transmission to immunodeficient animals. Parasitol. Res. 79:624-628. [DOI] [PubMed] [Google Scholar]
- 14.Gigliotti, F. 1992. Host species-specific antigenic variation of a mannosylated surface glycoprotein of Pneumocystis carinii. J. Infect. Dis. 165:329-336. [DOI] [PubMed] [Google Scholar]
- 15.Gigliotti, F., A. G. Harmsen, C. G. Haidaris, and P. J. Haidaris. 1993. Pneumocystis carinii is not universally transmissible between mammalian species. Infect. Immun. 61:2886-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottleib, M., R. Schroff, H. Schanker, et al. 1981. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N. Engl. J. Med. 305:1425-1431. [DOI] [PubMed] [Google Scholar]
- 17.Guillot, J., C. Demanche, J. P. Hugot, M. Berthelemy, A. E. Wakefield, E. Dei-Cas, and R. Chermette. 2001. Parallel phylogenies of Pneumocystis species and their mammalian hosts. J. Eukaryot. Microbiol. 38(Suppl.):113S-115S. [DOI] [PubMed] [Google Scholar]
- 18.Hanano, R., and S. H. E. Kaufmann. 1998. Pneumocystis carinii and the immune response in disease. Trends Microbiol. 6:71-76. [DOI] [PubMed] [Google Scholar]
- 19.Hsueh, J. Y. C., R. P. Bohm, Jr., P. J. Didier, X. Tang, M. E. Lasbury, B. Li, S. Jin, M. S. Bartlett, J. W. Smith, and C.-H. Lee. 2001. Internal transcribed spacer regions of rRNA genes of Pneumocystis carinii from monkeys. Clin. Diagn. Lab. Immunol. 8:503-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulskotte, E. G., A. M. Geretti, and A. Osterhaus. 1998. Towards an HIV-1 vaccine: lessons from studies in macaque models. Vaccine 16:904-915. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs, J. A., J. Hiemenz, A. Macher, D. Stover, H. Murray, J. Shelhamer, H. Lane, C. Urmacher, C. Honig, D. Longo, M. Parker, C. Natanson, J. Parrillo, A. Fauci, P. Pizzo, and H. Masur. 1984. Pneumocystis carinii pneumonia: a comparison between patients with the acquired immunodeficiency syndrome and patients with other immunodeficiencies. Ann. Intern. Med. 100:663-671. [DOI] [PubMed] [Google Scholar]
- 22.Kunz, S., U. Junker, J. Blaser, et al. 1995. The scid mouse as an experimental model for the evaluation of anti-Pneumocystis therapy. J. Antimicrob. Chemother. 36:137-155. [DOI] [PubMed] [Google Scholar]
- 23.Lane, B. R., J. C. Ast, P. A. Hossler, D. P. Mindell, M. S. Bartlett, J. W. Smith, and S. R. Meshnick. 1997. Dihydropteroate synthase polymorphisms in Pneumocystis carinii. J. Infect. Dis. 175:482-485. [DOI] [PubMed] [Google Scholar]
- 24.Latouche, S., P. Roux, J. L. Poirit, I. Lavrard, B. Hermelin, and V. Bertrand. 1994. Preliminary results of Pneumocystis carinii strain differentiation by using molecular biology. J. Clin. Microbiol. 32:3052-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy, J. A. 1996. The value of primate models for studying human immunodeficiency virus pathogenesis. J. Med. Primatol. 25:163-174. [DOI] [PubMed] [Google Scholar]
- 26.Lu, J. J., M. S. Bartlett, M. M. Shaw, S. F. Queener, J. W. Smith, M. Ortiz-Rivera, M. J. Leibowitz, and C.-H. Lee. 1994. Typing of Pneumocystis carinii strains that infect humans based on nucleotide sequence variations of internal transcribed spacers of rRNA genes. J. Clin. Microbiol. 32:2904-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansharamani, N., D. Balachandran, I. Vernovsky, R. Garland, and H. Koziel. 2000. Peripheral blood CD4+ T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest 118:712-720. [DOI] [PubMed] [Google Scholar]
- 28.Masur, H., M. Michelis, J. Greene, et al. 1981. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N. Engl. J. Med. 305:1431-1438. [DOI] [PubMed] [Google Scholar]
- 29.Nuesch, R., C. Bellini, and W. Zimmerli. 1999. Pneumocystis carinii pneumonia in human immunodeficiency virus (HIV)-positive and HIV-negative immunocompromised patients. Clin. Infect. Dis. 29:1519-1523. [DOI] [PubMed] [Google Scholar]
- 30.Palmer, R., M. Cushion, and A. E. Wakefield. 1993. Discrimination of rat-derived Pneumocystis carinii f. sp. carinii and Pneumocystis carinii f. sp. ratti using the polymerase chain reaction. Mol. Cell. Probes 13:147-155. [DOI] [PubMed] [Google Scholar]
- 31.The Pneumocystis Workshop. 1994. Revised nomenclature for Pneumocystis carinii. J. Eukaryot. Microbiol. 41:121S-122S. [PubMed]
- 32.Shellito, J. E., V. Suzara, W. Blumenfeld, J. M. Beck, H. Steger, and T. Ermack. 1990. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J. Clin. Investig. 85:1686-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinclair, K., A. E. Wakefield, S. Banerji, and J. M. Hopkin. 1991. Pneumocystis carinii organisms derived from rat and human hosts are genetically distinct. Mol. Biochem. Parasitol. 45:183-184. [DOI] [PubMed] [Google Scholar]
- 34.Smulian, A. G., S. P. Keely, S. M. Sunkin, and J. R. Stringer. 1997. Genetic and antigenic variation in Pneumocystis carinii organisms: tools for examining the epidemiology and pathogenesis of infection. J. Lab. Clin. Med. 130:461-468. [DOI] [PubMed] [Google Scholar]
- 35.Stringer, J. R., C. Beard, R. Miller, and A. E. Wakefield. 2002. A new name (Pneumocystis jiroveci) for Pneumocystis in humans. Emerg. Infect. Dis. 8:891-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stringer, J. R., and S. P. Keely. 2001. Genetics of surface antigen expression in Pneumocystis carinii. Infect. Immun. 69:627-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakefield, A. E., F. Pixley, S. Banerji, K. Sinclair, R. Miller, E. Moxon, and J. M. Hopkin. 1990. Amplification of mitochondrial ribosomal RNA sequences from Pneumocystis carinii DNA of rat and human origin. Mol. Biochem. Parasitol. 43:69-76. [DOI] [PubMed] [Google Scholar]
- 38.Wakefield, A. E., F. J. Pixley, S. Banerji, K. Sinclair, R. F. Miller, E. R. Moxon, and J. M. Hopkin. 1990. Detection of Pneumocystis carinii with DNA amplification. Lancet 336:451-453. [DOI] [PubMed] [Google Scholar]
- 39.Wright, T. W., F. Gigliotti, C. G. Haidaris, and P. J. Simpson-Haidaris. 1995. Cloning and characterization of a conserved region of human and rhesus macaque Pneumocystis carinii gpA. Gene 167:185-189. [DOI] [PubMed] [Google Scholar]
- 40.Yale, S., and A. Limper. 1996. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illnesses and prior corticosteroid therapy. Mayo Clin. Proc. 71:5-13. [DOI] [PubMed] [Google Scholar]